Effects of Acetaminophen Contamination on 5-Methylcytosine Content in Zea mays and Plant Physiological Parameters
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Conditions
2.2. Photosynthesis and Gas Exchange Parameters
2.3. Water Potential Analysis
2.4. Proline Content Analysis
2.5. Determination of 5-Methylcytosine Content
2.6. Microscopy
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ok, Y.S.; Kim, S.-C.; Kim, K.-R.; Lee, S.S.; Moon, D.H.; Lim, K.J.; Sung, J.-K.; Hur, S.-O.; Yang, J.E. Monitoring of selected veterinary antibiotics in environmental compartments near a composting facility in Gangwon Province, Korea. Environ. Monit. Assess. 2011, 174, 693–701. [Google Scholar] [CrossRef]
- Boxall, A.B.A.; Rudd, M.A.; Brooks, B.W.; Caldwell, D.J.; Choi, K.; Hickmann, S.; Innes, E.; Ostapyk, K.; Staveley, J.P.; Verslycke, T.; et al. Pharmaceuticals and Personal Care Products in the Environment: What Are the Big Questions? Environ. Health Perspect. 2012, 120, 1221–1229. [Google Scholar] [CrossRef]
- OECD. Available online: https://www.oecd-ilibrary.org/sites/5689c05c-en/index.html?itemId=/content/component/5689c05c-en (accessed on 16 May 2023).
- Doll, T.E.; Frimmel, F.H. Fate of pharmaceuticals––Photodegradation by simulated solar UV-light. Chemosphere 2003, 52, 1757–1769. [Google Scholar] [CrossRef]
- Jjemba, P.K. Excretion and ecotoxicity of pharmaceutical and personal care products in the environment. Ecotoxicol. Environ. Saf. 2006, 63, 113–130. [Google Scholar] [CrossRef]
- Choi, K.; Kim, Y.; Park, J.; Park, C.K.; Kim, M.; Kim, H.S.; Kim, P. Seasonal Variations of Several Pharmaceutical Residues in Surface Water and Sewage Treatment Plants of Han River, Korea. Sci. Total Environ. 2008, 405, 120–128. [Google Scholar] [CrossRef]
- Fatta-Kassinos, D.; Vasquez, M.I.; Kümmerer, K. Transformation products of pharmaceuticals in surface waters and wastewater formed during photolysis and advanced oxidation processes–degradation, elucidation of byproducts and assessment of their biological potency. Chemosphere 2011, 85, 693–709. [Google Scholar] [CrossRef]
- Brodin, T.; Piovano, S.; Fick, J.; Klaminder, J.; Heynen, M.; Jonsson, M. Ecological effects of pharmaceuticals in aquatic systems-impacts through behavioural alterations. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130580. [Google Scholar] [CrossRef]
- Rehman, M.S.U.; Rashid, N.; Ashfaq, M.; Saif, A.; Ahmad, N.; Han, J.I. Global risk of pharmaceutical contamination from highly populated developing countries. Chemosphere 2015, 138, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Couto, C.F.; Lange, L.C.; Amaral, M.C.S. Occurrence, fate and removal of pharmaceutically active compounds (PhACs) in water and wastewater treatment plants—A review. J. Water Process Eng. 2019, 32, 100927. [Google Scholar] [CrossRef]
- Zheng, C.; Liu, J.; Cai, Y.; Jing, C.; Jiang, R.; Zheng, X.; Lu, G. Pharmaceutically active compounds in biotic and abiotic media of rivers receiving urban sewage: Concentrations, bioaccumulation and ecological risk. Process Saf. Environ. Prot. 2022, 166, 491–499. [Google Scholar] [CrossRef]
- Kümmerer, K.; Steger-Hartmann, T.; Meyer, M. Biodegradability of the anti-tumour agent ifosfamide and its occurrence in hospital effluents and communal sewage. Water Res. 1997, 31, 2705–2710. [Google Scholar] [CrossRef]
- Kuhne, M.; Ihnen, D.; Moller, G.; Agthe, O. Stability of tetracycline in water and liquid manure. J. Vet. Med. Ser. 2000, 47, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Torsvik, V.; Goksøyr, J.; Daae, F.L. High diversity in DNA of soil bacteria. Appl. Environ. Microbiol. 1990, 56, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Westergaard, K.; Müller, A.K.; Christensen, S.; Bloem, J.; Sørensen, S.J. Effects of tylosin as a disturbance on the soil microbial community. Soil Biol. Biochem. 2001, 33, 2061–2071. [Google Scholar] [CrossRef]
- Leitão, I.; Martins, L.L.; Carvalho, L.; Oliveira, M.C.; Marques, M.M.; Mourato, M.P.; Spagnuolo, V.; Capozzi, F. Acetaminophen Induces an Antioxidative Response in Lettuce Plants. Plants 2021, 10, 1152. [Google Scholar] [CrossRef] [PubMed]
- Jjemba, P.K.; Robertson, B.K. Antimicrobial Agents with Improved Clinical Efficacy versus Their Persistence in the Environment: Synthetic 4-Quinolone as an Example. EcoHealth 2005, 2, 171–182. [Google Scholar] [CrossRef]
- Nunes, B.; Pinto, G.; Martins, L.; Gonçalves, F.; Antunes, S.C. Biochemical and standard toxic effects of acetaminophen on the macrophyte species Lemna minor and Lemna gibba. Environ. Sci. Pollut. Res. 2014, 21, 10815–10822. [Google Scholar] [CrossRef]
- Lukens, L.N.; Zhan, S. The plant genome’s methylation status and response to stress: Implications for plant improvement. Curr. Opin. Plant Biol. 2007, 10, 317–322. [Google Scholar] [CrossRef]
- Chen, M.; Lv, S.; Meng, Y. Epigenetic performers in plants. Dev. Growth Differ. 2010, 52, 555–566. [Google Scholar] [CrossRef]
- Grativol, C.; Hemerly, A.S.; Ferreira, P.C.G. Genetic and Epigenetic Regulation of Stress Responses in Natural Plant Populations. Biochim. Biophys. Acta-Gene Regul. Mech. 2012, 1819, 176–185. [Google Scholar] [CrossRef]
- Kohli, R.M.; Zhang, Y. TET Enzymes, TDG and the Dynamics of DNA Demethylation. Nature 2013, 502, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Wu, H.; Diep, D.; Yamaguchi, S.; D’Alessio, A.C.; Fung, H.L.; Zhang, K.; Zhang, Y. Genome-Wide Analysis Reveals TET- and TDG-Dependent 5-Methylcytosine Oxidation Dynamics. Cell 2013, 153, 692–706. [Google Scholar] [CrossRef]
- Espinas, N.A.; Saze, H.; Saijo, Y. Epigenetic control of defense signaling and priming in plants. Front. Plant Sci. 2016, 7, 1201. [Google Scholar] [CrossRef] [PubMed]
- Thiebaut, F.; Hemerly, A.S.; Ferreira, P.C.G. A Role for Epigenetic Regulation in the Adaptation and Stress Responses of Non-Model Plants. Front. Plant Sci. 2019, 10, 246. [Google Scholar] [CrossRef] [PubMed]
- Bossdorf, O.; Arcuri, D.; Richards, C.L.; Pigliucci, M. Experimental alteration of DNA methylation affects the phenotypic plasticity of ecologically relevant traits in Arabidopsis thaliana. Evol. Ecol. 2010, 24, 541–553. [Google Scholar] [CrossRef]
- Iwase, Y.; Shiraya, T.; Takeno, K. Flowering and Dwarfism Induced by DNA Demethylation in Pharbitis nil. Physiol. Plant. 2010, 139, 118–127. [Google Scholar] [CrossRef]
- Ba, Q.; Zhang, G.; Wang, J.; Niu, N.; Ma, S.; Wang, J. Gene expression and DNA methylation alterations in chemically induced male sterility anthers in wheat (Triticum aestivum L.). Acta Physiol. Plant. 2014, 36, 503–512. [Google Scholar] [CrossRef]
- Lechat, M.M.; Brun, G.; Montiel, G.; Véronési, C.; Simier, P.; Thoiron, S.; Pouvreau, J.B.; Delavault, P. Seed response to strigolactone is controlled by abscisic acid-independent DNA methylation in the obligate root parasitic plant, Phelipanche ramosa L. Pomel. J. Exp. Bot. 2015, 66, 3129–3140. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Snyder, W.C. Nutrition of Strawberry Plant under Controlled Conditions. (a) Effects of Deficiencies of Boron and Certain Other Elements, (b) Susceptibility to Injury from Sodium Salts. Proc. Am. Soc. Hortic. Sci. 1933, 30, 288–294. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. Growing plants without soil by the water-culture method. Grow. Plants Without Soil Water-Cult. Method 1938, 347, 16. [Google Scholar]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 32. [Google Scholar]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants; BBCH Monograph: Berlin, Germany, 1997. [Google Scholar]
- Kuklová, M.; Hniličková, H.; Kukla, J.; Hnilička, F. Environmental impact of the Al smelter on physiology and macronutrient contents in plants and Cambisols. Plant Soil Environ. 2015, 61, 72–78. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Dold, C. Water-use efficiency: Advances and challenges in a changing climate. Front. Plant Sci. 2019, 10, 103. [Google Scholar] [CrossRef]
- Banks, J.M.; Hirons, A.D. Alternative methods of estimating the water potential at turgor loss point in Acer genotypes. Plant Methods 2019, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Zemanová, V.; Popov, M.; Pavlíková, D.; Kotrba, P.; Hnilička, F.; Česká, J.; Pavlík, M. Effect of arsenic stress on 5-methylcytosine, photosynthetic parameters and nutrient content in arsenic hyperaccumulator Pteris cretica (L.) var. Albo-lineata. BMC Plant Biol. 2020, 20, 130. [Google Scholar] [CrossRef] [PubMed]
- Smilauer, P.; Leps, J. Multivariate Analysis of Ecological Data Using Canoco 5; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Sabourin, L.; Duenk, P.; Bonte-Gelok, S.; Payne, M.; Lapen, D.R.; Topp, E. Uptake of pharmaceuticals, hormones and parabens into vegetables grown in soil fertilized with municipal biosolids. Sci. Total Environ. 2012, 431, 233–236. [Google Scholar] [CrossRef]
- Calderon-Preciado, D.; Matamoros, V.; Save, R.; Munoz, P.; Biel, C.; Bayona, J.M. Uptake of microcontaminants by crops irrigated with reclaimed water and groundwater under realfield greenhouse conditions. Environ. Sci. Pollut. Res. 2013, 20, 3629–3638. [Google Scholar] [CrossRef]
- Vasquez, M.I.; Lambrianides, A.; Schneider, M.; Kümmerer, K.; Fatta-Kassinos, D. Environmental side effects of pharmaceutical cocktails: What we know and what we should know. J. Hazard. Mater. 2014, 279, 169–189. [Google Scholar] [CrossRef]
- Schroeder, L.M.; Blackwell, B.; Klein, D.; Morse, A.N. Rate uptake of three common pharmaceuticals in celery. Apium Graveolens. Water Air Soil Pollut. 2015, 226, 123. [Google Scholar] [CrossRef]
- Azanu, D.; Mortey, C.; Darko, G.; Weisser, J.J.; Styrishave, B.; Abaidoo, R.C. Uptake of antibiotics from irrigation water by plants. Chemosphere 2016, 157, 107–114. [Google Scholar] [CrossRef]
- Christou, A.; Karaolia, P.; Hapeshi, E.; Michael, C.; Fatta-Kassinos, D. Long-term wastewater irrigation of vegetables in real agricultural systems: Concentration of pharmaceuticals in soil, uptake and bioaccumulation in tomato fruits and human health risk assessment. Water Res. 2017, 109, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Di Baccio, D.; Pietrini, F.; Bertolotto, P.; Pérez, S.; Barcelò, D.; Zacchini, M.; Donati, E. Response of Lemna gibba L. to High and Environmentally Relevant Concentrations of Ibuprofen: Removal, Metabolism and Morpho-Physiological Traits for Biomonitoring of Emerging Contaminants. Sci. Total Environ. 2017, 584–585, 363–373. [Google Scholar] [CrossRef]
- Christou, A.; Kyriacou, M.C.; Georgiadou, E.C.; Papamarkou, R.; Hapeshi, E.; Karaolia, P.; Michael, C.; Fotopoulos, V.; Fatta-Kassinos, D. Uptake and bioaccumulation of three widely prescribed pharmaceutically active compounds in tomato fruits and mediated effects on fruit quality attributes. Sci. Total Environ. 2019, 647, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Rede, D.; Santos, L.H.M.L.M.; Ramos, S.; Oliva-Teles, F.; Antão, C.; Sousa, S.R.; Delerue-Matos, C. Individual and mixture toxicity evaluation of three pharmaceuticals to the germination and growth of Lactuca sativa seeds. Sci. Total Environ. 2019, 673, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Kudrna, J.; Hnilicka, F.; Kubes, J.; Vachova, P.; Hnilickova, H.; Kuklova, M. Effect of acetaminophen (APAP) on physiological Indicators in Lactuca sativa. Life 2020, 10, 303. [Google Scholar] [CrossRef]
- Kummerová, M.; Zezulka, S.; Babula, P.; Triska, J. Possible ecological risk of two pharmaceuticals diclofenac and paracetamol demonstrated on a model plant Lemna minor. J. Hazard. Mater. 2016, 302, 351–361. [Google Scholar] [CrossRef]
- Al-Muwayhi, M.A.R. Paracetamol Mediated Changes Modifies the Photosynthetic Efficiency of Vigna Radiata. Legum. Res. 2018, 41, 842–845. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Hayat, S.; Ahmad, A. Salicylic Acid Influences Net Photosynthetic Rate, Carboxylation Efficiency, Nitrate Reductase Activity, and Seed Yield in Brassica juncea. Photosynthetica 2003, 41, 281–284. [Google Scholar] [CrossRef]
- Khan, W.; Prithiviraj, B.; Smith, D.L. Photosynthetic responses of corn and soybean to foliar application of salicylates. J. Plant Physiol. 2003, 160, 485–492. [Google Scholar] [CrossRef]
- Taschina, M.; Copolovici, D.M.; Bungau, S.G.; Andreea, L.P.; Copolovici, L.; Iovan, C. The influence of residual acetaminophen on Phaseolus vulgaris L. Secondary metabolites. Farmacia 2017, 65, 709–713. [Google Scholar]
- Mahouachi, J.; Socorro, A.R.; Talon, M. Responses of papaya seedlings (Carica papaya L.) to water stress and re-hydration: Growth, photosynthesis and mineral nutrient imbalance. Plant Soil 2006, 281, 137–146. [Google Scholar] [CrossRef]
- Kumar, P.; Lakshmi, N.J.; Mani, V.P. Interactive effects of salicylic acid and phytohormones on photosynthesis and grain yield of soybean (Glycine max L.). Physiol. Mol. Biol. Plants 2000, 6, 179–186. [Google Scholar]
- Vernay, P.; Gauthier-Moussard, C.; Hitmi, A. Interaction of bioaccumulation of heavy metal chromium with water relation, mineral nutrition and photosynthesis in developed leaves of Lolium perenne L. Chemosphere 2007, 68, 1563–1575. [Google Scholar] [CrossRef]
- Kaznina, N.M.; Titov, A.F.; Batova, Y.V.; Laidinen, G.F. The resistance of plants Setaria veridis (L.) Beauv. to the influence of cadmium. Biol. Bull. 2014, 41, 428–433. [Google Scholar] [CrossRef]
- Barcelo, J.; Vazquez, M.D.; Poschenrieder, C.H. Structural and ultrastructural disorders in cadmium-treated bush bean plants (Phaseolus vulgaris L.). New Phytol. 1988, 108, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Steudle, E. Water uptake by plant roots: An integration of views. Plant Soil 2000, 226, 45–56. [Google Scholar] [CrossRef]
- Javot, H.; Maurel, C. The role of aquaporins in root water uptake. Ann. Bot. 2002, 90, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, A.; Sharma, I.; Tripathi, B.; Munjal, A.; Baunthiyal, M.; Sharma, V. Metal toxicity and photosynthesis. In Photosynthesis: Overviews on Recent Progress and Future Perspectives; Itoh, S., Mohanty, P., Guruprasad, K.N., Eds.; IK International Publishing House: New Delhi, India, 2012; pp. 229–236. [Google Scholar]
- Ahmed, M.B.M.; Rajapaksha, A.U.; Lim, J.E.; Vu, N.T.; Kim, I.S.; Kang, H.M.; Lee, S.S.; Ok, Y.S. Distribution and Accumulative Pattern of Tetracyclines and Sulfonamides in Edible Vegetables of Cucumber, Tomato, and Lettuce. J. Agric. Food Chem. 2015, 63, 398–405. [Google Scholar] [CrossRef]
- Yakubu, O.H. Pharmaceutical wastewater effluent-source of contaminants of emerging concern: Phytotoxicity of metronidazole to soybean (Glycine max). Toxics 2017, 5, 10. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, L.; Natarajan, S.K.; Becker, D.F. Proline Mechanisms of Stress Survival. Antioxid. Redox Signal. 2013, 19, 998–1011. [Google Scholar] [CrossRef] [PubMed]
- Silva-Ortega, C.O.; Ochoa-Alfaro, A.E.; Reyes-Agüero, J.A.; Aguado-Santacruz, G.A.; Jiménez-Bremont, J.F. Salt stress increases the expression of p5cs gene and induces proline accumulation in cactus pear. Plant Physiol. Biochem. 2008, 46, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Soshinkova, T.N.; Radyukina, N.L.; Korolkova, D.V.; Nosov, A.V. Proline and functioning of the antioxidant system in Thellungiella salsuginea plants and cultured cells subjected to oxidative stress. Russ. J. Plant Physiol. 2012, 60, 41–54. [Google Scholar] [CrossRef]
- Szabados, L.; Savoure, A. Proline: A multifunctional amino acid. Trends Plant Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Villamor, J.G.; Verslues, P.E. Essential role of tissue-specific proline synthesis and catabolism in growth and redox balance at low water potential. Plant Physiol. 2011, 157, 292–304. [Google Scholar] [CrossRef]
- Gharsallah, C.; Fakhfakh, H.; Grubb, D.; Gorsane, F. Effect of salt stress on ion concentration, proline content, antioxidant enzyme activities and gene expression in tomato cultivars. AoB Plants 2016, 8, plw055. [Google Scholar] [CrossRef]
- Jungklang, J.; Saengnil, K.; Uthaibutra, J. Effects of water-deficit stress and paclobutrazol on growth, relative water content, electrolyte leakage, proline content and some antioxidant changes in Curcuma alismatifolia Gagnep. cv. Chiang Mai Pink. Saudi J. Biol. Sci. 2017, 24, 1505–1512. [Google Scholar] [CrossRef]
- Martins, M.; Sousa, B.; Lopes, J.; Soares, C.; Machado, J.; Carvalho, S.; Fidalgo, F.; Teixeira, J. Diclofenac shifts the role of root glutamine synthetase and glutamate dehydrogenase for maintaining nitrogen assimilation and proline production at the expense of shoot carbon reserves in Solanum lycopersicum L. Environ. Sci. Pollut. Res. 2020, 27, 29130–29142. [Google Scholar] [CrossRef]
- Marco, F.; Bitrián, M.; Carrasco, P.; Rajam, M.V.; Alcázar, R.; Tiburcio, A.F. Genetic engineering strategies for abiotic stress tolerance in plants. In Plant Biology and Biotechnology; Springer: New Dehli, India, 2015; pp. 579–609. [Google Scholar]
- Khan, A.; Yadav, N.S.; Morgenstern, Y.; Zemach, A.; Grafi, G. Activation of Tag1 transposable elements in Arabidopsis dedifferentiating cells and their regulation by CHROMOMETHYLASE 3-mediated CHG methylation. Biochim. Biophys. Acta 2016, 1859, 1289–1298. [Google Scholar] [CrossRef]
- Wang, H.; He, L.; Song, J.; Cui, W.; Zhang, Y.; Jia, C.; Francis, D.; Rogers, H.J.; Sun, L.; Tai, P.; et al. Cadmium-induced genomic instability in Arabidopsis: Molecular toxicological biomarkers for early diagnosis of cadmium stress. Chemosphere 2016, 150, 258–265. [Google Scholar] [CrossRef]
- Hao, X.F.; Jin, Z.P.; Wang, Z.Q.; Qin, Q.S.; Pei, Y.X. Hydrogen sulfide mediates DNA methylation to enhance osmotic stress tolerance in Setaria italica L. Plant Soil 2020, 453, 355–370. [Google Scholar] [CrossRef]
- Chakrabarti, M.; Mukherjee, A. Investigating the Underlying Mechanism of Cadmium-Induced Plant Adaptive Response to Genotoxic Stress. Ecotoxicol. Environ. Saf. 2021, 209, 111817. [Google Scholar] [CrossRef] [PubMed]
- Luo, D.; Lu, H.; Wang, C.; Mubeen, S.; Cao, S.; Yue, Y.; Pan, J.; Wu, X.; Wu, Q.; Zhang, H.; et al. Physiological and DNA methylation analysis provides epigenetic insights into kenaf cadmium tolerance heterosis. Plant Sci. 2023, 331, 111663. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Zheng, F.; Norton, G.J.; Beesley, L.; Zhang, Z.; Lin, H.; Zhi, S.; Liu, X.; Ding, Y. Physiological responses and transcriptome analyses of upland rice following exposure to arsenite and arsenate. Environ. Exp. Bot. 2021, 183, 104366. [Google Scholar] [CrossRef]


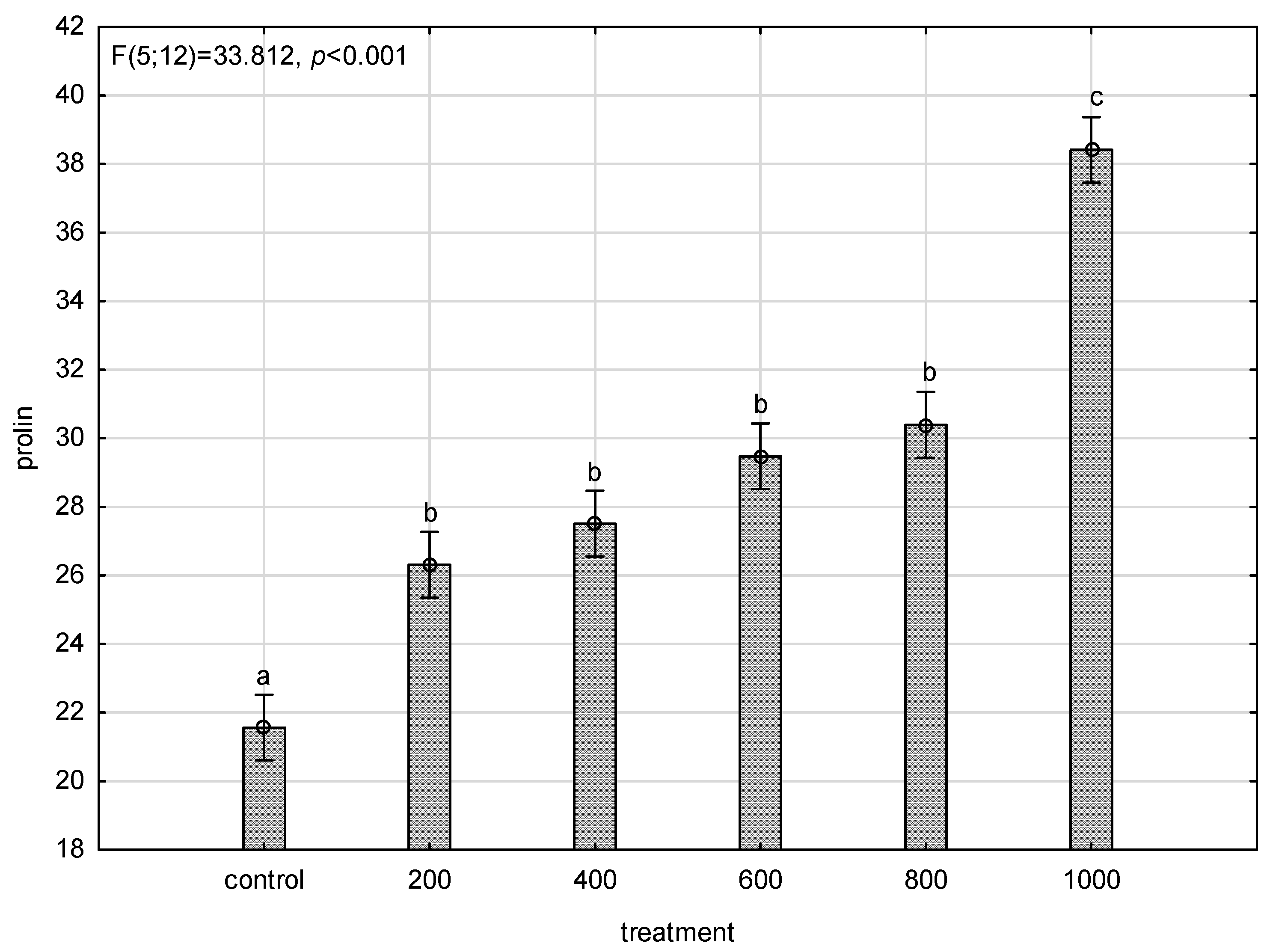
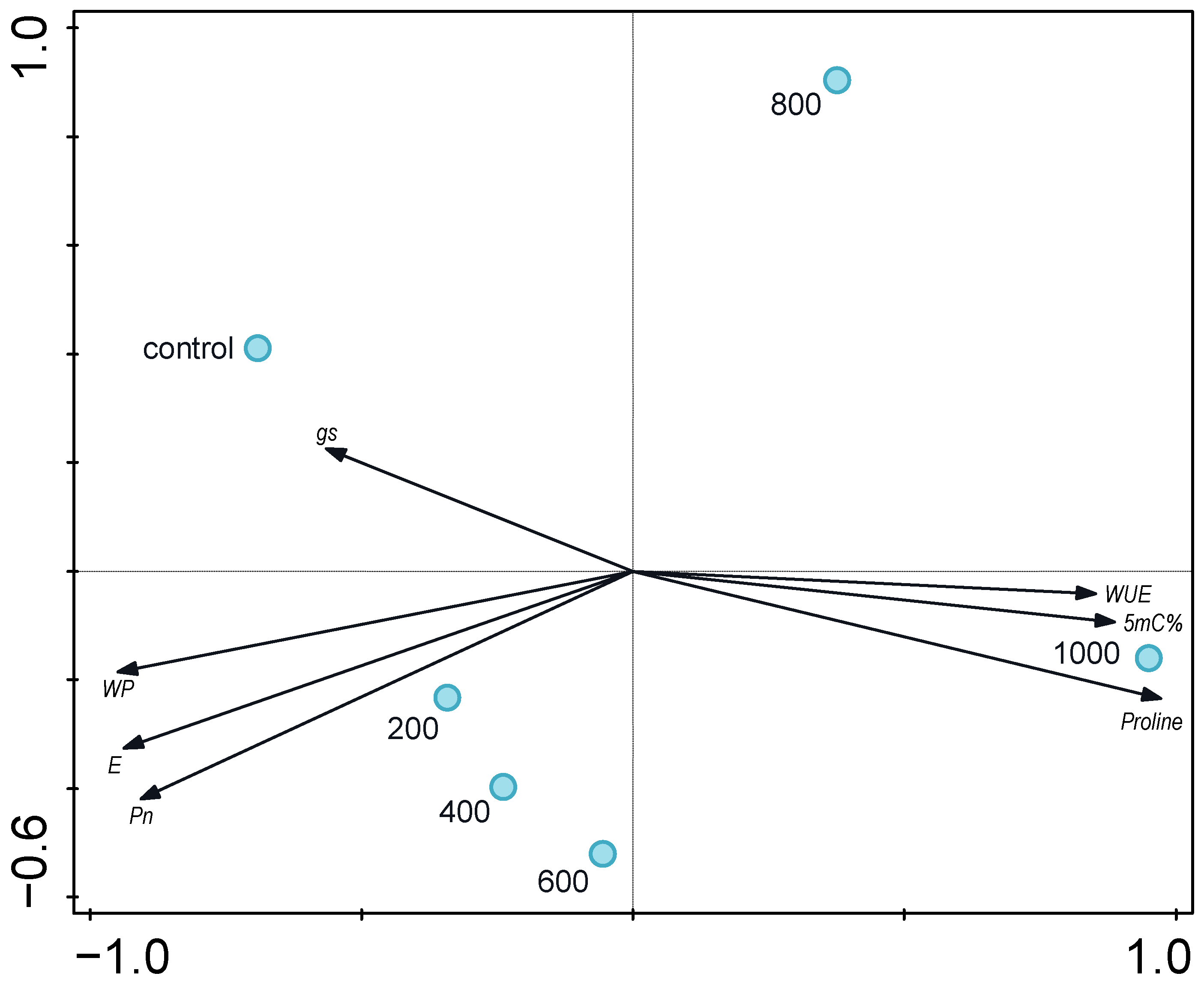
| Water Potential | E | gs | Pn | WUE | |
|---|---|---|---|---|---|
| control | −0.95 ± 0.02 a | 0.98 ± 0.00 *f | 0.03 ± 0.01 b | 12.01 ± 0.01 e | 12.25 ± 0.01 a |
| 200 | −1.04 ± 0.11 a | 0.89 ± 0.00 *e | 0.01 ± 0.00 *a | 11.60 ± 0.02 d | 13.08 ± 0.05 b |
| 400 | −1.38 ± 0.15 a | 0.81 ± 0.00 *d | 0.02 ± 0.01 ab | 11.55 ± 0.02 d | 14.26 ± 0.02 c |
| 600 | −1.94 ± 0.20 a | 0.72 ± 0.00 *c | 0.01 ± 0.00 *a | 10.78 ± 0.02 c | 14.98 ± 0.02 d |
| 800 | −2.73 ± 0.30 ab | 0.22 ± 0.00 *b | 0.01 ± 0.00 *a | 3.37 ± 0.01 a | 15.32 ± 0.03 e |
| 1000 | −4.64 ± 0.21 b | 0.18 ± 0.00 *a | 0.01 ± 0.00 *a | 2.78 ± 0.01 a | 15.44 ± 0.03 e |
| p | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudrna, J.; Popov, M.; Hnilička, F.; Lhotská, M.; Zemanová, V.; Vachová, P.; Kubeš, J.; Česká, J.; Tunklová, B. Effects of Acetaminophen Contamination on 5-Methylcytosine Content in Zea mays and Plant Physiological Parameters. Agriculture 2023, 13, 1333. https://doi.org/10.3390/agriculture13071333
Kudrna J, Popov M, Hnilička F, Lhotská M, Zemanová V, Vachová P, Kubeš J, Česká J, Tunklová B. Effects of Acetaminophen Contamination on 5-Methylcytosine Content in Zea mays and Plant Physiological Parameters. Agriculture. 2023; 13(7):1333. https://doi.org/10.3390/agriculture13071333
Chicago/Turabian StyleKudrna, Jiří, Marek Popov, František Hnilička, Marie Lhotská, Veronika Zemanová, Pavla Vachová, Jan Kubeš, Jana Česká, and Barbora Tunklová. 2023. "Effects of Acetaminophen Contamination on 5-Methylcytosine Content in Zea mays and Plant Physiological Parameters" Agriculture 13, no. 7: 1333. https://doi.org/10.3390/agriculture13071333
APA StyleKudrna, J., Popov, M., Hnilička, F., Lhotská, M., Zemanová, V., Vachová, P., Kubeš, J., Česká, J., & Tunklová, B. (2023). Effects of Acetaminophen Contamination on 5-Methylcytosine Content in Zea mays and Plant Physiological Parameters. Agriculture, 13(7), 1333. https://doi.org/10.3390/agriculture13071333







