Root Architectural Adaptations to Phosphorus Deficiency: Unraveling Genotypic Variability in Wheat Seedlings
Abstract
1. Introduction
2. Materials and Methods
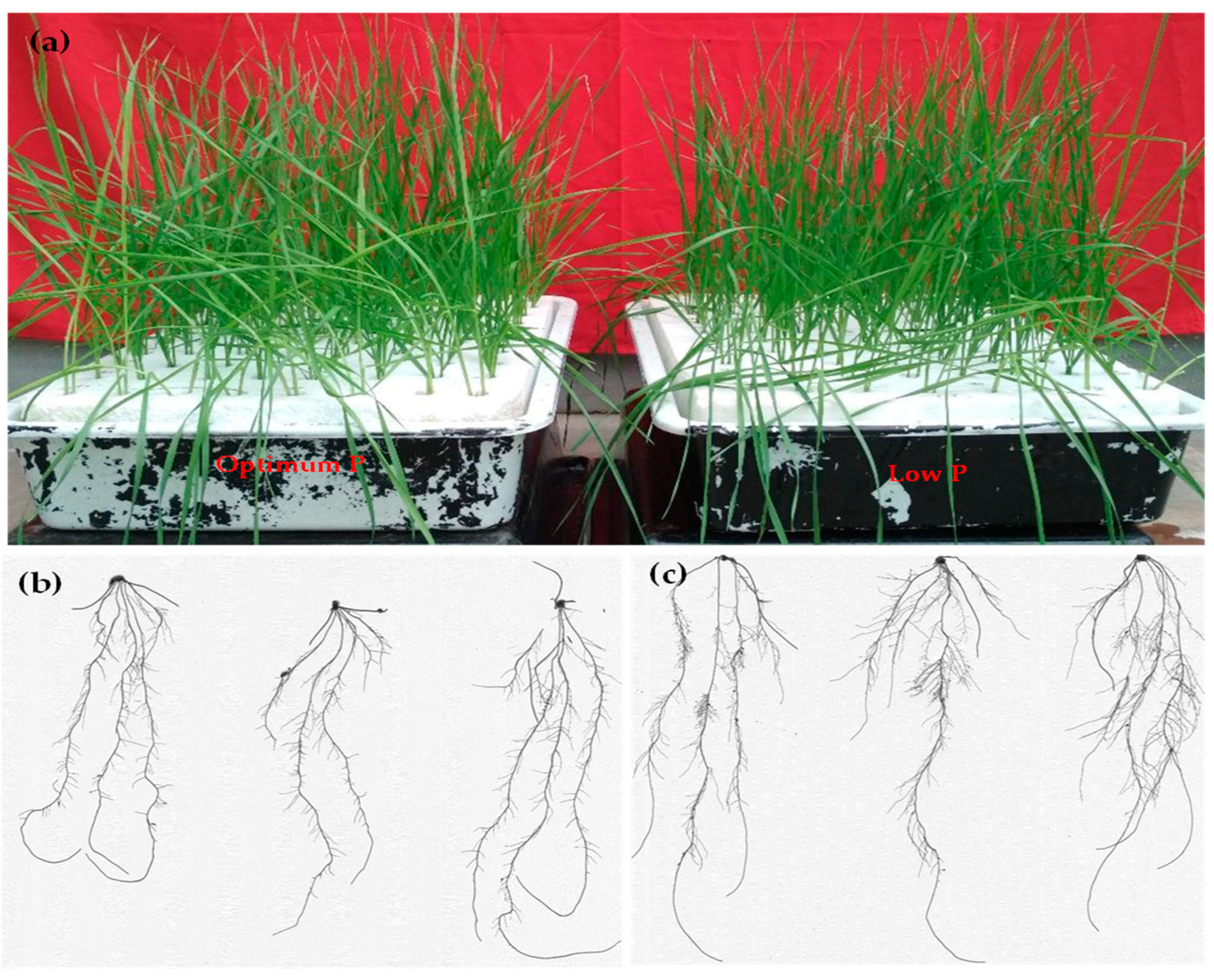
2.1. Plant Material and Growth Conditions
2.2. Root Trait Measurements
2.3. Tissue P Status and P-Related Indices
2.4. Statistical Analysis
2.5. Identification of P Use-Efficient and -Inefficient Genotypes
3. Results
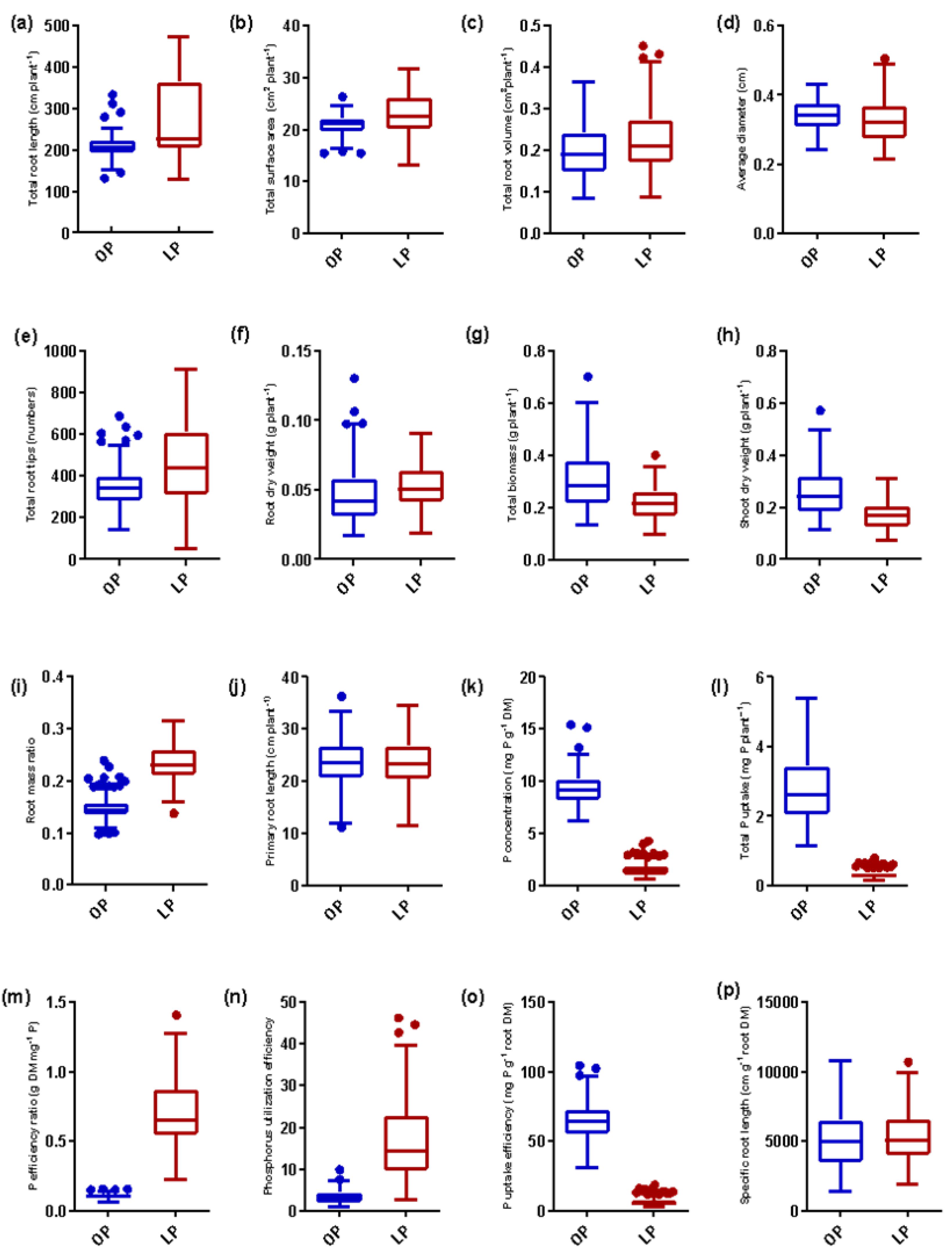
3.1. Variability in Root System, Biomass, P Concentration, and P Indices
3.2. Phenotypic and Genotypic Correlations
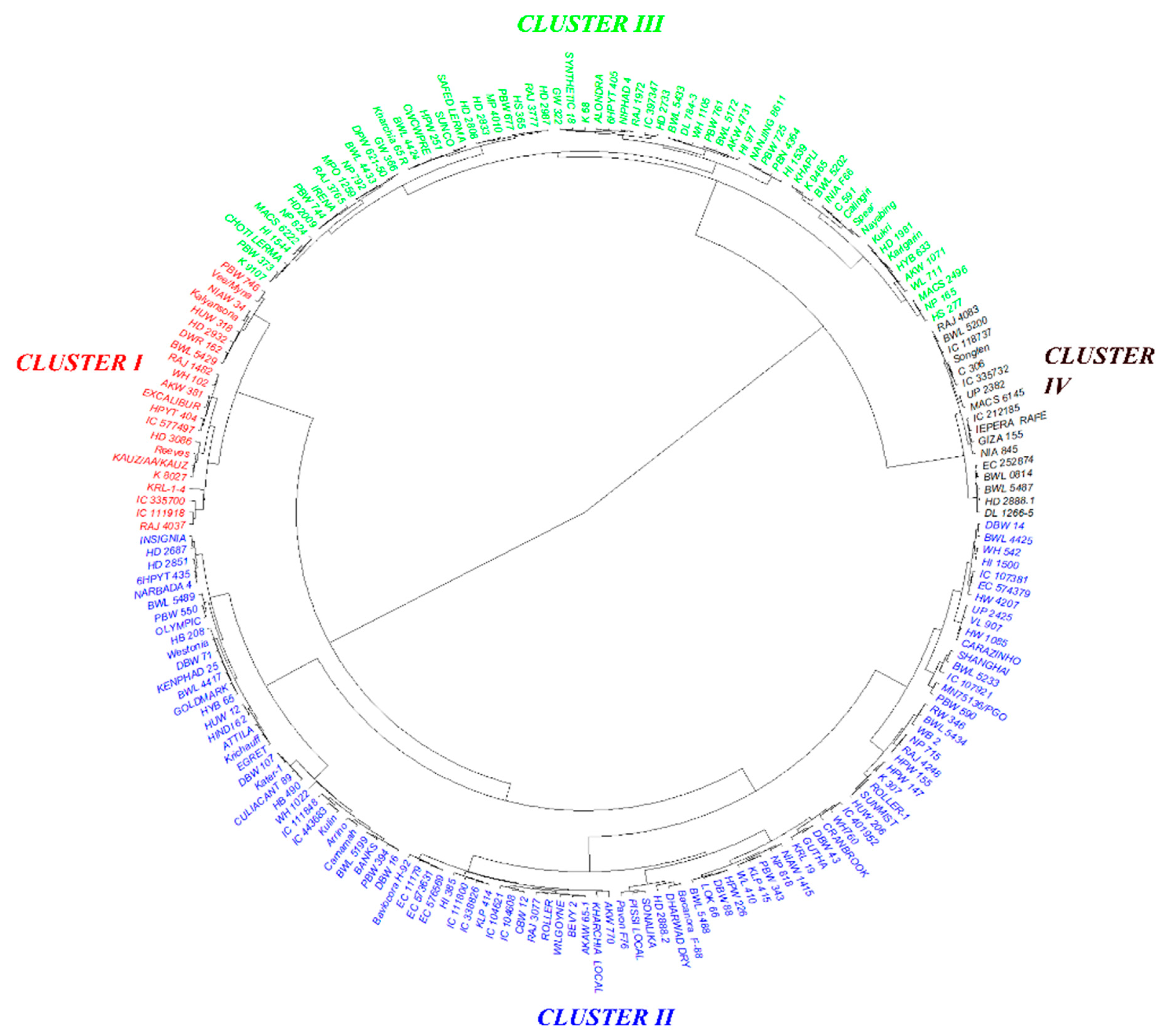
3.3. Trait-Based Diversity Pattern among the Genotypes
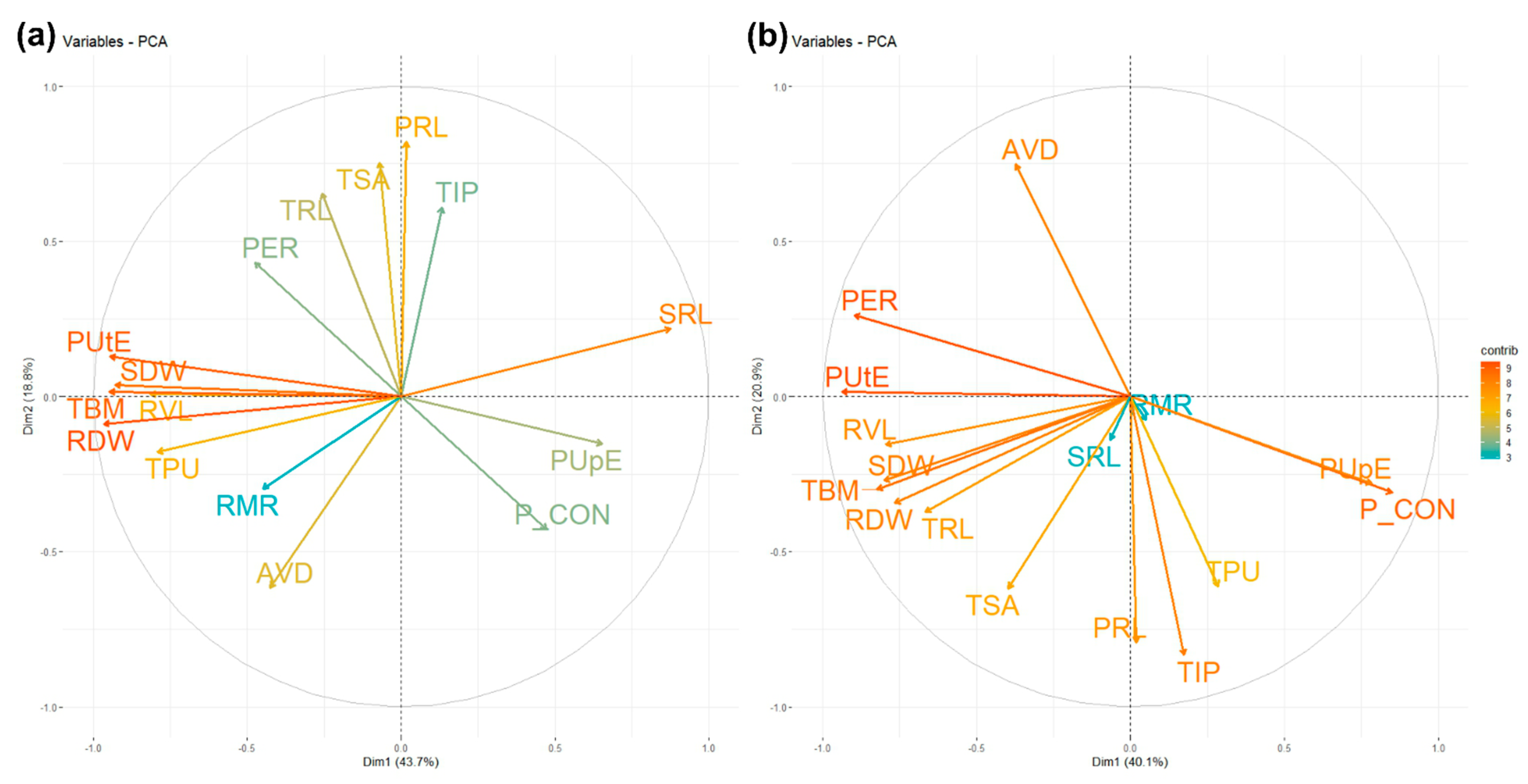
3.4. Principal Component Analysis for Root, Biomass, P Concentration, and Phosphorus Indices
3.5. Identification and Differential Responses of Contrasting Groups to Low and Optimum P
3.6. Grouping Genotypes Based on Root, Biomass, and P Indices
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tan, Y.; Chai, Q.; Li, G.; Zhao, C.; Yu, A.; Fan, Z.; Yin, W.; Hu, F.; Fan, H.; Wang, Q.; et al. Improving wheat grain yield via promotion of water and nitrogen utilization in arid areas. Sci. Rep. 2021, 11, 13821. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.K.; Hasan, M.A.; Bahadur, M.M.; Islam, R.; Hakim, A.; Iqbal, M.A.; Javed, T.; Raza, A.; Shabbir, R.; Sorour, S.; et al. Evaluation of drought tolerance of some wheat (Triticum aestivum L.) genotypes through phenology, growth, and physiological indices. Agronomy 2021, 11, 1792. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Z.; Fan, X.; Zhou, Q.; Cao, J.; Wang, F.; Ji, G.; Yang, L.; Feng, B.; Wang, T. A genome-wide association study of wheat spike related traits in China. Front. Plant Sci. 2018, 871, 1584. [Google Scholar] [CrossRef] [PubMed]
- Regmi, K.C.; Yogendra, K.; Farias, J.G.; Li, L.; Kandel, R.; Yadav, U.P.; Sha, S.; Trittermann, C.; Short, L.; George, J.; et al. Improved yield and photosynthate partitioning in AVP1 expressing wheat (Triticum aestivum) plants. Front. Plant Sci. 2020, 11, 273. [Google Scholar] [CrossRef] [PubMed]
- Van Duivenbooden, N.; De Wit, C.T.; Van Keulen, H. Nitrogen, Phosphorus and Potassium Relations in Five Major Cereals Reviewed in Respect to Fertilizer Recommendations Using Simulation Modelling. Fertil. Res. 1996, 44, 37–49. [Google Scholar] [CrossRef]
- Vinod, K.K.; Heuer, S. Approaches towards nitrogen- and phosphorus-efficient rice. AoB Plants 2012, 2012, pls028. [Google Scholar] [CrossRef] [PubMed]
- Pongrac, P.; Castillo-Michel, H.; Reyes-Herrera, J.; Hancock, R.D.; Fischer, S.; Kelemen, M.; Thompson, J.A.; Wright, G.; Likar, M.; Broadley, M.R.; et al. Effect of phosphorus supply on root traits of two Brassica oleracea L. genotypes. BMC Plant Biol. 2020, 20, 368. [Google Scholar] [CrossRef]
- Qiu, H.; Mei, X.; Liu, C.; Wang, J.; Wang, G.; Wang, X.; Liu, Z.; Cai, Y. Fine mapping of quantitative trait loci for acid phosphatase activity in maize leaf under low phosphorus stress. Mol. Breed. 2013, 32, 629–639. [Google Scholar] [CrossRef]
- Salim, N.; Raza, A. Nutrient Use Efficiency (NUE) for sustainable wheat production: A review. J. Plant Nutr. 2020, 43, 297–315. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, G.; Hu, H.; Yang, X.; Zhang, Z.; Jiang, X.; Wu, F.; Shi, H.; Wang, Q.; Zhou, K.; et al. Phenotypic and genetic variation in phosphorus-deficiency-tolerance traits in chinese wheat landraces. BMC Plant Biol. 2020, 20, 330. [Google Scholar] [CrossRef]
- Hasan, B.R. Phosphorus Status of Soils in India. Crops 1996, 10, 4–5. [Google Scholar]
- Alewell, C.; Ringeval, B.; Ballabio, C.; Robinson, D.A.; Panagos, P.; Borrelli, P. Global Phosphorus Shortage Will Be Aggravated by Soil Erosion. Nat. Commun. 2020, 11, 4546. [Google Scholar] [CrossRef] [PubMed]
- Kauwenbergh, S.J.V. World Phosphate Rock Reserves and Resources. Available online: https://Pdf.Usaid.Gov/Pdf_docs/PNADW835.Pdf (accessed on 15 December 2023).
- Iqbal, A.; Gui, H.; Zhang, H.; Wang, X.; Pang, N.; Dong, Q.; Song, M. Genotypic Variation in Cotton Genotypes for Phosphorus-Use Efficiency. Agronomy 2019, 9, 689. [Google Scholar] [CrossRef]
- Gaxiola, R.A.; Edwards, M.; Elser, J.J. A Transgenic Approach to Enhance Phosphorus Use Efficiency in Crops as Part of a Comprehensive Strategy for Sustainable Agriculture. Chemosphere 2011, 84, 840–845. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, G.K.; Bennett, E.M.; Potter, P.A.; Ramankutty, N. Agronomic Phosphorus Imbalances across the World’s Croplands. Proc. Natl. Acad. Sci. USA 2011, 108, 3086–3091. [Google Scholar] [CrossRef] [PubMed]
- Herder, G.D.; Van Isterdael, G.; Beeckman, T.; De Smet, I. The Roots of a New Green Revolution. Trends Plant Sci. 2010, 15, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Dhanapal, A.P.; York, L.M.; Hames, K.A.; Fritschi, F.B. Genome-Wide Association Study of Topsoil Root System Architecture in Field-Grown Soybean [Glycine mx (L.) Merr.]. Front. Plant Sci. 2021, 11, 590179. [Google Scholar] [CrossRef]
- Manavalan, L.P.; Prince, S.J.; Musket, T.A.; Chaky, J.; Deshmukh, R.; Vuong, T.D.; Song, L.; Cregan, P.B.; Nelson, J.C.; Shannon, J.G.; et al. Identification of Novel QTL Governing Root Architectural Traits in an Interspecific Soybean Population. PLoS ONE 2015, 10, e0120490. [Google Scholar] [CrossRef]
- Nguyen, V.L.; Stangoulis, J. Variation in root system architecture and morphology of two wheat genotypes is a predictor of their tolerance to phosphorus deficiency. Acta Physiol. Plant 2019, 41, 109. [Google Scholar] [CrossRef]
- Bates, T.R.; Lynch, J.P. Stimulation of Root Hair Elongation in Arabidopsis Thaliana by Low Phosphorus Availability. Plant. Cell Environ. 1996, 19, 529–538. [Google Scholar] [CrossRef]
- Williamson, L.C.; Ribrioux, S.P.C.P.; Fitter, A.H.; Leyser, H.M.O. Phosphate availability regulates root system architecture in arabidopsis. Plant Physiol. 2001, 126, 875–882. [Google Scholar] [CrossRef]
- Huang, G.; Liang, W.; Sturrock, C.J.; Pandey, B.K.; Giri, J.; Mairhofer, S.; Wang, D.; Muller, L.; Tan, H.; York, L.M.; et al. Rice actin binding protein RMD controls crown root angle in response to external phosphate. Nat. Commun. 2018, 9, 2346. [Google Scholar] [CrossRef] [PubMed]
- Gruber, B.D.; Giehl, R.F.H.; Friedel, S.; von Wirén, N. Plasticity of the arabidopsis root system under nutrient deficiencies. Plant Physiol. 2013, 163, 161–179. [Google Scholar] [CrossRef]
- Niu, Y.F.; Chai, R.S.; Jin, G.L.; Wang, H.; Tang, C.X.; Zhang, Y.S. Responses of root architecture development to low phosphorus availability: A Review. Ann. Bot. 2013, 112, 391–408. [Google Scholar] [CrossRef] [PubMed]
- Kohli, P.S.; Kumar Verma, P.; Verma, R.; Parida, S.K.; Thakur, J.K.; Giri, J. Genome-Wide Association Study for Phosphate Deficiency Responsive Root Hair Elongation in Chickpea. Funct. Integr. Genom. 2020, 20, 775–786. [Google Scholar] [CrossRef]
- Wang, Y.S.; Jensen, L.S.; Magid, J. Differential Responses of Root and Root Hair Traits of Spring Wheat Genotypes to Phosphorus Deficiency in Solution Culture. Plant. Soil Environ. 2016, 62, 540–546. [Google Scholar] [CrossRef]
- Li, R.; Zeng, Y.; Xu, J.; Wang, Q.; Wu, F.; Cao, M.; Lan, H.; Liu, Y.; Lu, Y. Genetic Variation for Maize Root Architecture in Response to Drought Stress at the Seedling Stage. Breed. Sci. 2015, 65, 298–307. [Google Scholar] [CrossRef] [PubMed]
- Bilal, H.M.; Aziz, T.; Maqsood, M.A.; Farooq, M.; Yan, G. Categorization of Wheat Genotypes for Phosphorus Efficiency. PLoS ONE 2018, 13, e0205471. [Google Scholar] [CrossRef]
- Campos, P.; Borie, F.; Cornejo, P.; López-Ráez, J.A.; López-García, Á.; Seguel, A. Phosphorus acquisition efficiency related to root traits: Is mycorrhizal symbiosis a key factor to wheat and barley cropping? Front. Plant Sci. 2018, 9, 752. [Google Scholar] [CrossRef]
- van de Wiel, C.C.; van der Linden, C.G.; Scholten, O.E. Improving phosphorus use efficiency in agriculture: Opportunities for breeding. Euphytica 2016, 207, 1–22. [Google Scholar] [CrossRef]
- Soumya, P.R.; Sharma, S.; Meena, M.K.; Pandey, R. Response of Diverse Bread Wheat Genotypes in Terms of Root Architectural Traits at Seedling Stage in Response to Low Phosphorus Stress. Plant Physiol. Rep. 2021, 26, 152–161. [Google Scholar] [CrossRef]
- Soumya, P.R.; Singh, D.; Sharma, S.; Singh, A.M.; Pandey, R. Evaluation of Diverse Wheat (Triticum aestivum) and Triticale (× Triticosecale) Genotypes for Low Phosphorus Stress Tolerance in Soil and Hydroponic Conditions. J. Soil Sci. Plant Nutr. 2021, 21, 1236–1251. [Google Scholar] [CrossRef]
- Li, L.; Peng, Z.; Mao, X.; Wang, J.; Chang, X.; Reynolds, M.; Jing, R. Genome-Wide Association Study Reveals Genomic Regions Controlling Root and Shoot Traits at Late Growth Stages in Wheat. Ann. Bot. 2019, 124, 993–1006. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhao, D.; Tang, X.; Yuan, M.; Zhang, D.; Xu, M.; Duan, Y.; Ren, H.; Zeng, Q.; Wu, J.; et al. Genome-Wide Association Study on Root System Architecture and Identification of Candidate Genes in Wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2022, 23, 1843. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, G.; Rodríguez, F.M.; Pacheco, A.; Burgueño, J.; Crossa, J.; Vargas, M.; Pérez-Rodríguez, P.; Lopez-Cruz, M.A. META-R: A Software to Analyze Data from Multi-Environment Plant Breeding Trials. Crop. J. 2020, 8, 745–756. [Google Scholar] [CrossRef]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Prentice Hall: Harlow, UK, 1996. [Google Scholar]
- Hallauer, A.R.; Carena, M.J.; Miranda Filho, J. Quantitative Genetics in Maize Breeding (Handbook of Plant Breeding); Springer Science & Business Media: New York, NY, USA; Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; The University of Illinois Press: Urbana, IL, USA, 1963. [Google Scholar]
- Hutcheson, K.A. A test for comparing diversities based on the Shannon formula. J. Theor. Biol. 1970, 29, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Aski, M.S.; Rai, N.; Reddy, V.R.P.; Gayacharan; Dikshit, H.K.; Mishra, G.P.; Singh, D.; Kumar, A.; Pandey, R.; Singh, M.P.; et al. Assessment of root phenotypes in mungbean mini-core collection (MMC) from the World Vegetable Center (AVRDC) Taiwan. PLoS ONE 2021, 16, e0247810. [Google Scholar] [CrossRef] [PubMed]
- Meena, S.K.; Pandey, R.; Sharma, S.; Gayacharan; Kumar, T.; Singh, M.P.; Dikshit, H.K. Physiological basis of combined stress tolerance to low phosphorus and drought in a diverse set of mungbean germplasm. Agronomy 2021, 11, 99. [Google Scholar] [CrossRef]
- Ramaekers, L.; Remans, R.; Rao, I.M.; Blair, M.W.; Vanderleyden, J. Strategies for Improving Phosphorus Acquisition Efficiency of Crop Plants. Field Crops Res. 2010, 117, 169–176. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.G. QTL Mapping for Traits Related to P-Deficient Tolerance Using Three Related RIL Populations in Wheat. Euphytica 2015, 203, 505–520. [Google Scholar] [CrossRef]
- Wang, Q.; Yuan, Y.; Liao, Z.; Jiang, Y.; Wang, Q.; Zhang, L.; Gao, S.; Wu, F.; Li, M.; Xie, W.; et al. Genome-Wide Association Study of 13 Traits in Maize Seedlings under Low Phosphorus Stress. Plant Genome 2019, 12, 190039. [Google Scholar] [CrossRef]
- Manske, G.G.B.; Ortiz-Monasterio, J.I.; Van Ginkel, M.; González, R.M.; Rajaram, S.; Molina, E.; Vlek, P.L.G. Traits associated with improved P-uptake efficiency in CIMMYT's semidwarf spring bread wheat grown on an acid Andisol in Mexico. Plant Soil 2000, 221, 189–204. [Google Scholar] [CrossRef]
- Gladman, N.; Hufnagel, B.; Regulski, M.; Liu, Z.; Wang, X.; Chougule, K.; Kochian, L.; Magalhães, J.; Ware, D. Sorghum Root Epigenetic Landscape during Limiting Phosphorus Conditions. Plant Direct 2022, 6, e393. [Google Scholar] [CrossRef]
- Strock, C.F.; De La Riva, L.M.; Lynch, J.P. Reduction in root secondary growth as a strategy for phosphorus acquisition. Plant Physiol. 2018, 176, 691–703. [Google Scholar] [CrossRef]
- Lynch, J. Root architecture and plant productivity. Plant Physiol. 1995, 109, 7. [Google Scholar] [CrossRef]
- Yang, M.; Wang, C.; Hassan, M.A.; Li, F.; Xia, X.; Shi, S.; Xiao, Y.; He, Z. QTL Mapping of Root Traits in Wheat under Different Phosphorus Levels Using Hydroponic Culture. BMC Genom. 2021, 22, 174. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, L.; Deng, M.; Li, Z.; Lu, Y.; Wang, J.; Wei, Y.; Zheng, Y. Genome-Wide Association Study of Phosphorus-Deficiency-Tolerance Traits in Aegilops Tauschii. Theor. Appl. Genet. 2015, 128, 2203–2212. [Google Scholar] [CrossRef] [PubMed]
- Horst, W.J.; Abdou, M.; Wiesler, F. Genotypic Differences in Phosphorus Efficiency of Wheat. Plant Soil 1993, 155–156, 293–296. [Google Scholar] [CrossRef]
- Steingrobe, B.; Schmid, H.; Claassen, N. Root production and root mortality of winter barley and its implication with regard to phosphate acquisition. Plant Soil 2001, 237, 239–248. [Google Scholar] [CrossRef]
- Lynch, J.P.; Brown, K.M. Root strategies for phosphorus acquisition. Ecophysiol. Plant-Phosphorus Interact. 2008, 7, 83–116. [Google Scholar] [CrossRef]
- Imada, S.; Yamanaka, N.; Tamai, S. Water Table Depth Affects Populus Alba Fine Root Growth and Whole Plant Biomass. Funct. Ecol. 2008, 22, 1018–1026. [Google Scholar] [CrossRef]
- Xie, Q.; Fernando, K.M.C.; Mayes, S.; Sparkes, D.L. Identifying Seedling Root Architectural Traits Associated with Yield and Yield Components in Wheat. Ann. Bot. 2017, 119, 1115–1129. [Google Scholar] [CrossRef]
- Aski, M.; Mehra, R.; Mishra, G.P.; Singh, D.; Yadav, P.; Rai, N.; Reddy, V.R.P.; Arun Kumar, M.B.; Pandey, R.; Singh, M.P.; et al. Genotypic Variation in Root Architectural Traits under Contrasting Phosphorus Levels in Mediterranean and Indian Origin Lentil Genotypes. PeerJ 2022, 10, e12766. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Mohan, A.; Gill, K.S.; Vara Prasad, P.V. Variability of Root Traits in Spring Wheat Germplasm. PLoS ONE 2014, 9, e100317. [Google Scholar] [CrossRef]
- Wissuwa, M.; Kondo, K.; Fukuda, T.; Mori, A.; Rose, M.T.; Pariasca-Tanaka, J.; Kretzschmar, T.; Haefele, S.M.; Rose, T.J. Unmasking Novel Loci for Internal Phosphorus Utilization Efficiency in Rice Germplasm through Genome-Wide Association Analysis. PLoS ONE 2015, 10, e0124215. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Gao, M.; Zhang, M.; Zheng, H.; Zhou, X.; Guo, Y.; Zhao, Y.; Kong, F.; Li, S. QTL Mapping for Phosphorus Efficiency and Morphological Traits at Seedling and Maturity Stages in Wheat. Front. Plant Sci. 2017, 8, 614. [Google Scholar] [CrossRef]
- Yano, K.; Kume, T. Root Morphological Plasticity for Heterogeneous Phosphorus Supply in Zea mays L. Plant Prod. Sci. 2005, 8, 427–432. [Google Scholar] [CrossRef]
- Wang, X.; Veneklaas, E.J.; Pearse, S.J.; Lambers, H. Interactions among Cluster-Root Investment, Leaf Phosphorus Concentration, and Relative Growth Rate in Two Lupinus Species. Am. J. Bot. 2015, 102, 1529–1537. [Google Scholar] [CrossRef]
- Chen, Y.; Rengel, Z.; Palta, J.; Siddique, K.H.M. Efficient Root Systems for Enhancing Tolerance of Crops to Water and Phosphorus Limitation. Indian J. Plant Physiol. 2018, 23, 689–696. [Google Scholar] [CrossRef]
- Chen, Y.; Palta, J.; Prasad, P.V.V.; Siddique, K.H.M. Phenotypic Variability in Bread Wheat Root Systems at the Early Vegetative Stage. BMC Plant Biol. 2020, 20, 185. [Google Scholar] [CrossRef]
- Rose, T.J.; Rose, M.T.; Pariasca-Tanaka, J.; Heuer, S.; Wissuwa, M. The Frustration with Utilization: Why Have Improvements in Internal Phosphorus Utilization Efficiency in Crops Remained so Elusive? Front. Plant Sci. 2011, 2, 73. [Google Scholar] [CrossRef]
- Salma Kosar, H.; Gill, M.A.; Aziz, T.; Tahir, M.A. Relative phosphorus utilization efficiency of wheat genotypes in hydroponics. Pak. J. Agric. Sci. 2003, 40, 28–32. [Google Scholar]
- Safdar, L.B.; Umer, M.J.; Almas, F.; Uddin, S.; Safdar, Q.T.A.; Blighe, K.; Quraishi, U.M. Identification of Genetic Factors Controlling Phosphorus Utilization Efficiency in Wheat by Genome-Wide Association Study with Principal Component Analysis. Gene 2021, 768, 145301. [Google Scholar] [CrossRef] [PubMed]
- Raju, B.R.; Narayanaswamy, B.R.; Mohankumar, M.V.; Sumanth, K.K.; Rajanna, M.P.; Mohanraju, B.; Udayakumar, M.; Sheshshayee, M.S. Root Traits and Cellular Level Tolerance Hold the Key in Maintaining Higher Spikelet Fertility of Rice under Water Limited Conditions. Funct. Plant Biol. 2014, 41, 930–939. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Abdel-Ghani, A.H.; Reyes-Matamoros, J.; Hochholdinger, F.; Lübberstedt, T. Genotypic Variation for Root Architecture Traits in Seedlings of Maize (Zea mays L.). Inbred Lines. Plant Breed. 2012, 131, 465–478. [Google Scholar] [CrossRef]
- Reddy, V.R.P.; Aski, M.S.; Mishra, G.P.; Dikshit, H.K.; Singh, A.; Pandey, R.; Singh, M.P.; Gayacharan; Ramtekey, V.; Priti; et al. Genetic variation for root architectural traits in response to phosphorus deficiency in mungbean at the seedling stage. PLoS ONE 2020, 15, e0221008. [Google Scholar] [CrossRef]
- Lin, Y.; Yi, X.; Tang, S.; Chen, W.; Wu, F.; Yang, X.; Jiang, X.; Shi, H.; Ma, J.; Chen, G.; et al. Dissection of Phenotypic and Genetic Variation of Drought-Related Traits in Diverse Chinese Wheat Landraces. Plant Genome 2019, 12, 190025. [Google Scholar] [CrossRef] [PubMed]
- Zobel, R.W.; Kinraide, T.B.; Baligar, V.C. Fine Root Diameters Can Change in Response to Changes in Nutrient Concentrations. Plant Soil 2007, 297, 243–254. [Google Scholar] [CrossRef]
- Yadav, R.K.; Gautam, S.; Palikhey, E.; Joshi, B.K.; Ghimire, K.H.; Gurung, R.; Adhikari, A.R.; Pudasaini, N.; Dhakal, R. Agro-Morphological Diversity of Nepalese Naked Barley Landraces. Agric. Food Secur. 2018, 7, 86. [Google Scholar] [CrossRef]
- Nagar, C.K.; Gayatri, G.; Sinha, S.K.; Venkatesh, K.; Mandal, P.K. Nitrogen stress induced changes in root system architecture (RSA) in diverse wheat (T. aestivum L.) Genotypes at Seedling Stage. Wheat Barley Res. 2018, 10, 93–101. [Google Scholar] [CrossRef][Green Version]
- Gourley, C.J.P.; Allan, D.L.; Russelle, M.P. Plant nutrient efficiency: A comparison of definitions and suggested improvement. Plant Soil 1994, 158, 29–37. [Google Scholar] [CrossRef]


| Trait | P Level | Genotype | P Level | Genotype × P Level | Std. Deviation | Mean | Minimum | Maximum | CV (%) | Heritability (h2) |
|---|---|---|---|---|---|---|---|---|---|---|
| TRL | OP | 5.219 *** | 306.6 *** | 20.32 *** | 33.81 | 207.8 | 133.6 | 335.1 | 33.8 | 0.71 |
| LP | 69.45 | 271.2 | 129.2 | 474.8 | 35.7 | |||||
| TSA | OP | 5.033 *** | 118.7 *** | 13.21 *** | 2.41 | 20.98 | 15.61 | 26.9 | 11.5 | 0.68 |
| LP | 4.23 | 22.81 | 13.28 | 31.9 | 18.5 | |||||
| AVD | OP | 16.96 *** | 43.29 *** | 12.04 *** | 0.045 | 0.34 | 0.24 | 0.43 | 13.2 | 0.91 |
| LP | 0.063 | 0.33 | 0.22 | 0.51 | 19.5 | |||||
| RVL | OP | 12.17 *** | 69.7 *** | 2.297 *** | 0.065 | 0.20 | 0.09 | 0.37 | 33.0 | 0.88 |
| LP | 0.078 | 0.23 | 0.09 | 0.45 | 34.3 | |||||
| TIP | OP | 5.58 *** | 185.7 *** | 9.252 *** | 111.2 | 347.0 | 143.2 | 691.0 | 32.1 | 0.73 |
| LP | 211.5 | 460.5 | 52.5 | 914.8 | 37.9 | |||||
| RDW | OP | 11.02 *** | 39.6 *** | 5.30 *** | 0.02 | 0.05 | 0.017 | 0.11 | 37.3 | 0.86 |
| LP | 0.017 | 0.05 | 0.019 | 0.09 | 31.9 | |||||
| TBM | OP | 8.881 *** | 316.2 *** | 5.362 *** | 0.119 | 0.31 | 0.14 | 0.61 | 38.7 | 0.87 |
| LP | 0.068 | 0.22 | 0.10 | 0.41 | 30.7 | |||||
| SDW | OP | 6.998 *** | 524.9 *** | 5.559 *** | 0.1 | 0.26 | 0.12 | 0.50 | 38.3 | 0.85 |
| LP | 0.054 | 0.17 | 0.08 | 0.31 | 31.9 | |||||
| RMR | OP | 1.143 *** | 3108 *** | 7.239 *** | 0.026 | 0.15 | 0.10 | 0.24 | 17.3 | 0.69 |
| LP | 0.037 | 0.24 | 0.14 | 0.32 | 15.5 | |||||
| PRL | OP | 12.103 *** | 3.526 NS | 4.637 *** | 5.11 | 23.8 | 11.3 | 36.4 | 21.5 | 0.72 |
| LP | 5.39 | 23.5 | 11.6 | 34.8 | 23.1 | |||||
| P CON | OP | 0.223 *** | 13797 *** | 29.69 *** | 1.53 | 9.35 | 6.22 | 15.5 | 16.4 | 0.64 |
| LP | 0.58 | 1.60 | 0.71 | 4.36 | 31.2 | |||||
| TPU | OP | 0.577 *** | 3550 *** | 11.05 *** | 1.02 | 2.81 | 1.16 | 5.41 | 36.4 | 0.70 |
| LP | 0.119 | 0.71 | 0.23 | 1.28 | 35.6 | |||||
| PER | OP | 0.619 *** | 4354 *** | 42.29 *** | 0.017 | 0.11 | 0.07 | 0.16 | 16.2 | 0.78 |
| LP | 0.225 | 0.71 | 0.23 | 1.29 | 28.6 | |||||
| PUtE | OP | 1.989 *** | 1264 *** | 14.61 *** | 11.59 | 33.46 | 1.06 | 10.0 | 39.2 | 0.71 |
| LP | 9.04 | 16.67 | 2.89 | 46.3 | 41.2 | |||||
| PUpE | OP | 8.972 *** | 9157 *** | 6.755 *** | 14.72 | 64.9 | 31.7 | 104.9 | 22.7 | 0.81 |
| LP | 2.78 | 6.70 | 2.91 | 19.10 | 41.1 | |||||
| SRL | OP | 3.201 *** | 4.485 * | 5.91 *** | 2292.8 | 5220 | 1520 | 10,291 | 39.1 | 0.62 |
| LP | 2054.6 | 5376 | 2112 | 10,224 | 32.3 |
| Traits | Treatment | Performance of All 204 Wheat Genotypes | |||
|---|---|---|---|---|---|
| Low | Medium | High | H′ | ||
| TRL | OP | 19 | 75 | 9 | 0.73 |
| LP | 16 | 76 | 11 | 0.73 | |
| TSA | OP | 21 | 65 | 17 | 0.81 |
| LP | 28 | 63 | 12 | 0.80 | |
| AVD | OP | 9 | 78 | 16 | 0.71 |
| LP | 10 | 64 | 29 | 0.79 | |
| RVL | OP | 18 | 69 | 16 | 0.78 |
| LP | 11 | 85 | 7 | 0.64 | |
| TIP | OP | 5 | 84 | 14 | 0.64 |
| LP | 28 | 64 | 11 | 0.79 | |
| RDW | OP | 16 | 76 | 11 | 0.73 |
| LP | 20 | 72 | 11 | 0.75 | |
| TBM | OP | 16 | 76 | 11 | 0.73 |
| LP | 26 | 67 | 10 | 0.78 | |
| SDW | OP | 15 | 77 | 11 | 0.72 |
| LP | 26 | 67 | 10 | 0.78 | |
| RMR | OP | 5 | 86 | 12 | 0.62 |
| LP | 13 | 64 | 26 | 0.80 | |
| PRL | OP | 12 | 80 | 11 | 0.69 |
| LP | 28 | 58 | 17 | 0.84 | |
| P CON | OP | 10 | 70 | 23 | 0.76 |
| LP | 6 | 92 | 5 | 0.55 | |
| TPU | OP | 12 | 81 | 10 | 0.68 |
| LP | 9 | 88 | 6 | 0.60 | |
| PER | OP | 23 | 69 | 11 | 0.77 |
| LP | 7 | 86 | 10 | 0.63 | |
| PUtE | OP | 19 | 71 | 13 | 0.76 |
| LP | 10 | 83 | 10 | 0.66 | |
| PUpE | OP | 9 | 80 | 14 | 0.68 |
| LP | 6 | 93 | 4 | 0.38 | |
| SRL | OP | 12 | 78 | 13 | 0.59 |
| LP | 20 | 74 | 9 | 0.60 | |
| Traits | Optimum P | Low P | ||
|---|---|---|---|---|
| PC1 | PC2 | PC1 | PC2 | |
| TRL | 0.10 | 0.38 | 0.26 | 0.20 |
| TSA | 0.03 | 0.44 | 0.16 | 0.34 |
| AVD | 0.31 | 0.01 | 0.32 | 0.08 |
| RVL | 0.16 | −0.36 | 0.15 | −0.41 |
| TIP | −0.05 | 0.35 | −0.07 | 0.46 |
| RDW | 0.37 | −0.05 | 0.30 | 0.19 |
| TBM | 0.36 | 0.01 | 0.32 | 0.16 |
| SDW | 0.35 | 0.02 | 0.32 | 0.15 |
| RMR | 0.17 | −0.17 | −0.02 | 0.04 |
| PRL | −0.01 | 0.47 | −0.01 | 0.43 |
| PCON | −0.18 | −0.25 | −0.34 | 0.17 |
| TPU | 0.30 | −0.10 | −0.11 | 0.33 |
| PER | 0.18 | 0.25 | 0.35 | −0.14 |
| PUtE | 0.36 | 0.07 | 0.37 | −0.01 |
| PUpE | −0.25 | −0.09 | −0.31 | 0.15 |
| SRL | −0.33 | 0.13 | 0.03 | 0.08 |
| Variation proportion | ||||
| Standard deviation | 2.7 | 1.73 | 2.53 | 1.83 |
| Variance percent | 43.7 | 18.8 | 40.0 | 21.0 |
| Cumulative variant % | 43.7 | 62.5 | 40.0 | 61.0 |
| Most contributing traits | TRL, TSA, AVD, RVL, TIP, RDW, TBM, SDW, PRL, PUtE, SRL | TSA, AVD, RVL, TIP, TBM, SDW, PRL, P CON, TPU, PER, PUtE, PUpE | ||
| S.No. | P Use-Efficient Genotypes | P Use-Inefficient Genotypes |
|---|---|---|
| 1 | SHANGHAI | HS 365 |
| 2 | Pavon F76 | WH760 |
| 3 | BWL 5233 | IEPERA RAFE |
| 4 | SONALIKA | IC 212185 |
| 5 | KHARCHIA LOCAL | NIA 845 |
| 6 | WH 102 | K 9465 |
| 7 | BWL 4425 | MACS 2496 |
| 8 | HD 2888.2 | IC 335732 |
| 9 | CBW 12 | IC 118737 |
| 10 | MN75136/PGO | HB 490 |
| 11 | KRL 19 | BWL 0814 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajamanickam, V.; Vinod, K.K.; Vengavasi, K.; Kumar, T.; Chinnusamy, V.; Pandey, R. Root Architectural Adaptations to Phosphorus Deficiency: Unraveling Genotypic Variability in Wheat Seedlings. Agriculture 2024, 14, 447. https://doi.org/10.3390/agriculture14030447
Rajamanickam V, Vinod KK, Vengavasi K, Kumar T, Chinnusamy V, Pandey R. Root Architectural Adaptations to Phosphorus Deficiency: Unraveling Genotypic Variability in Wheat Seedlings. Agriculture. 2024; 14(3):447. https://doi.org/10.3390/agriculture14030447
Chicago/Turabian StyleRajamanickam, Vijay, Kunnummal Kurungara Vinod, Krishnapriya Vengavasi, Tarun Kumar, Viswanathan Chinnusamy, and Renu Pandey. 2024. "Root Architectural Adaptations to Phosphorus Deficiency: Unraveling Genotypic Variability in Wheat Seedlings" Agriculture 14, no. 3: 447. https://doi.org/10.3390/agriculture14030447
APA StyleRajamanickam, V., Vinod, K. K., Vengavasi, K., Kumar, T., Chinnusamy, V., & Pandey, R. (2024). Root Architectural Adaptations to Phosphorus Deficiency: Unraveling Genotypic Variability in Wheat Seedlings. Agriculture, 14(3), 447. https://doi.org/10.3390/agriculture14030447







