Agronomic, Physiological, Genetic and Phytochemical Characteristics of Onion Varieties Influenced by Daylength Requirements
Abstract
1. Introduction
2. Materials and Methods
2.1. Growing Conditions and Experimental Design
2.2. Biometrical and Agronomic Parameters
2.2.1. Bulb Dimensions
2.2.2. Total Fresh and Dry Weight
2.2.3. Leaf and Scale Number
2.3. Chlorophyll Index
2.4. Genomic DNA Isolation and ISSR Analysis
2.5. Phytochemical Analysis
2.5.1. Total Polyphenols
2.5.2. Total Flavonoids
2.5.3. Anthocyanines
2.5.4. DPPD Assay
2.6. Data Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Morphological Screening
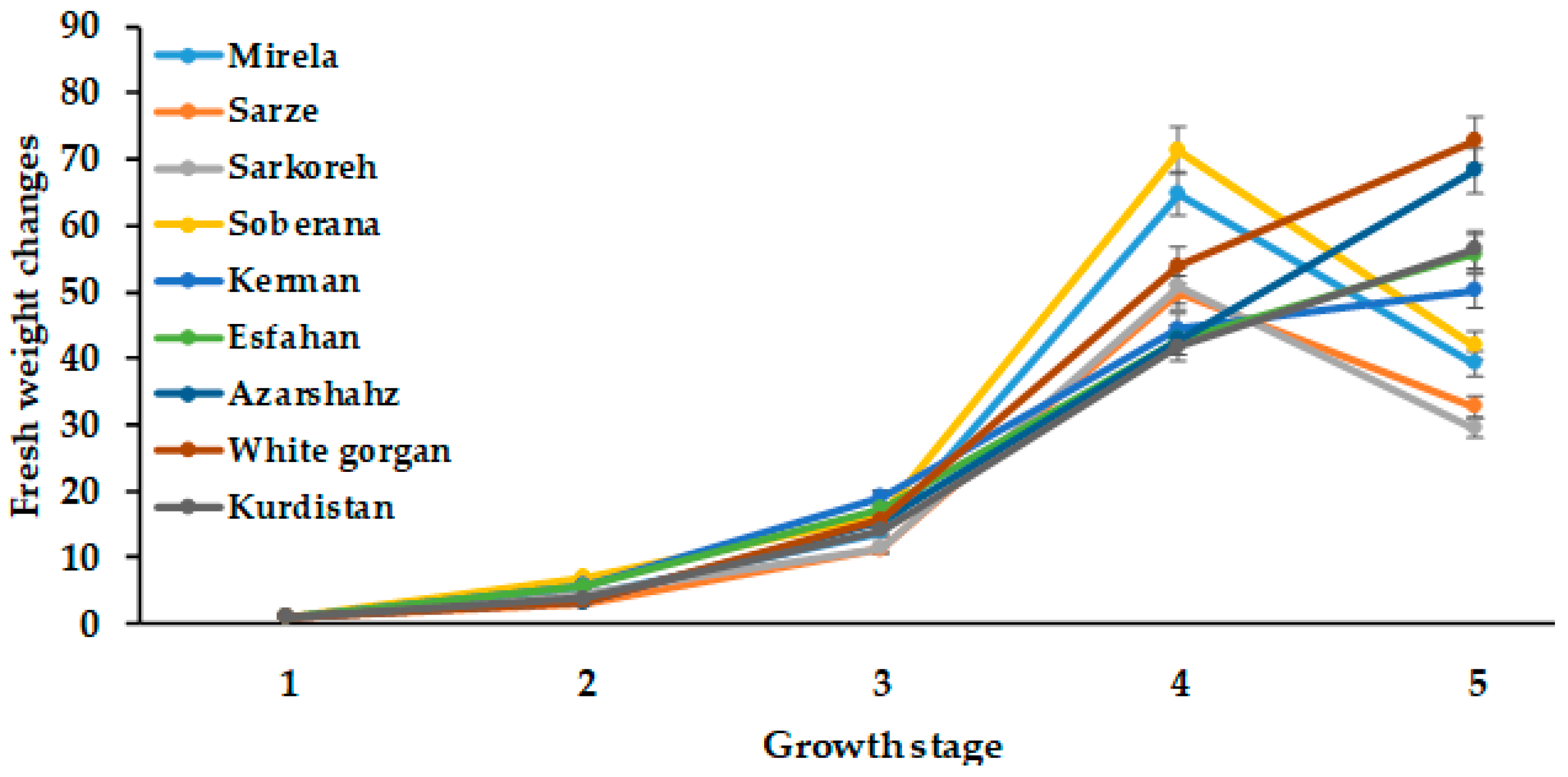
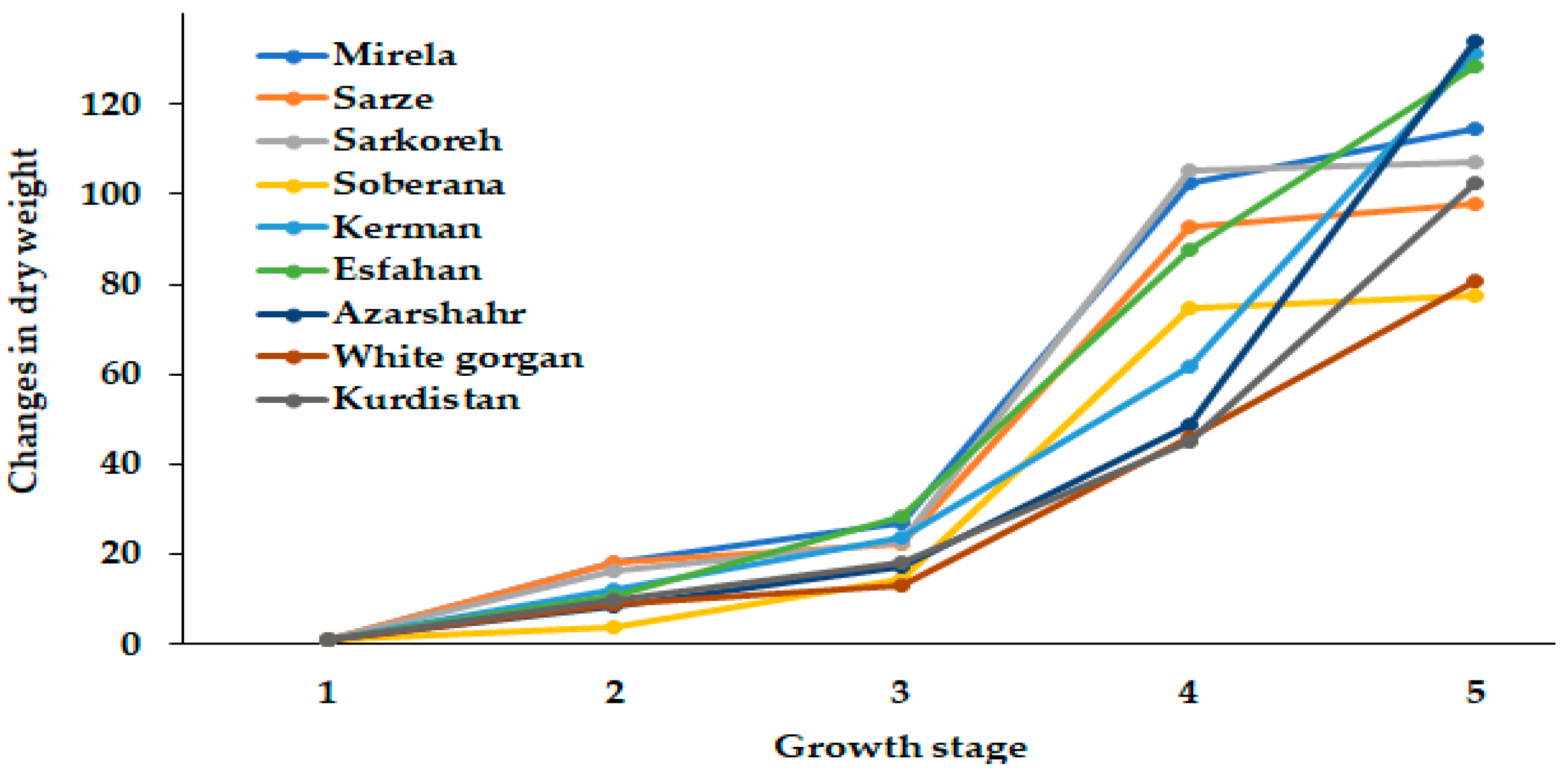
3.1.1. Plant Fresh and Dry Weight
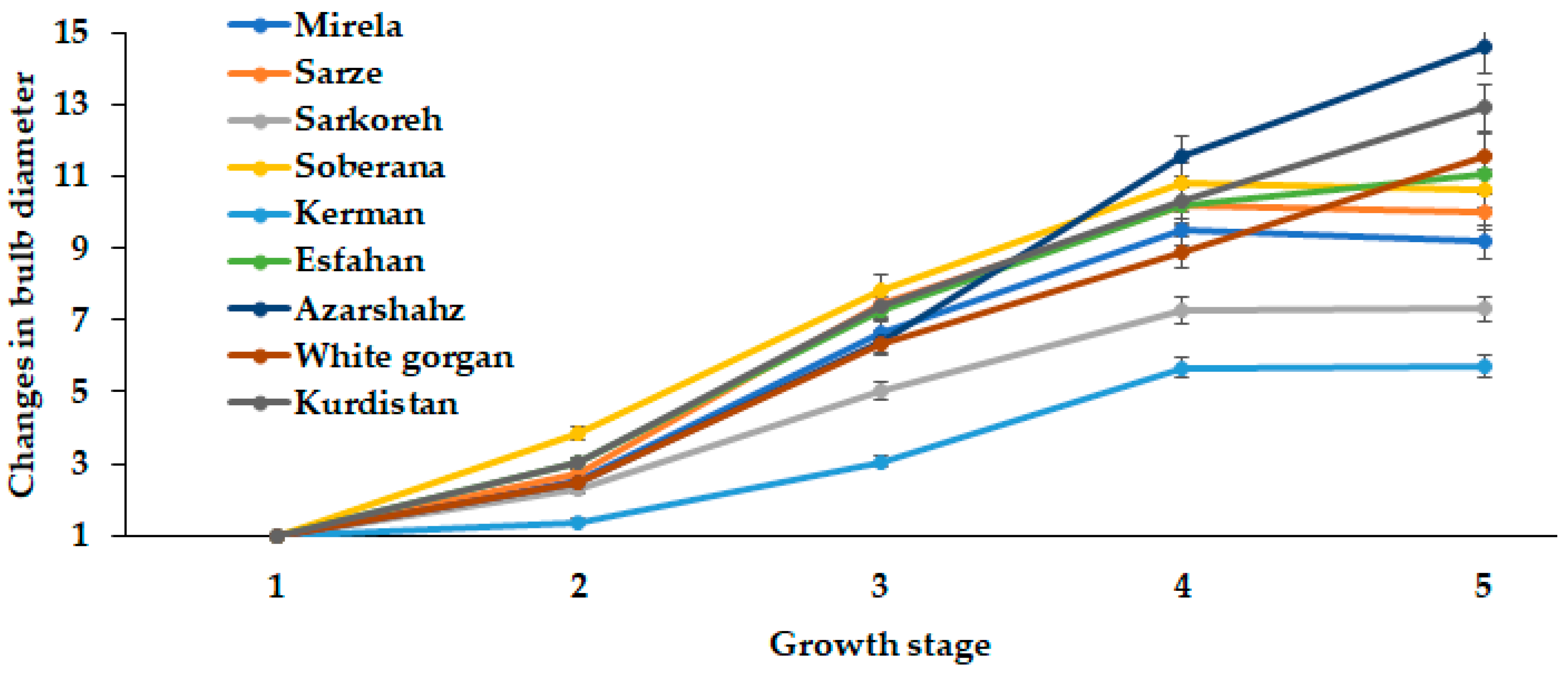
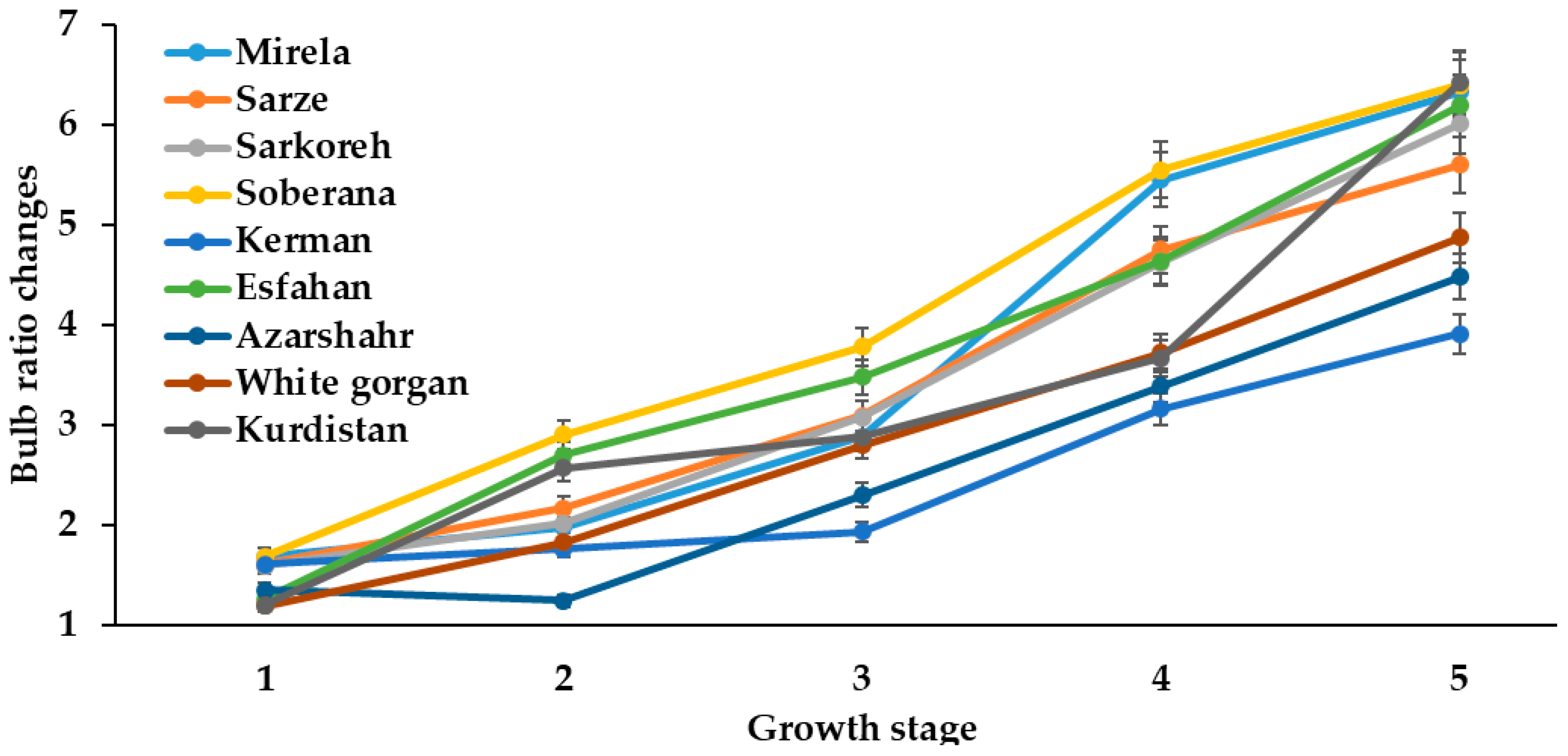
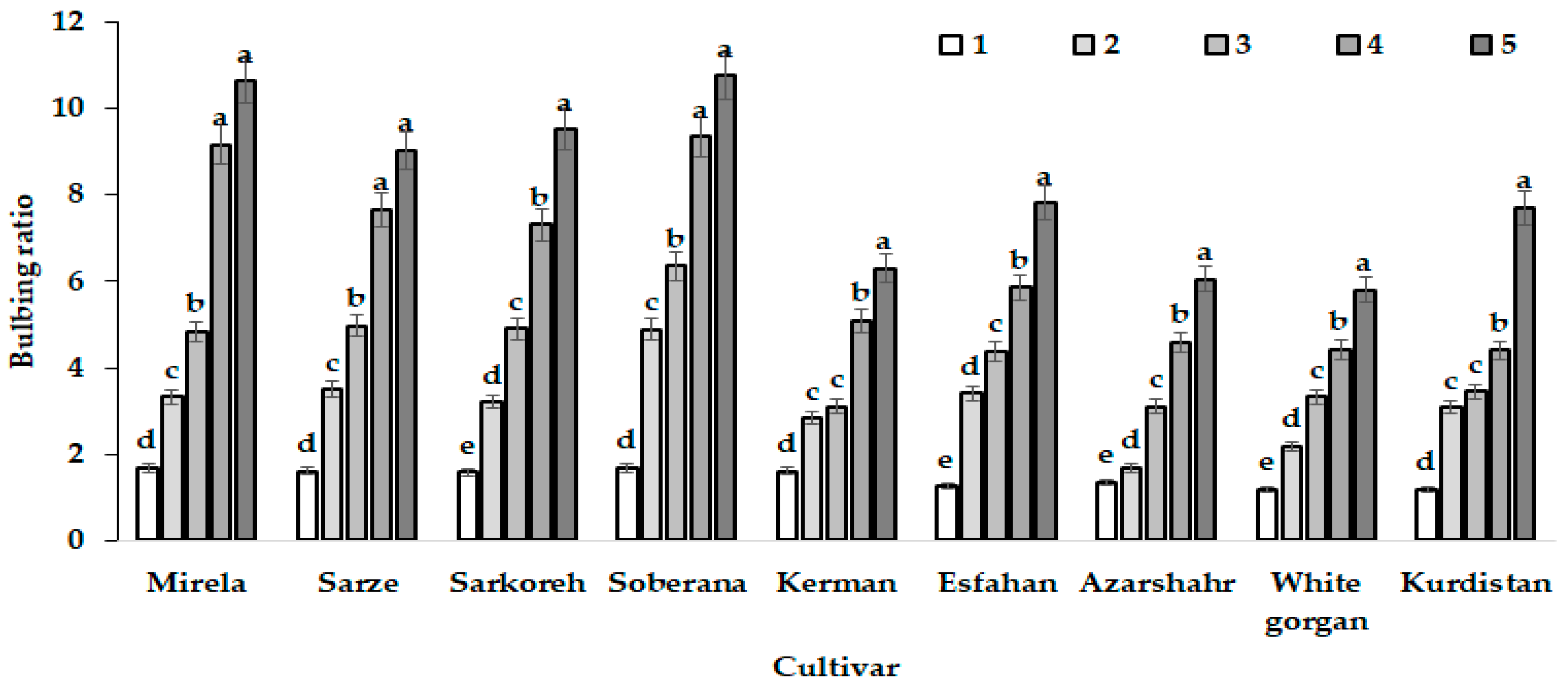
3.1.2. Bulbing Ratio and Bulb Diameter
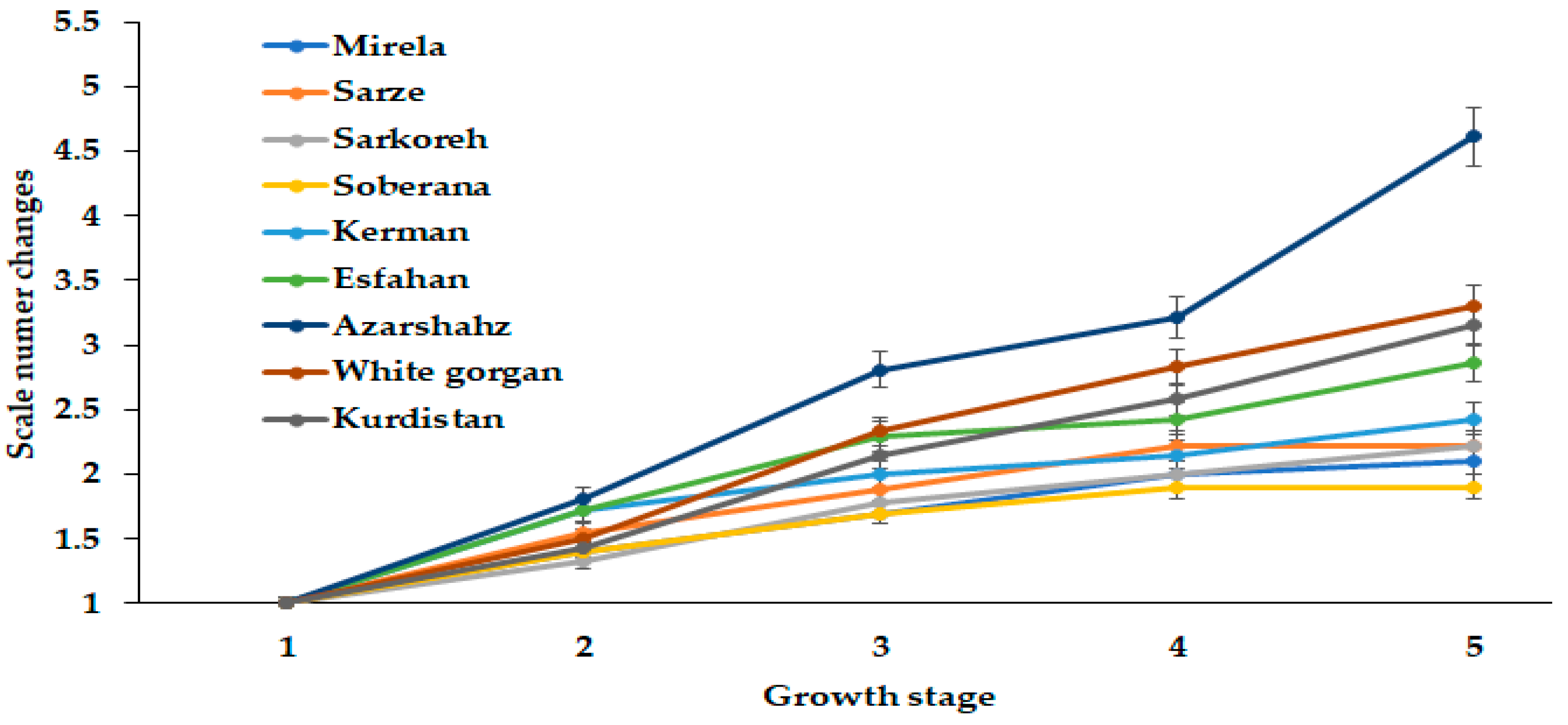
3.1.3. Scale Number
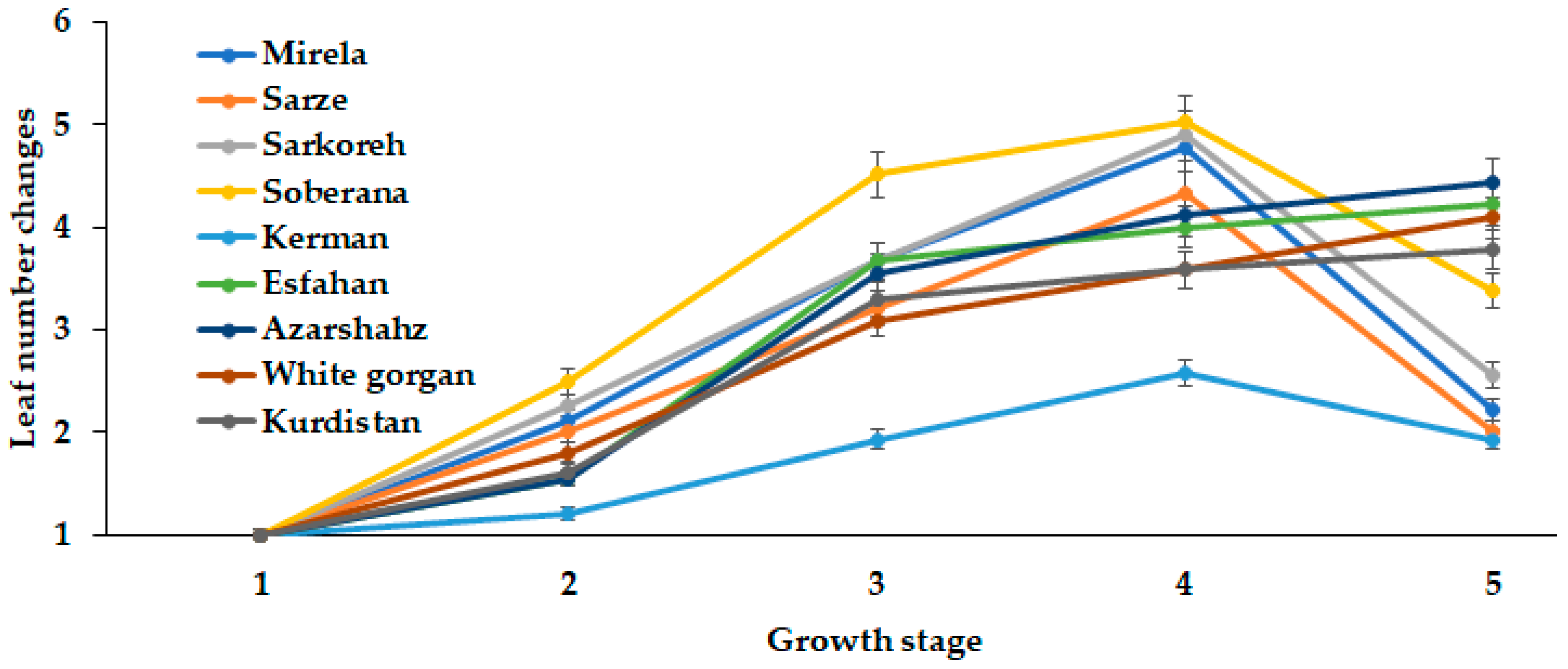
3.1.4. Leaf Number and Length
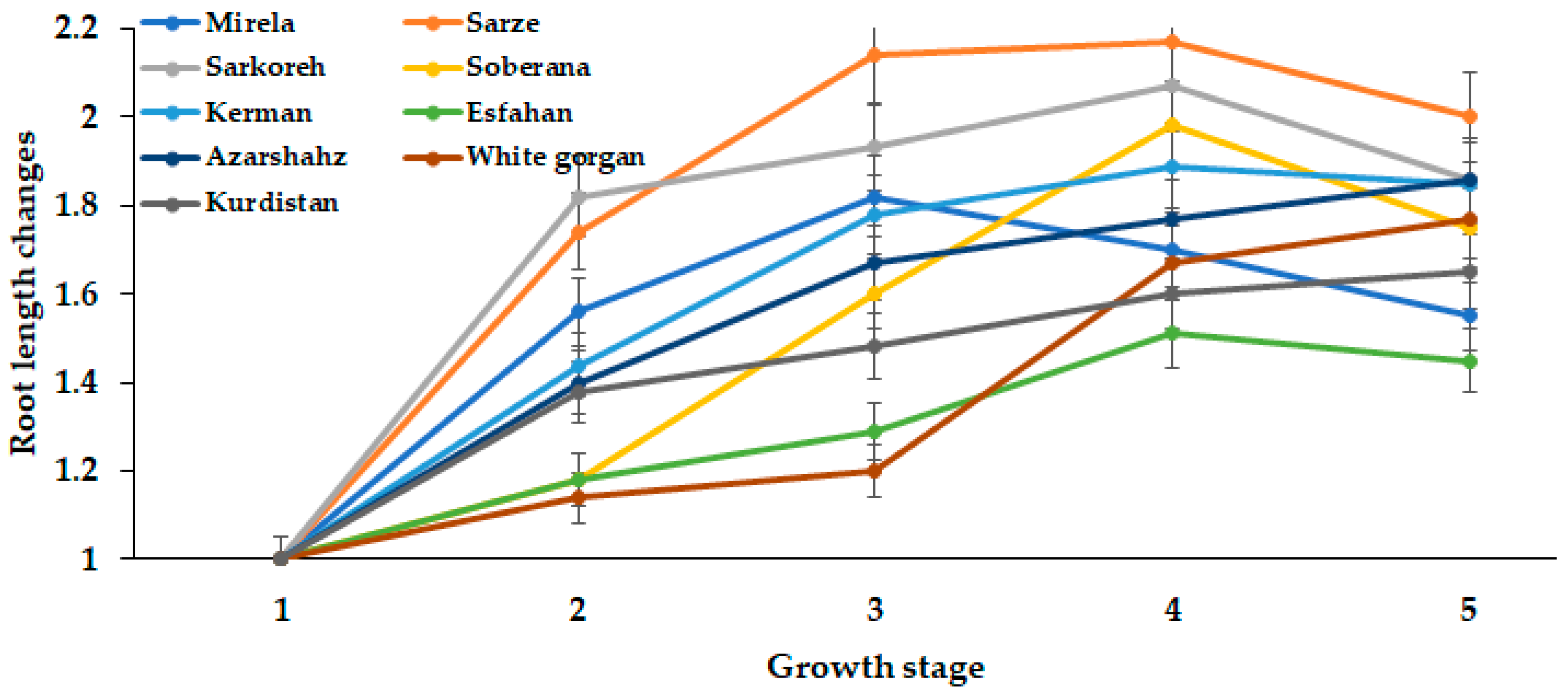
3.1.5. Root Length
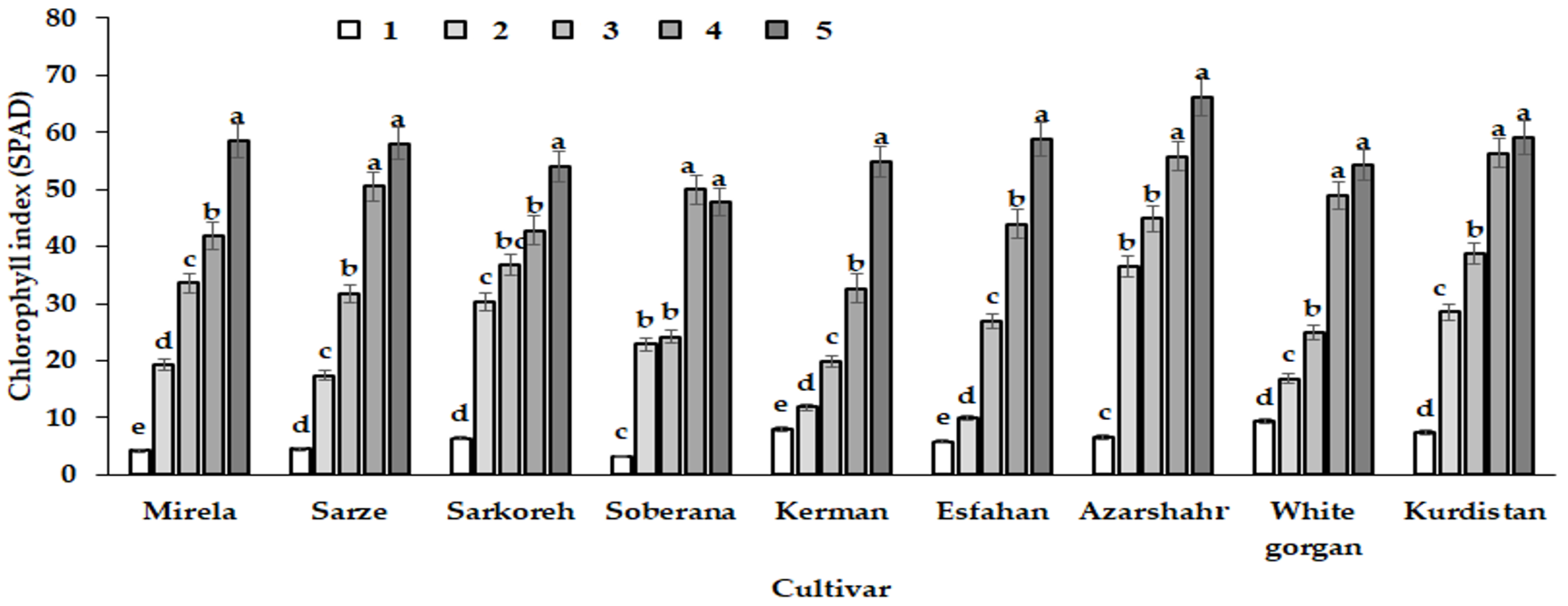
3.2. Chlorophyll Index
3.3. Antioxidant Status
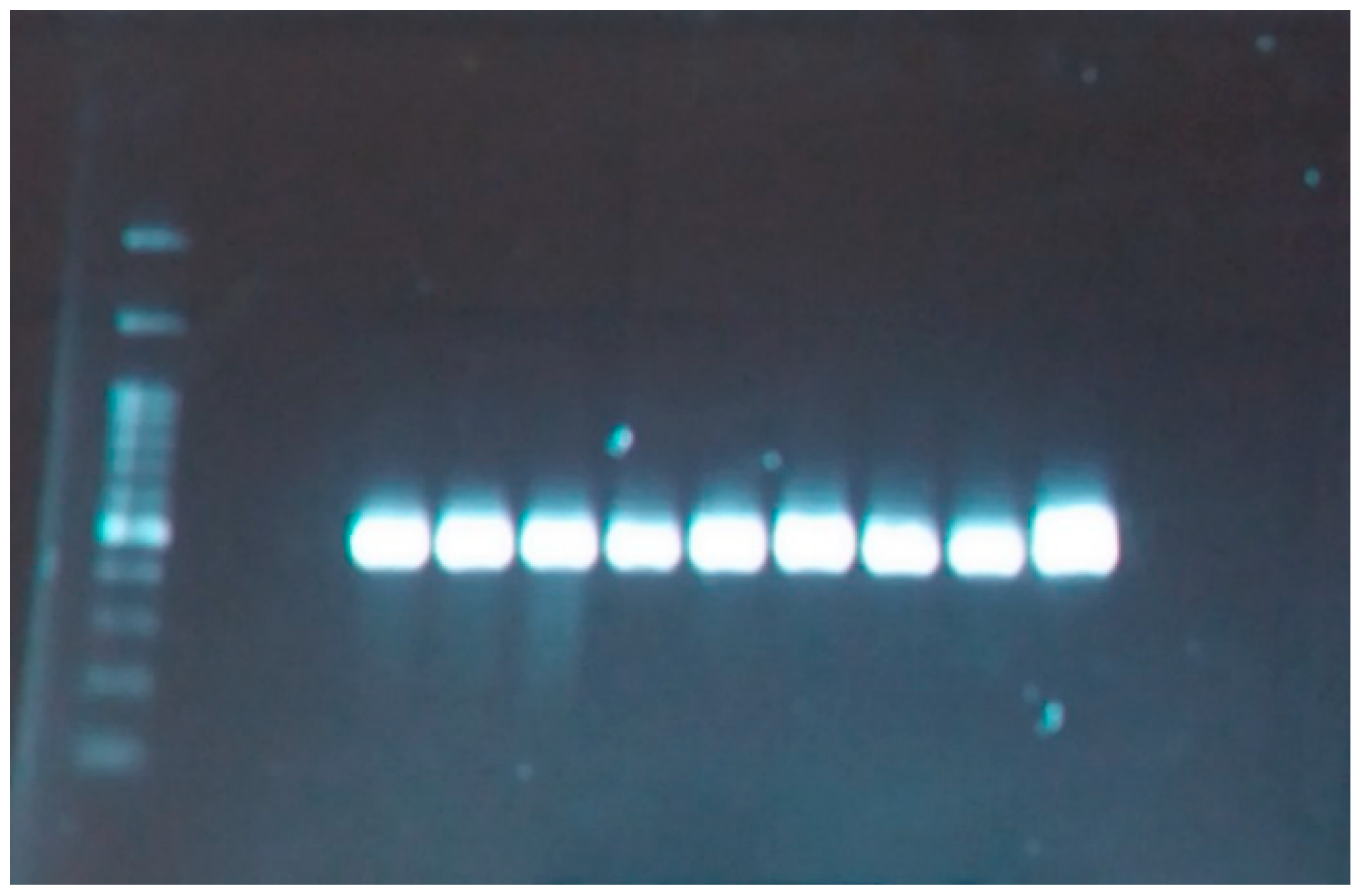
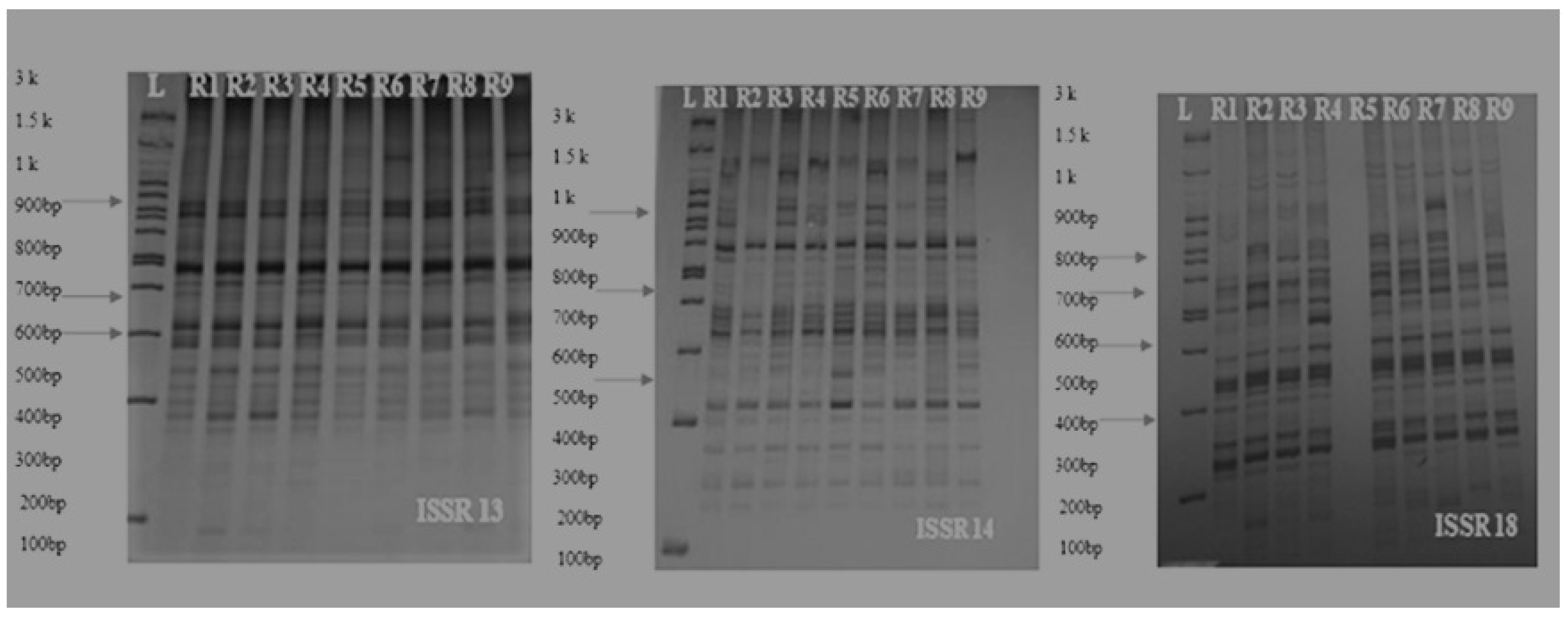
3.4. Results of Molecular Experiments
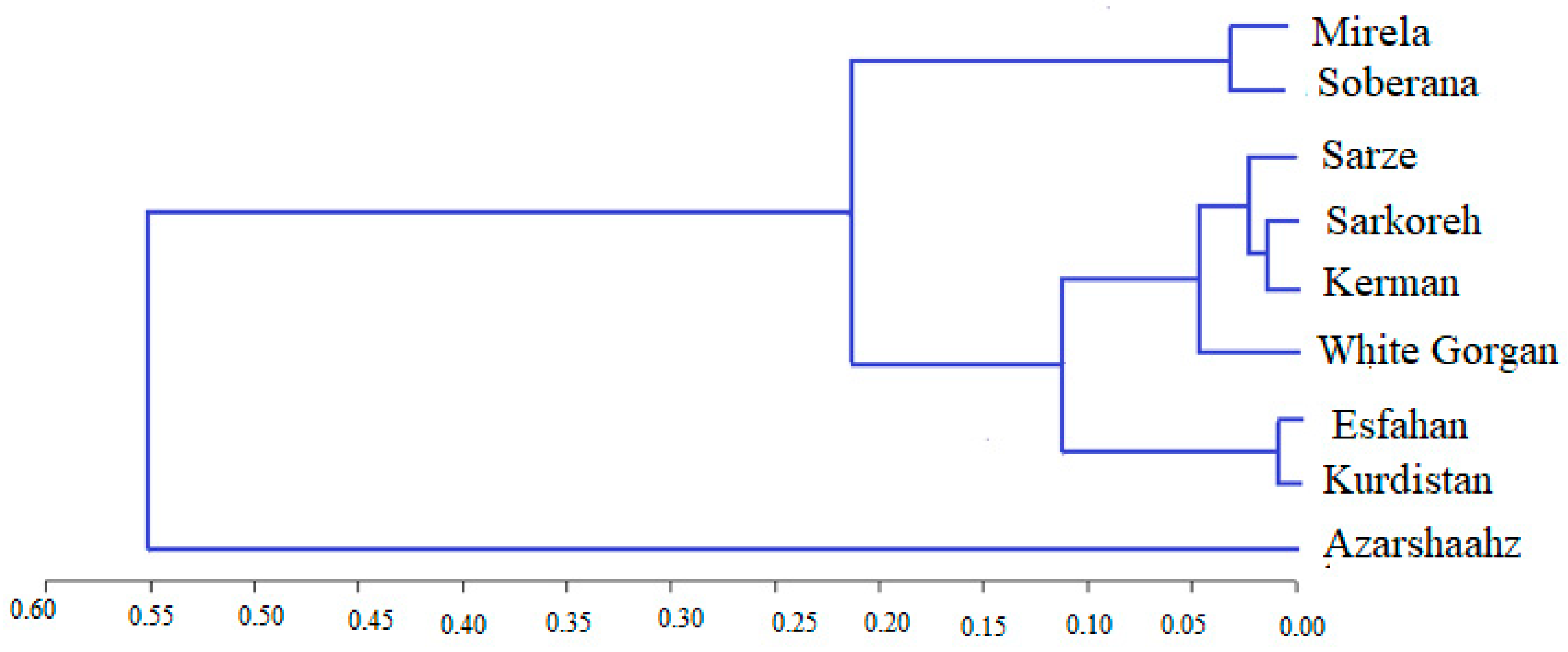
3.4.1. ISSR Analysis
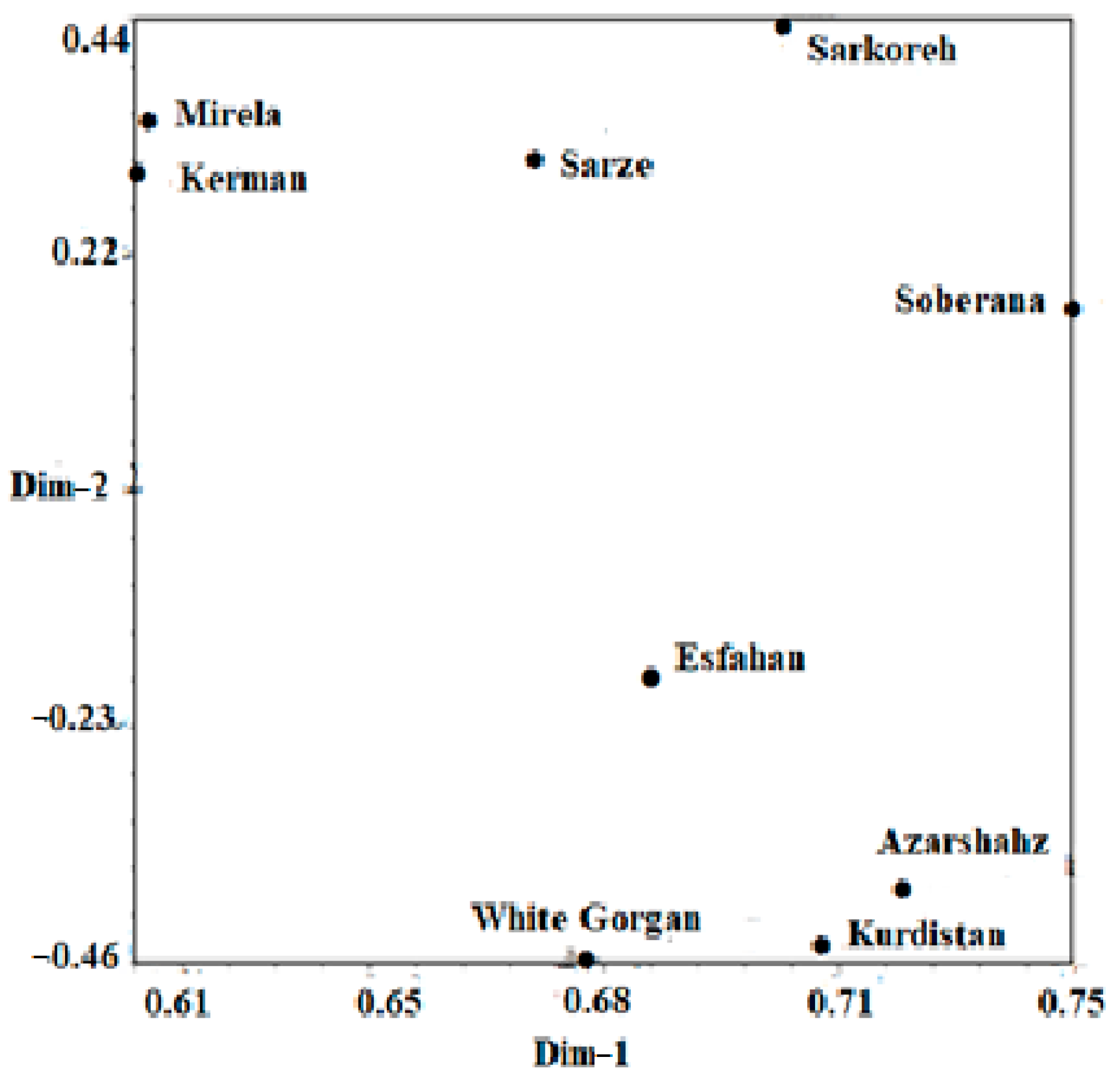
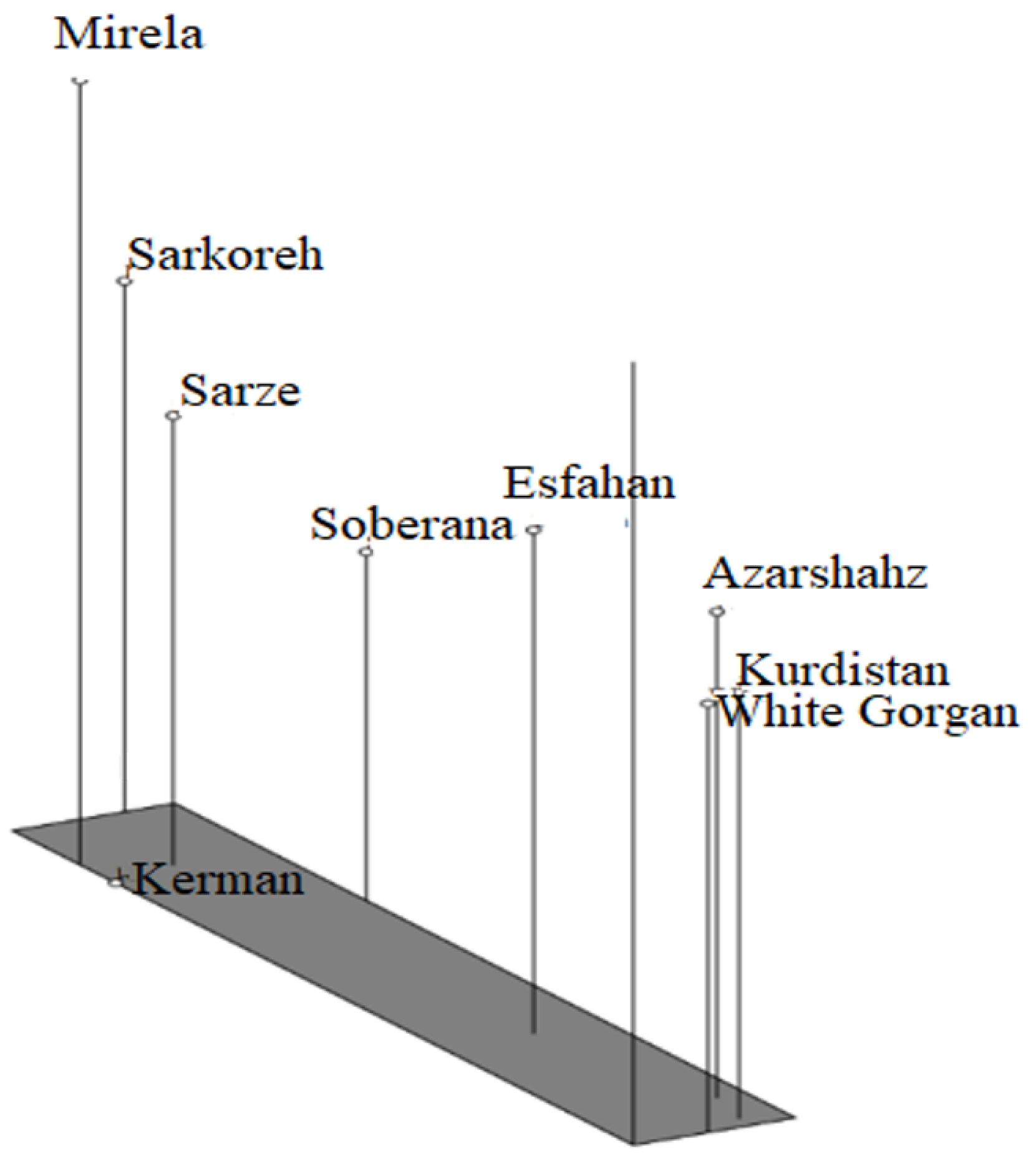
3.4.2. Relationships between the Iranian Onion Landraces and the Commercial Short-Day Species
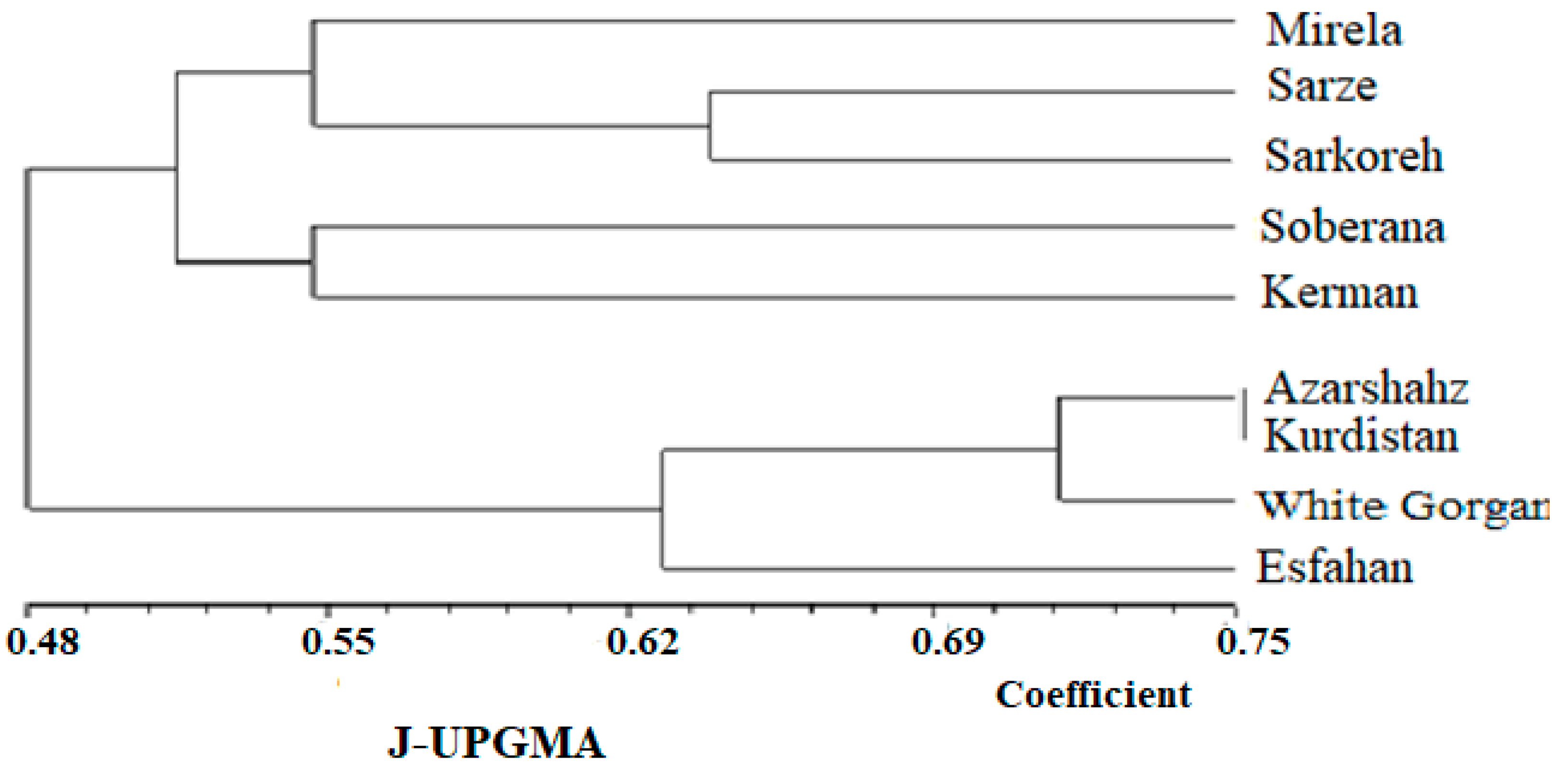
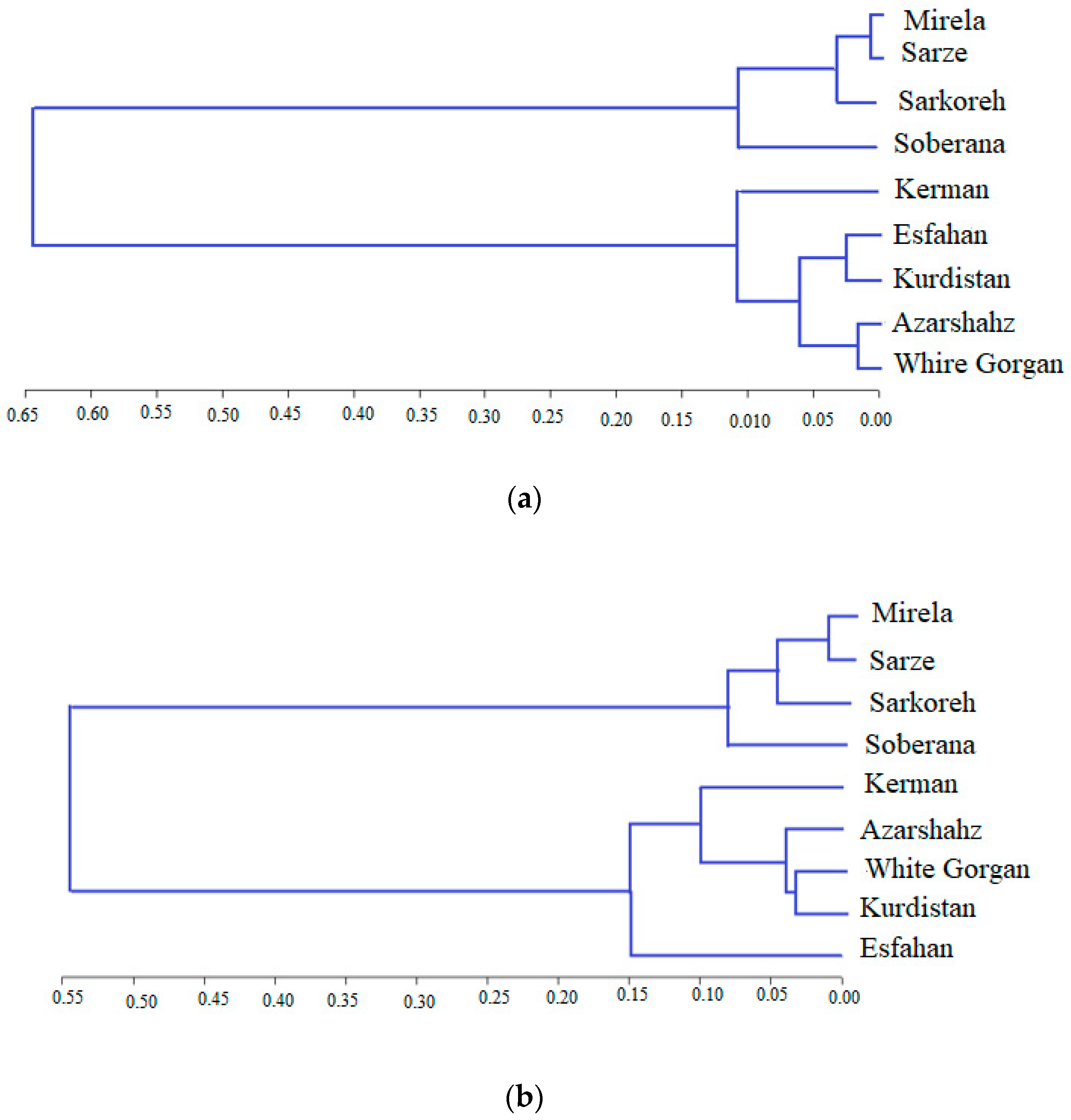
3.4.3. Cluster Analysis of Biometrical and Agronomic Traits
3.4.4. Cluster Analysis of Phytochemical Traits
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- King, J.J.; Bradeen, J.M.; Bark, O.; McCallum, J.A.; Havey, M.J. A low-density genetic map of onion reveals a role for tandem duplication in the evolution of an extremely large diploid genome. Theor. Appl. Genet. 1998, 96, 52–62. [Google Scholar] [CrossRef]
- Vavilov, N.I. Origin and Geography of Cultivated Plants; English Translation by D Love 1992; Cambridge University Press: Cambridge, UK, 1926. [Google Scholar]
- Brewster, J.L. Environmental physiology of the onion: Towards quantitative models for the effects of photoperiod, temperature and irradiance on bulbing, flowering and growth. Acta Hortic. 1997, 433, 347–374. [Google Scholar] [CrossRef]
- Malik, G.; Dhatt, A.; Malik, A.A. A Review of Genetic Understanding and Amelioration of Edible Allium Species. Food Rev. 2020, 37, 415–446. [Google Scholar] [CrossRef]
- Astley, D. Conservation of genetic resources. In Onions and Allied Crops; Rabinowitch, H.D., Brewster, J.L., Eds.; CRC Press: Boca Raton, FL, USA, 1990; Volume 1, pp. 177–198. [Google Scholar]
- Mallor, C.; Arnedo-Andres, M.S.; Garces-Claver, A. Assessing the genetic diversity of Spanish Allium cepa landraces for onion breeding using microsatellite markers. Sci. Hortic. 2014, 170, 24–31. [Google Scholar] [CrossRef]
- Mettananda, K.A.; Fordham, R. The effects of 12 and 16 h daylength treatments on the onset of bulbing in 21 onion cultivars (Allium cepa L.) and its application to screening germplasm for use in the tropics. J. Hortic. Sci. Biotechnol. 1997, 72, 981–988. [Google Scholar] [CrossRef]
- Khokhar, K.M. Environmental and genotypic effects on bulb development in onion. A review. J. Hortic. Sci. Biotechnol. 2017, 92, 448–454. [Google Scholar] [CrossRef]
- Hall, K.D.; Holloway, R.L.; Smith, D.T.; Anciso, J.; Troxclair, N.; Black, M. Texas Crop Profile-Onions; AgriLife Extension Service Publication E-18; Texas A&M University: Austin, TX, USA, 2011; Available online: http://upshur.agrilife.org/files/2011/03/Onions.pdf (accessed on 3 February 2023).
- Lee, E.J.; Yoo, K.S.; Leskovar, D.; Patil, B.S. Effects of leaf cutting on bulb weight and pungency of short-day onions after lifting the plants. Sci. Hortic. 2019, 257, 108720. [Google Scholar] [CrossRef]
- Mobli, M.; Aslani, L. Research on edible onion in Iran. Strateg. Res. J. Agric. Sci. Nat. Resour. 2019, 3, 153–168. [Google Scholar]
- Kik, C. Allium genetic resources with particular reference to onion. Acta Hortic. 2008, 770, 135–138. [Google Scholar] [CrossRef]
- Rashid, M.H.; Islam, M.A.K.; Mian, M.A.K.; Hossain, T.; Kabir, M.E. Multivariate analysis in onion (Allium cepa L.). Bangladesh J. Agric. Res. 2013, 37, 573–582. [Google Scholar] [CrossRef]
- Fabriki Ourang, S.; Shams Bakhsh, M.; Jalali Javara, M.; Ahmadi, J. Analysis of genetic diversity of Iranian melons (Cucumis melo L.) using ISSR markers. Iran. J. Biol. 2009, 22, 57–68. [Google Scholar]
- Lancaster, J.E.; Triggs, C.M.; Deruiter, J.M.; Gandar, P.W. Bulbing in onions: Photoperiod and temperature requirements and prediction of bulb size and maturity. Ann. Bot. 1996, 78, 423–430. [Google Scholar] [CrossRef]
- Ikeda, H.; Tsukazaki, H. Expression analysis of bulb development-related genes in onion cultivars. Acta Hortic. 2021, 1312, 31–36. [Google Scholar] [CrossRef]
- Dantata, I.J. Bulb Moisture, Ash and Dry Matter Contents of Onion Provenances in Northern Bauchi, Nigeria. Asian J. Appl. Sci. 2014, 2, 368–374. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and pralin contents in Burkinafasan honey, as well as their scavenging activity. Food Chem. J. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Masood, S.; Ihsan, M.A.; Shahzad, K.; Sabir, M.; Alam, S.; Ahmed, W.; Shah, Z.H.; Alghabari, F.; Mehmood, A.; Chung, G. Antioxidant potential and α-glucosidase inhibitory activity of onion (Allium cepa L.) peel and bulb extracts. Braz. J. Biol. 2023, 83, e247168. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Wrolstad, R.E. Current Protocols in Food Analytical Chemistry F1.2.1–1.2.13; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. J. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Nei, M.; Li, W.H. Mathematical model for studying the genetic variation in terms of restriction endonuclease. Proc. Natl. Acad. Sci. USA 1979, 76, 5269–5273. [Google Scholar] [CrossRef]
- Rohlf, F. NTSYS PC: Numerical Taxonomy and Multivariate Analysis for the IBM PC Microcomputers (and Compatibles); Version 2.1; Applied Biostatistics Inc.: Stony Brook, NY, USA, 2000. [Google Scholar]
- Feller, C.; Bleiholder, H.; Buhr, L.; Hack, H.; Hess, M.; Klose, R.; Meier, U.; Stauss, R.; Van den Boom, T.; Weber, E. Phenological growth stages of vegetable crops. I. Bulb root tuber and leaf vegetables. Coding and description according to the expanded BBCH scale with illustrations. Nachr. Dtsch. Pflanzenschutzd. 1995, 47, 193–206. [Google Scholar]
- Przygocka-Cyna, K.; Barłóg, P.; Grzebisz, W.; Spiżewski, T. Onion (Allium cepa L.) Yield and Growth Dynamics Response to In-Season Patterns of Nitrogen and Sulfur Uptake. Agronomy 2020, 10, 1146. [Google Scholar] [CrossRef]
- Lee, E.J.; Suh, J.K. Effect of temperature on the growth, pyruvic acid and sugar contents in onion bulbs. Korean J. Hortic. Sci. Biotechnol. 2009, 27, 554–558. [Google Scholar]
- Ikeda, H.; Yamamoto, T.; Kinoshita, T.; Tsukazaki, H.; Yamasaki, A. A Comparative Study on the Growth and Bulb Development of Several Onion (Allium cepa L.) Cultivars Sown in Spring in the Northeast Region of Japan. Jpn. Soc. Hortic. Sci. 2020, 89, 586–592. [Google Scholar] [CrossRef]
- Rabinowitch, H.D.; Currah, L. Allium Crop Science, Recent Advances; Centre for Agriculture and Biosciences International: Wallingford, UK, 2002. [Google Scholar]
- Kopsell, D.A.; Lefsrud, M.G.; Auge, R.M.; Both, A.J. Biomass production and pigment accumulation in kale grown under increasing photoperiods. HortScience 2006, 41, 603–606. [Google Scholar]
- Koontz, H.V.; Prince, R.P.; Koontz, R.F. Comparison of fluorescent and high-pressure sodium lamps on growth of leaf lettuce. HortScience 1987, 22, 424–425. [Google Scholar] [CrossRef]
- Duysens, L.M.N.; Sonneveld, A.; Rademaker, H. Chlorophyll a fluorescence as a monitor of nanosecond reduction of the photooxidized primary donor P-680+ of Photosystem II. Biochim. Biophys. Acta (BBA)-Bioenerg. 1979, 548, 536–551. [Google Scholar]
- Golubkina, N.; Caruso, G. Nutritional and antioxiant properties of Allium cepa L. In Nutritional Composition and Antioxidant Properties of Fruits and Vegetables; Jaiswal, A.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 73–89. [Google Scholar]
- Marefatia, N.; Ghorania, V.; Shakeric, F.; Boskabadye, M.; Kianiang, F.; Rezaeeh, R.; Hosein, M. Boskabady A review of anti-inflammatory, antioxidant, and immunomodulatory effects of Allium cepa and its main constituents. Pharm. Biol. 2021, 59, 285–300. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.B.; Lebelle, J.; Birsan, R.; Rai, D.K. Enrichment and Assessment of the Contributions of the Major Polyphenols to the Total Antioxidant Activity of Onion Extracts: A Fractionation by Flash Chromatography Approach. Antioxidants 2018, 7, 175. [Google Scholar] [CrossRef] [PubMed]
- Metrani, R.; Singh, J.; Acharya, P.; Jayaprakasha, G.K.; Patil, B.S. Comparative Metabolomics Profiling of Polyphenols, Nutrients and Antioxidant Activities of Two Red Onion (Allium cepa L.) Cultivars. Plants 2020, 9, 1077. [Google Scholar] [CrossRef]
- Keighobadi, K.; Golabadi, M.; Khozaei, M.; Rezai, A. Phytochemical Screening of the Aqueous Extracts of Iranian Onion (Allium cepa L.) Landraces. J. Food Biosci. Technol. 2021, 11, 99–106. [Google Scholar]
- Baldwin, S.; Pither-Joyce, M.; Wright, K.; Chen, L.; McCallum, J. Development of robust genomic simple sequence repeat markers for estimation of genetic diversity within and among bulb onion (Allium cepa L.) populations. Mol. Breed. 2012, 30, 1401–1411. [Google Scholar] [CrossRef]
- Khar, A.; Lawande, K.E.; Negi, K.S. Microsatellite marker based analysis of genetic diversity in short-day tropical Indian onion and cross amplification in related Allium spp. Genet. Resour. Crop Evol. 2011, 58, 741–752. [Google Scholar] [CrossRef]
- Gupta, V.P.; Sekhon, M.S.; Satija, D.R. Studies on genetic diversity, heterosis and combining ability in Indian mustard (Brassica juncea (L.) Czern and Coss.). Indian J. Genet. 1991, 51, 448–453. [Google Scholar]
- Dehdari, A.; Rezaei, A.; Mobli, M. Evaluation of physical and agronomic characteristics of some Iranian onion genotypes. J. Agric. Sci. Technol. Nat. Res. 2001, 5, 109–123. [Google Scholar]
- Kutty, M.S.; Veere Gowda, R.; Anand, L. Analysis of genetic diversity among Indian short-day onion (Allium cepa L.) cultivars using RAPD markers. J. Hortic. Sci. Biotechnol. 2006, 81, 774–778. [Google Scholar] [CrossRef]
- Seyyed Tabatabaei, B.E.; Karimi Nafchi, Z.; Mobli, M. Genetic diversity of some onion genotypes using SSR markers. Iranian J. Hortic. Sci. 2012, 42, 11–20. [Google Scholar]
- Huang, X.; Han, B. Natural variations and genome-wide association studies in crop plants. Annu. Rev. Plant Biol. 2014, 65, 531–551. [Google Scholar] [CrossRef]
- Chinnappareddy, L.R.D.; Khandagale, K.; Chennareddy, A.; Ramappa, V.G. Molecular markers in the improvement of Allium crops. Czech. J. Genet. Plant Breed. 2013, 49, 131–139. [Google Scholar] [CrossRef]
- Shanmugam, A.S.; Rangasamy, S.R.S. Multivariate analysis of genetic divergences of blackgram (Vigna mungo (L.) Hepper). Madras Agric. J. 1982, 69, 701–706. [Google Scholar]
- Elsharkawy, G.A.; Hegazi, H.H.; Azab, E.; Gobouri, A.A.; Sayed, S.A. Assessment of genetic diversity among Egyptian garlic landraces based on morphological characteristics and ISSR markers. Eur. J. Hortic. Sci. 2021, 86, 579–589. [Google Scholar] [CrossRef]




| Cultivar | Place of Origin | Day Length Requirement | Skin Colour | Latitude (UTM) |
|---|---|---|---|---|
| Mirela | Hybrid F1 | Short-day | Yellow | - |
| Soberana | Hybrid F1 | Short-day | Yellow | - |
| Sarze | Iranshahr | Short-day | Pink | 27°12′ N |
| Sarkoreh | Booshehr | Short-day | White | 29°26′ N |
| Kerman | Kerman | Short-day | Orange | 30°59′ N |
| Esfahan | Esfahan | Long-day | Pink | 32°37′ N |
| Azarshahr | East Azerbaijan | Long-day | Red | 46°37′ N |
| White Gorgan | Gorgan | Long-day | White | 36°80′ N |
| Kurdistan | Kurdistan | Long-day | Pink | 34°47′ N |
| Sampling Number | 2019 | 2020 | ||
|---|---|---|---|---|
| Day Duration (Hours) | Growing Degree Days (°C) | Day Duration (Hours) | Growing Degree Days (°C) | |
| (1) | 11.2 | 452 | 11.0 | 428 |
| (2) | 12.2 | 609 | 12.2 | 590 |
| (3) | 14.3 | 1097 | 14.3 | 1176 |
| (4) | 15.3 | 1634 | 15.3 | 1735 |
| (5) | 15.6 | 2330 | 15.7 | 2570 |
| Culivar | Leaf Length (cm) | Leaf Number | Root Length (cm) | Bulb Scale Number | Bulb Diameter (mm) | Bulbing Ratio | Bulb Dry Weight (g) | Bulb Fresh Weight (g) |
|---|---|---|---|---|---|---|---|---|
| Mirela | 38.85 bc | 3.00 cd | 7.73 b | 7.00 a | 70.66 a | 10.63 a | 9.50 a | 53.33 ab |
| Soberana | 38.33 bc | 4.66 b | 8.75 ab | 6.33 ab | 67.66 ab | 10.75 a | 9.16 a | 45.66 cd |
| Sarze | 42.50 bc | 2.66 d | 9.50 a | 6.66 ab | 68.66 ab | 9.02 a | 9.50 a | 52.33 ab |
| Sarkoreh | 35.00 c | 3.66 c | 8.67 ab | 6.66 ab | 70.00 a | 9.50 a | 9.00 a | 51.00 bc |
| Kerman | 45.83 ab | 5.00 b | 8.33 ab | 5.66 b | 56.66 b | 6.30 c | 9.33 a | 40.66 d |
| Esfahan | 48.16 a | 6.33 a | 7.50 b | 6.66 ab | 64.33 ab | 7.80 b | 7.33 b | 49.00 c |
| Azarshahr | 51.33 a | 6.66 a | 9.92 a | 7.66 a | 65.33 ab | 6.05 c | 9.66 a | 55.33 ab |
| White Gorgan | 53.66 a | 6.66 a | 11.67 a | 7.00 a | 65.66 ab | 5.80 c | 8.16 ab | 63.33 a |
| Kurdistan | 52.00 a | 6.33 a | 10.75 a | 7.33 a | 65.00 ab | 7.70 b | 9.83 a | 55.33 ab |
| M ± SD | 45.07 ± 6.74 | 5.00 ± 1.60 | 9.20 ± 1.39 | 6.77 ± 0.58 | 66.00 ± 4.17 | 8.17 ± 1.91 | 9.05 ± 0.81 | 51.77 ± 6.43 |
| CV (%) | 15.0 | 32.0 | 15.1 | 8.6 | 6.3 | 23.4 | 9.0 | 12.4 |
| Range | 35.00–53.66 | 2.66–6.66 | 7.50–8.75 | 5.66–7.66 | 56.66–70.66 | 5.80–10.75 | 7.33–9.83 | 40.66–63.33 |
| Source of Variation | df | Leaf Length | Leaf Number | Root Length | Bulb Scale Number | Bulb Diameter | Bulbing Ratio | Total Fresh Weight | Total Dry Weight | Chlorophyll Index |
|---|---|---|---|---|---|---|---|---|---|---|
| Block (r) | 2 | 0.427 ns | 0.443 ns | 6.03 ns | 0.163 ns | 0.227 ns | 0.06 ns | 17.19 ns | 0.915 ns | 4.50 ns |
| Sowing date (S) | 1 | 1262.39 ** | 1.203 * | 28.52 ** | 0.750 ns | 222.17 ** | 29.28 ** | 2.66 ns | 1.194 ns | 222.16 ** |
| Sampling date (Sam) | 4 | 23,364.008 ** | 231.92 ** | 175.39 ** | 147.64 ** | 40,509.05 ** | 446.05 ** | 47,699.08 ** | 589.63 ** | 24,570.57 ** |
| Cultivar (C) | 8 | 26.59 ** | 0.447 ns | 20.55 ** | 7.37 ** | 791.21 ** | 37.37 ** | 838.72 ** | 12.509 ** | 754.32 ** |
| S × Sam | 4 | 104.05 ** | 0.570 ns | 6.093 ** | 0.333 ns | 29.10 ** | 4.82 ** | 57.60 ** | 1.55 ** | 124.9 * |
| C × S | 8 | 32.32 ** | 0.447 ns | 14.89 ** | 0.690 ns | 3.64 * | 2.62 ** | 11.107 ** | 0.182 ns | 52.87 ** |
| C × Sam | 32 | 116.48 ** | 0.966 ** | 3.26 ** | 1.21 ** | 111.42 ** | 4.25 ** | 674.35 ** | 6.238 ** | 141.63 ** |
| C × S × Sam | 32 | 14.89 ** | 0.416 ns | 2.13 ** | 0.264 ns | 3.27 ns | 0.792 ** | 7.33 ** | 0.134 ns | 59.48 ** |
| Experimental Error (%) | 4.71 | 0.305 | 1.42 | 0.382 | 2.5 | 0.372 | 2.66 | 0.173 | 42.13 | |
| Coefficient of variation (%) | 6.38 | 12.85 | 14.95 | 12.47 | 10.36 | 12.81 | 10.01 | 11.53 | 17.37 | |
| Culivar | Antioxidant Activity (mg mL−1) | Phenolics (mg GAE g−1 f.w.) | Flavonoids (mg QE g−1 f.w.) | Anthocyanins (µM g−1 f.w.) |
|---|---|---|---|---|
| Mirela | 87.66 a | 7.00 e | 15.95 bc | 0.026 f |
| Sarze | 90.00 a | 24.76 bc | 12.86 d | 0.103 b |
| Sarkoreh | 90.00 a | 16.33 d | 13.79 cd | 0.080 c |
| Soberana | 86.66 a | 13.66 d | 13.42 cd | 0.056 e |
| Kerman | 88.87 a | 20.66 c | 12.30 d | 0.070 cd |
| Esfahan | 91.33 a | 24.30 bc | 16.74 b | 0.080 c |
| Azarshahr | 92.66 a | 39.79 a | 21.40 a | 0.163 a |
| White Gorgan | 88.66 a | 14.66 d | 9.70 e | 0.063 de |
| Kurdistan | 90.00 a | 25.00 b | 16.79 b | 0.086 bc |
| M ± SD | 89.54 ± 1.82 | 20.68 ± 9.39 | 14.77 ± 3.38 | 0.081 ± 0.038 |
| CV (%) | 2.03 | 45.41 | 22.88 | 46.90 |
| Range | 86.66–92.66 | 7.00–39.79 | 9.70–21.40 | 0.026–0.163 |
| Polyphenols | Flavonoids | Anthocyanins | |
|---|---|---|---|
| Antioxidant activity | 0.871 (p < 0.001) | 0.638 (p < 0.05) | 0.846 (p < 0.001) |
| Polyphenols | 1 | 0.628 (p < 0.05) | 0.968 (p < 0.001) |
| Flavonoids | 1 | 0.591 (p > 0.05) |
| Source of Variation | df | Mean Squares | |||
|---|---|---|---|---|---|
| Polyphenols | Flavonoids | Anthocyanins | Antioxidant Activity | ||
| Block | 2 | 2.7 ns | 11.60 ns | 0.000018 ns | 15.08 ns |
| Cultivar | 8 | 388.19 ** | 41.02 ** | 0.0080 ** | 146.19 ** |
| Experimental Error (%) | 38 | 4.60 | 4.97 | 0.0003 | 1.24 |
| Coefficient of variation (%) | 12.41 | 16.32 | 23.83 | 6.7 | |
| Primer Name | Sequence (5′-3′) | Tm (°C) | Annealing Temp* (°C) | No. of Amplicons | No. of Polymorphic Bands | Percent Polymorphism (%) | PIC Value |
|---|---|---|---|---|---|---|---|
| ISSR-13 | 5′AGAGAGAGAGAGAGAGG3′ | 52.8 | 50 | 32 | 19 | 59 | 0.46 |
| ISSR-14 | 5′GAGAGAGAGAGAGAGAT3′ | 50.4 | 48 | 41 | 28 | 67 | 0.39 |
| ISSR-15 | 5′GAGAGAGAGAGAGAGAC3′ | 52.8 | 50 | 6 | 1 | 84 | 0.40 |
| ISSR-18 | 5′CTCTCTCTCTCTCTCTG3′ | 52.8 | 50 | 46 | 32 | 70 | 0.43 |
| ISSR-19 | 5′CTCTCTCTCTCTCTCTT3′ | 50.4 | 48 | 13 | 5 | 38 | 0.40 |
| ISSR-23 | 5′GTGTGTGTGTGTGTGTC3′ | 52.8 | 50 | 12 | 5 | 45 | 0.41 |
| ISSR-26 | 5′GTGTGTGTGTGTGTGTC3′ | 52.8 | 50 | 12 | 5 | 45 | 0.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiani, Z.; Mashayekhi, K.; Golubkina, N.; Mousavizadeh, S.J.; Nezhad, K.Z.; Caruso, G. Agronomic, Physiological, Genetic and Phytochemical Characteristics of Onion Varieties Influenced by Daylength Requirements. Agriculture 2023, 13, 697. https://doi.org/10.3390/agriculture13030697
Kiani Z, Mashayekhi K, Golubkina N, Mousavizadeh SJ, Nezhad KZ, Caruso G. Agronomic, Physiological, Genetic and Phytochemical Characteristics of Onion Varieties Influenced by Daylength Requirements. Agriculture. 2023; 13(3):697. https://doi.org/10.3390/agriculture13030697
Chicago/Turabian StyleKiani, Zahra, Kambiz Mashayekhi, Nadezhda Golubkina, Seyyed Javad Mousavizadeh, Khalil Zaynali Nezhad, and Gianluca Caruso. 2023. "Agronomic, Physiological, Genetic and Phytochemical Characteristics of Onion Varieties Influenced by Daylength Requirements" Agriculture 13, no. 3: 697. https://doi.org/10.3390/agriculture13030697
APA StyleKiani, Z., Mashayekhi, K., Golubkina, N., Mousavizadeh, S. J., Nezhad, K. Z., & Caruso, G. (2023). Agronomic, Physiological, Genetic and Phytochemical Characteristics of Onion Varieties Influenced by Daylength Requirements. Agriculture, 13(3), 697. https://doi.org/10.3390/agriculture13030697







