Effects of Melatonin Dose on Fruit Yield, Quality, and Antioxidants of Strawberry Cultivars Grown in Different Crop Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Growing Conditions
2.2. Yield and Quality Analysis
2.3. Data Statistical Analysis
3. Results
3.1. Yield Parameters
3.2. Quality and Phytochemical Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Özgüven, A.I.; Yilmaz, C. Bazı Çilek Çeşitlerinin Adana Ekolojik Koşullarındaki Morfolojik ve Pomolojik Özellikleri Alatarım. Eskiseh. Osman. Univ. Res. Inf. Syst. 2009, 8, 17–21. [Google Scholar]
- Darrow, G.M. The strawberry. History, breeding and physiology. In The Strawberry. History, Breeding and Physiology; Holt, Rinehart & Winston: New York, NY, USA, 1966. [Google Scholar]
- Padmanabhan, P.; Mizran, A.; Sullivan, J.A.; Paliyath, G. Strawberries’ Encyclopedia of Food and Health; Academic Press: Oxford, UK, 2016; pp. 193–198. [Google Scholar]
- Kader, A.A. Quality and its maintenance in relation to the postharvest physiology of strawberry. In The Strawberry into the 21st Century; Timber Press: Portland, Oregon, 1991; pp. 145–152. [Google Scholar]
- Salamé-Donoso, T.P.; Santos, B.M.; Chandler, C.K.; Sargent, S.A. Effect of high tunnels on the growth, yields, and soluble solids of strawberry cultivars in Florida. Int. J. Fruit Sci. 2010, 10, 249–263. [Google Scholar] [CrossRef]
- Naumann, W.D.; Seipp, D. Grundlagen für anbau und vermarktung. In Erdbeeren; Ulmer Fachbuch: Stuttgart, Germany, 1990; p. 256. [Google Scholar]
- Kahramanoğlu, İ. Effects of lemongrass oil application and modified atmosphere packaging on the postharvest life and quality of strawberry fruits. Sci. Hortic. 2019, 256, 108527. [Google Scholar] [CrossRef]
- Chandler, C.K.; Herrington, M.; Slade, A. Effect of harvest date on soluble solids and titratable acidity in fruit of strawberry grown in winter, annual hill production system. In XXVI International Horticultural Congress: Berry Crop Breeding, Production and Utilization for a New Century; Toronto, Canada, 2002; Volume 626, pp. 345–346. [Google Scholar]
- Türemiş, N.; Özgüven, A.I.; Paydaş, S. Güneydoğu Anadolu Bölgesinde Çilek Yetiştiriciliği. Türkiye Bilimsel ve Teknik Araştırma Kurumu. In Türkiye Tarımsal Araştırma Projesi Yay; Adana, Turkey, 2000; p. 36. [Google Scholar]
- Baby, B.; Antony, P.; Vijayan, R. Antioxidant and anticancer properties of berries. Crit. Rev. Food Sci. Nutr. 2017, 58, 2491–2507. [Google Scholar] [CrossRef] [PubMed]
- Oszmiański, J.; Wojdyło, A. Comparative study of phenolic content and antioxidant activity of strawberry puree, clear, and cloudy juices. Eur. Food Res. Technol. 2009, 228, 623–631. [Google Scholar] [CrossRef]
- Singh, S.; Singh, R.P. In Vitro methods of assay of antioxidants: An overview. Food Rev. Int. 2008, 24, 392–415. [Google Scholar] [CrossRef]
- MoA. Turkey. Alata Bahçe Kültürleri Araştırma İstasyonu. Çilek yetiştirciliği. 2012. Available online: https://arastirma.tarimorman.gov.tr/alata/Belgeler/Diger-belgeler/%C3%87ilekYeti%C5%9Ftiricili%C4%9Fi%C3%87Nacar.pdf (accessed on 15 January 2022).
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of melatonin, the pineal gland factor that lightens melanocytes. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Arnao, M.B. Phytomelatonin: Discovery, content, and role in plants. In Advances in Botany; Hindawi Publishing Corporation: London, UK, 2014. [Google Scholar]
- Pandi-Perumal, S.R.; Trakht, I.; Srinivasan, V.; Spence, D.W.; Maestroni, G.J.; Zisapel, N.; Cardinali, D.P. Physiological effects of melatonin: Role of melatonin receptors and signal transduction pathways. Prog. Neurobiol. 2008, 85, 335–353. [Google Scholar] [CrossRef]
- Hardeland, R.; Madrid, J.A.; Tan, D.X.; Reiter, R.J. Melatonin, the circadian multioscillator system and health: The need for detailed analyses of peripheral melatonin signaling. J. Pineal Res. 2012, 52, 139–166. [Google Scholar] [CrossRef]
- Wei, W.; Li, Q.T.; Chu, Y.N.; Reiter, R.J.; Yu, X.M.; Zhu, D.H.; Zhang, W.K.; Ma, B.; Lin, Q.; Zhang, J.S.; et al. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef]
- Carrillo-Vico, A.; Lardone, P.J.; Álvarez-Sánchez, N.; Rodríguez-Rodríguez, A.; Guerrero, J.M. Melatonin: Buffering the immune system. Int. J. Mol. Sci. 2013, 14, 8638–8683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Zhao, B.; Zhang, H.J.; Weeda, S.; Yang, C.; Yang, Z.C.; Guo, Y.D. Melatonin promotes water-stress tolerance, lateral root formation, and seed germination in cucumber (Cucumis sativus L.). J. Pineal Res. 2013, 54, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: Plant growth regulator and/or biostimulator during stress? Trends Plant Sci. 2014, 19, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Yin, L.; Liang, D.; Li, C.; Ma, F.; Yue, Z. Delayed senescence of apple leaves by exogenous melatonin treatment: Toward regulating the ascorbate–glutathione cycle. J. Pineal Res. 2012, 53, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, D.E.; Jang, H.; Byeon, Y.; Kim, Y.S.; Back, K. Melatonin-rich transgenic rice plants exhibit resistance to herbicide-induced oxidative stress. J. Pineal Res. 2013, 54, 258–263. [Google Scholar] [CrossRef]
- Vitalini, S.; Gardana, C.; Simonetti, P.; Fico, G.; Iriti, M. Melatonin, melatonin isomers and stilbenes in Italian traditional grape products and their antiradical capacity. J. Pineal Res. 2013, 54, 322–333. [Google Scholar] [CrossRef]
- Agathokleous, E.; Zhou, B.; Xu, J.; Ioannou, A.; Feng, Z.; Saitanis, C.J.; Frei, M.; Calabrese, E.J.; Fotopoulos, V. Exogenous application of melatonin to plants, algae, and harvested products to sustain agricultural productivity and enhance nutritional and nutraceutical value: A meta-analysis. Environ. Res. 2021, 200, 111746. [Google Scholar] [CrossRef]
- Hışıl, Y. Enstrümantal Gıda Analizleri Laboratuar Kılavuzu Ege Üniversitesi Yayınları; 1993; Volume 55. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Boskou, D.; Tsimidou, M.; Blekas, G. Polar phenolic compounds. In Olive Oil, 2nd ed.; Boskou, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 73–92. [Google Scholar]
- Kolář, J.; Macháčková, I.; Eder, J.; Prinsen, E.; Van Dongen, W.; Van Onckelen, H.; Illnerová, H. Melatonin: Occurrence and daily rhythm in Chenopodium rubrum. Phytochemistry 1997, 44, 1407–1413. [Google Scholar] [CrossRef]
- Zhang, H.J.; Zhang, N.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA4 interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279. [Google Scholar] [CrossRef]
- Altaf, M.A.; Shahid, R.; Ren, M.X.; Altaf, M.M.; Jahan, M.S.; Khan, L.U. Melatonin mitigates nickel toxicity by improving nutrient uptake fluxes, root architecture system, photosynthesis, and antioxidant potential in tomato seedling. J. Soil Sci. Plant Nutr. 2021, 21, 1842–1855. [Google Scholar] [CrossRef]
- Buttar, Z.A.; Wu, S.N.; Arnao, M.B.; Wang, C.; Ullah, I.; Wang, C. Melatonin suppressed the heat stress-induced damage in wheat seedlings by modulating the antioxidant machinery. Plants 2020, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Abdelhamid, M.T.; Pessarakli, M.; Sekara, A.; Caruso, G.; Rady, M.M.; El-Metwally, I.M. Role of melatonin in improving the tolerance of plants to salinity stress. In Handbook of Plant and Crop Physiology; CRC Press: Boca Raton, FL, USA, 2021; pp. 158–173. [Google Scholar]
- Abbas, S.M.; Sarhan, I.A. The role of melatonin in improving the vegetative growth characteristics of four genotypes of Faba Bean (Vicia faba L.). In IOP Conference Series: Earth and Environmental Science (Vol. 904, No. 1, p. 012028); IOP Publishing: Bristol, UK, 2021. [Google Scholar]
- Altaf, M.A.; Shahid, R.; Ren, M.; Mora-Poblete, F.; Arnao, M.B.; Naz, S.; Anwar, M.; Shahid, S.; Shakoor, A.; Sohail, H.; et al. Phytomelatonin: An overview of the importance and mediating functions of melatonin against environmental stresses. Physiol. Plant. 2020, 172, 820–846. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Ruiz, J.; Arnao, M.B. Melatonin stimulates the expansion of etiolated lupin cotyledons. Plant Growth Regul. 2008, 55, 29–34. [Google Scholar] [CrossRef]
- Kim, S.K.; Bae, R.N.; Na, H.; Dal Ko, K.; Chun, C. Changes in physicochemical characteristics during fruit development in June-bearing strawberry cultivars. Hortic. Environ. Biotechnol. 2013, 54, 44–51. [Google Scholar] [CrossRef]
- Saini, S.; Kumar, P.; Sharma, N.C.; Sharma, N.; Balachandar, D. Nano-enabled Zn fertilization against conventional Zn analogues in strawberry (Fragaria × ananassa Duch.). Sci. Hortic. 2021, 282, 110016. [Google Scholar] [CrossRef]
- Gasperotti, M.; Masuero, D.; Guella, G.; Palmieri, L.; Martinatti, P.; Pojer, E.; Mattivi, F.; Vrhovsek, U. Evolution of ellagitannin content and profile during fruit ripening in Fragaria spp. J. Agric. Food Chem. 2013, 61, 8597–8607. [Google Scholar] [CrossRef]
- Liu, L.; Ji, M.L.; Chen, M.; Sun, M.Y.; Fu, X.L.; Li, L.; Gao, D.S.; Zhu, C.Y. The flavor and nutritional characteristic of four strawberry varieties cultured in soilless system. Food Sci. Nutr. 2016, 4, 858–868. [Google Scholar] [CrossRef]
- Li, X.; Li, M.H.; Deng, W.W.; Ahammed, G.J.; Wei, J.P.; Yan, P.; Zhang, L.P.; Fu, J.Y.; Han, W.Y. Exogenous melatonin improves tea quality under moderate high temperatures by increasing epigallocatechin-3-gallate and theanine biosynthesis in Camellia sinensis L. J. Plant Physiol. 2020, 253, 153273. [Google Scholar] [CrossRef]
- Tan, D.; Reiter, R.J.; Manchester, L.C.; Yan, M.; El-Sawi, M.; Sainz, R.M.; Mayo, J.C.; Kohen, R.; Allegra, M.; Hardeland, R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002, 2, 181–197. [Google Scholar] [CrossRef]
- Weeda, S.; Zhang, N.; Zhao, X.; Ndip, G.; Guo, Y.D.; Buck, G.; Fu, C.; Ren, S. Arabidopsis transcriptome analysis reveals key roles of melatonin in plant defense systems. PLoS ONE 2014, 9, e93462. [Google Scholar] [CrossRef] [PubMed]
- Voća, S.; Dobričević, N.; Dragović-Uzelac, V.; Duralija, B.; Družić, J.; Čmelik, Z.; Skendrović Babojelić, M. Fruit quality of new early ripening strawberry cultivars in Croatia. Food Technol. Biotechnol. 2008, 46, 292–298. [Google Scholar]
- Arnao, M.B.; Hernández-Ruiz, J.; Cano, A.; Reiter, R.J. Melatonin and carbohydrate metabolism in plant cells. Plants 2021, 10, 1917. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, R.; Pareek, S.; González-Aguilar, G.A.; Domínguez-Avila, J.A. Changes in the activity of proline-metabolising enzymes is associated with increased cultivar-dependent chilling tolerance in mangos, in response to pre-storage melatonin application. Postharvest Biol. Technol. 2021, 182, 111702. [Google Scholar] [CrossRef]



| Experimental Treatment | Fruit Yield (t·ha−1) | Fruit Number per Plant | Mean Fruit Weight (g) | Plant Dry Matter (g·m−2) |
|---|---|---|---|---|
| Crop system—CS | ||||
| Open field | 38.1 | 72.8 | 10.6 | 357.4 |
| Tunnel | 76.7 | 97.4 | 15.5 | 678.7 |
| * | * | * | * | |
| Cultivar (C) | ||||
| Kabarla | 83.1 a | 141.1 a | 11.8 b | 794.2 a |
| Fortuna | 54.7 b | 75.0 b | 12.7 b | 518.4 b |
| Sweet Ann | 56.4 b | 68.9 b | 14.6 a | 523.6 b |
| Festival | 35.4 c | 55.3 c | 13.0 b | 341.5 c |
| Melatonin dose (MD) | ||||
| Untreated control | 41.4 c | 62.7 c | 12.9 | 412.7 c |
| 1 p.p.m. | 56.5 b | 81.3 b | 13.2 | 514.8 b |
| 5 p.p.m. | 61.5 b | 98.7 a | 12.5 | 559.3 b |
| 10 p.p.m. | 70.1 a | 97.7 a | 13.5 | 646.1 a |
| n.s. | ||||
| Interaction | ||||
| CS × C | n.s. | n.s. | n.s. | n.s. |
| CS × MD | * | * | n.s. | n.s |
| C × MD | * | * | n.s. | n.s. |
| CS × C × MD | n.s. | n.s. | n.s. | n.s. |
| Experimental Treatment | Dry Matter (%) | Soluble Solids (°Brix) | Glucose (mg·g−1 d.w.) | Fructose (mg·g−1 d.w.) | Sucrose (mg·g−1 d.w.) | Titratable Acidity (g Citric Acid·100 g−1 f.w.) |
|---|---|---|---|---|---|---|
| Crop system—CS | ||||||
| Open field | 11.1 | 9.5 | 260.2 | 234.7 | 156.3 | 0.48 |
| Tunnel | 10.9 | 9.3 | 252.9 | 226.7 | 153.5 | 0.47 |
| n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | |
| Cultivar (C) | ||||||
| Kabarla | 11.0 ab | 9.4 ab | 255.7 ab | 230.2 ab | 154.3 | 0.49 |
| Fortuna | 11.3 a | 9.8 a | 266.7 a | 239.4 a | 161.5 | 0.47 |
| Sweet Ann | 10.7 b | 9.1 b | 247.8 b | 221.4 b | 150.2 | 0.49 |
| Festival | 10.9 ab | 9.4 ab | 256.1 ab | 231.8 ab | 153.5 | 0.46 |
| n.s. | n.s. | |||||
| Melatonin dose (MD) | ||||||
| Untreated control | 11.4 a | 9.7 | 262.0 | 236.4 | 158.7 | 0.50 a |
| 1 p.p.m. | 11.0 ab | 9.4 | 256.6 | 232.2 | 154.4 | 0.47 ab |
| 5 p.p.m. | 10.9 ab | 9.4 | 257.1 | 229.5 | 155.4 | 0.48 ab |
| 10 p.p.m. | 10.7 b | 9.2 | 250.6 | 224.8 | 151.2 | 0.46 b |
| n.s. | n.s. | n.s. | n.s. | |||
| Interaction | ||||||
| CS × C | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| CS × MD | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
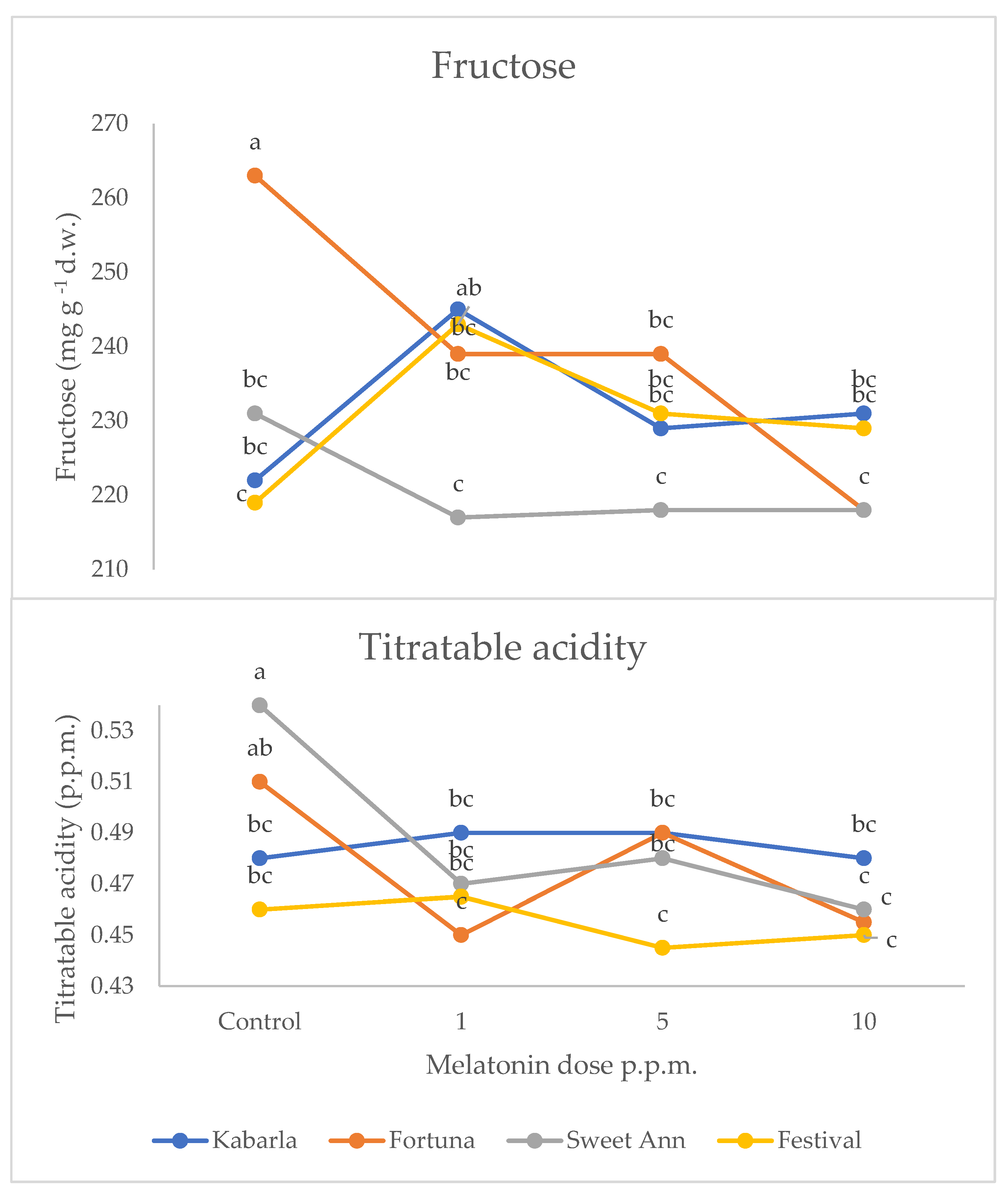
| C × MD | n.s. | n.s. | n.s. | * | n.s. | * |
| CS × C × MD | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. |
| Experimental Treatment | Total Phenolics (µ GAE·g−1) | Ascorbic Acid (mg·100 mL−1) | Antioxidant Activity (DPPH) |
|---|---|---|---|
| Crop system—CS | |||
| Open field | 307.5 | 69.8 | 31.8 |
| Tunnel | 311.6 | 59.0 | 30.6 |
| n.s. | * | n.s. | |
| Cultivar (C) | |||
| Kabarla | 315.4 ab | 69.0 a | 30.5 |
| Fortuna | 292.9 c | 62.3 bc | 31.0 |
| Sweet Ann | 325.6 a | 58.1 c | 31.3 |
| Festival | 304.4 bc | 68.1 ab | 32.1 |
| n.s. | |||
| Melatonin dose (MD) | |||
| Untreated control | 297.6 b | 62.2 b | 31.4 |
| 1 p.p.m. | 315.2 a | 56.6 b | 30.0 |
| 5 p.p.m. | 309.5 ab | 70.2 a | 32.6 |
| 10 p.p.m. | 316.0 a | 68.4 a | 30.8 |
| n.s. | |||
| Interaction | |||
| CS × C | n.s. | n.s. | n.s. |
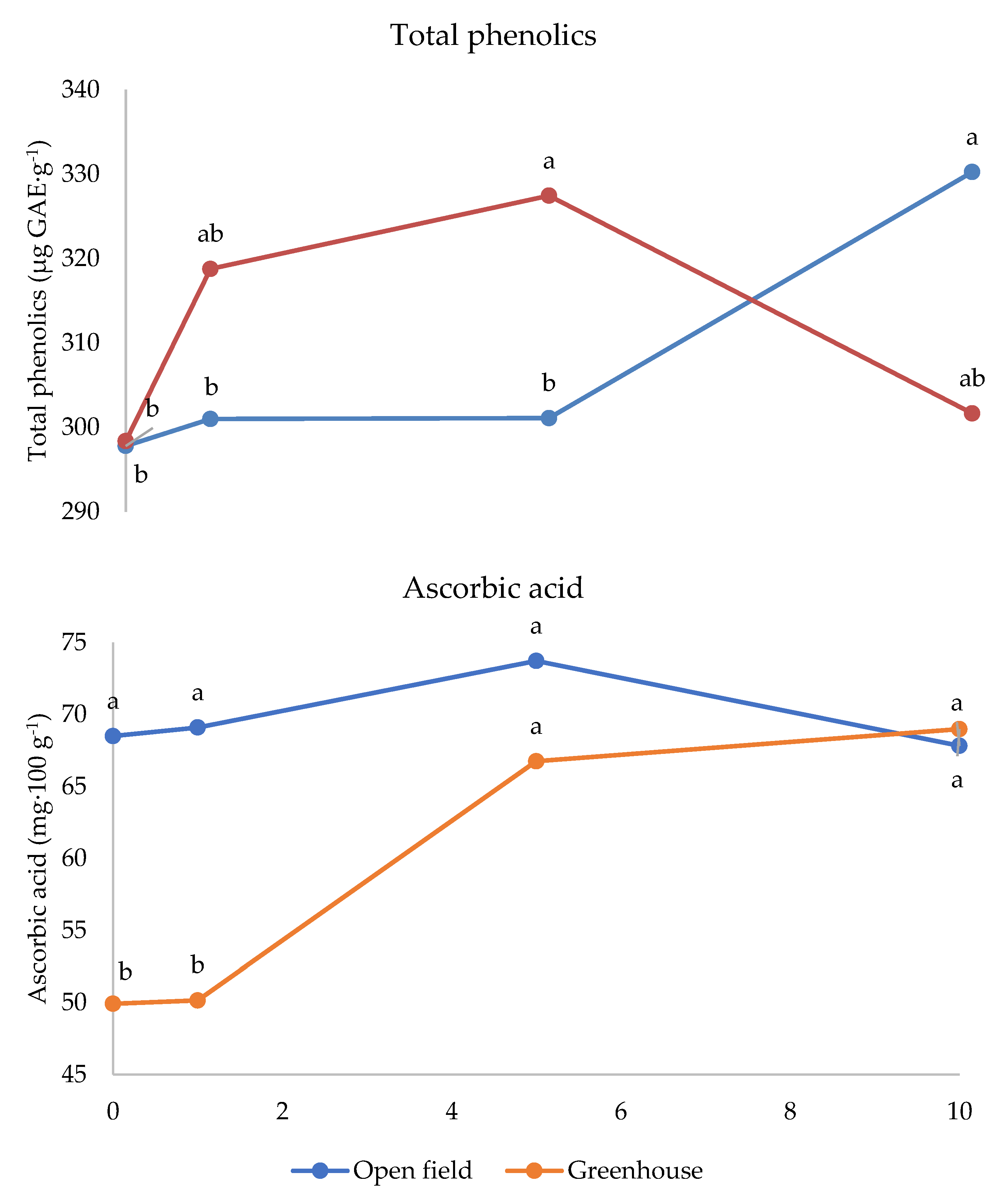
| CS × MD | * | * | n.s. |
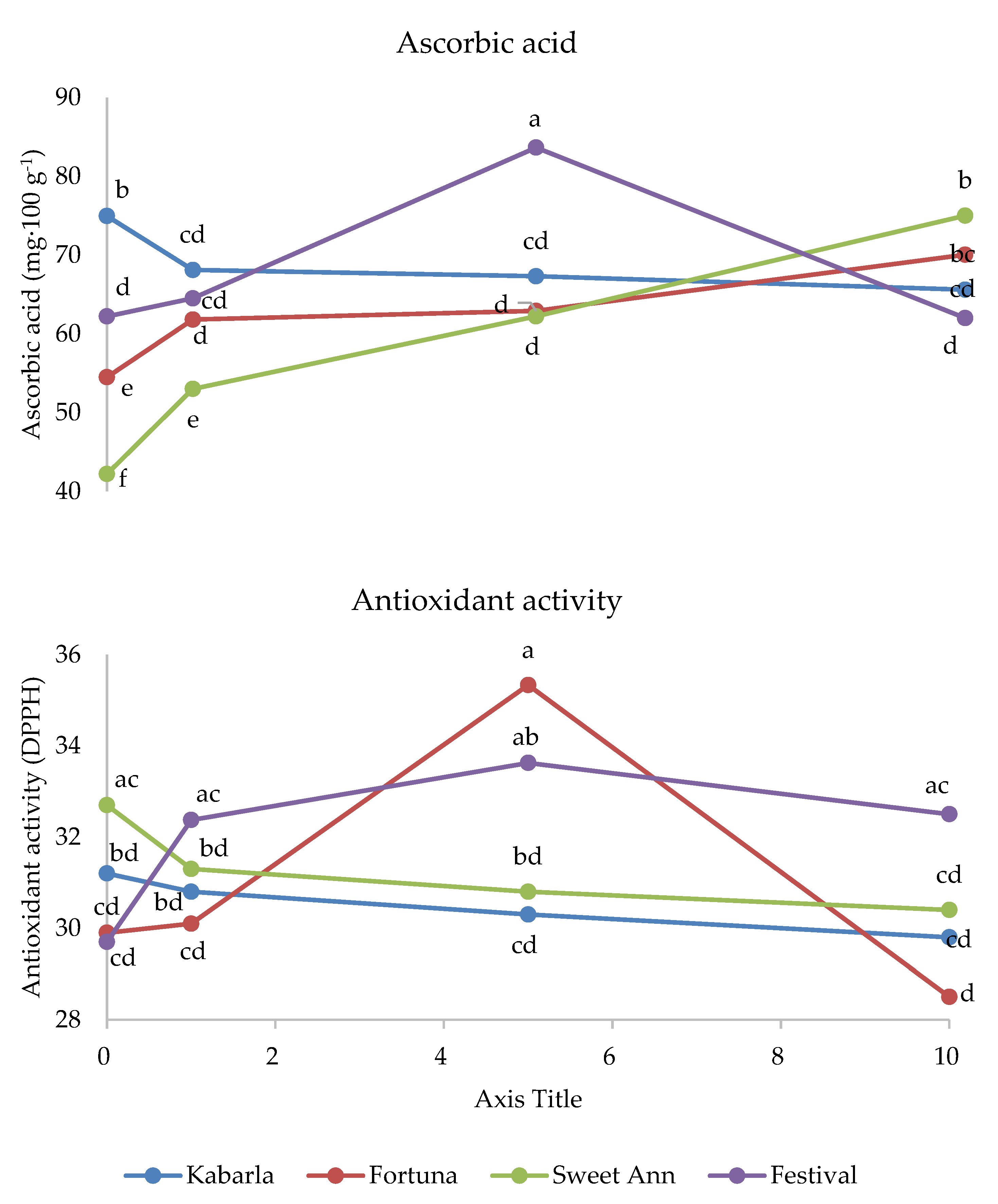
| C × MD | * | * | * |
| CS × C × MD | n.s. | n.s. | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okatan, V.; Aşkın, M.A.; Polat, M.; Bulduk, I.; Çolak, A.M.; Güçlü, S.F.; Kahramanoğlu, İ.; Tallarita, A.V.; Caruso, G. Effects of Melatonin Dose on Fruit Yield, Quality, and Antioxidants of Strawberry Cultivars Grown in Different Crop Systems. Agriculture 2023, 13, 71. https://doi.org/10.3390/agriculture13010071
Okatan V, Aşkın MA, Polat M, Bulduk I, Çolak AM, Güçlü SF, Kahramanoğlu İ, Tallarita AV, Caruso G. Effects of Melatonin Dose on Fruit Yield, Quality, and Antioxidants of Strawberry Cultivars Grown in Different Crop Systems. Agriculture. 2023; 13(1):71. https://doi.org/10.3390/agriculture13010071
Chicago/Turabian StyleOkatan, Volkan, Mehmet Atilla Aşkın, Mehmet Polat, Ibrahim Bulduk, Ayşen Melda Çolak, Sultan Filiz Güçlü, İbrahim Kahramanoğlu, Alessio Vincenzo Tallarita, and Gianluca Caruso. 2023. "Effects of Melatonin Dose on Fruit Yield, Quality, and Antioxidants of Strawberry Cultivars Grown in Different Crop Systems" Agriculture 13, no. 1: 71. https://doi.org/10.3390/agriculture13010071
APA StyleOkatan, V., Aşkın, M. A., Polat, M., Bulduk, I., Çolak, A. M., Güçlü, S. F., Kahramanoğlu, İ., Tallarita, A. V., & Caruso, G. (2023). Effects of Melatonin Dose on Fruit Yield, Quality, and Antioxidants of Strawberry Cultivars Grown in Different Crop Systems. Agriculture, 13(1), 71. https://doi.org/10.3390/agriculture13010071








