Selenium Biofortification: Strategies, Progress and Challenges
Abstract
1. Introduction
2. Methodology
3. Sources and Pathways of Se
4. Se Biofortification Strategies
4.1. Se Forms
4.2. Se Biofortification Strategies in Plants
4.2.1. Foliar Application
4.2.2. Soil Application
4.2.3. Microbial-Assisted Biofortification
4.2.4. Genetic Biofortification
4.2.5. Crop Breeding
4.3. Se Biofortification Strategies in Livestock
5. Se Biofortification in Plants
5.1. Se Biofortification Effects in Plants
5.1.1. Se and Salinity
5.1.2. Se and Heavy Metals
5.1.3. Se and Extreme Temperatures
5.1.4. Se and Photosynthesis
| Plant | Se Forms and Dosage | Se Effects | Reference |
|---|---|---|---|
| Maize | Na2SeO4 20 and 40 mg/L Foliar Spray | Increased plant development due to higher salt tolerance during the reproductive stage by reducing oxidative damage and enhancing the activity of antioxidant enzymes. | [122] |
| Na2SeO4 40 mg/L Foliar Spray | Increased fodder yield by 15% | [144] | |
| Na2SeO4 0.8–1.0 g/L Foliar Spray | At the jointing stage, fresh ear yield went up by 2.3%; at the large bell stage, it went up by 2%. | [145] | |
| Na2SeO3 1, 5 and 25 μM Addition to nutrient solution | Enhanced salt resistance via changes in photosynthetic capacity, antioxidant activity, and Na+ homeostasis | [146] | |
| Na2SeO3 5–15 μM Addition to nutrient solution. | Improved the activity of antioxidant system components | [147] | |
| Wheat | Na2SeO4 0.4 mg Na2SeO4/kg soil Direct soil application | Height and weight of the plant increased | [147] |
| Na2SeO4 5 μM Direct addition to soil | In normal and NaCl-stressed seedlings, Se increased proline and sugar build-up and supplied additional osmolarity to preserve relative water content and safeguard photosynthesis. | [148] | |
| Na2SeO4 10 mL/pot Foliar spray | Enhanced antioxidant enzyme activity; improved plant growth, photosynthetic capacity, relative water content, and chlorophyll content | [149] | |
| Rice | Na2SeO3 25μM Addition to nutrient solution. | Increased phenolic chemicals and decreased arsenic accumulation | [150] |
| Na2SeO4 10 μM Addition to nutrient solution. | Increased plant growth and biomass, and increased protein content. The activities of MDA, H2O2, APX, CAT, and SOD reduced in the shoots. | [151] | |
| Na2SeO3 0.8 and 1.0 mg/L | As-induced toxicity significantly decreased germination by 70%, and Se supplementation by seed priming increased germination by 9% and root, shoot, and seedling biomass accumulation by 1.3, 1.6, and 1.4 folds, respectively. | [152] | |
| Tomato | Na2SeO3 or Na2SeO4 1 μM Addition to nutrient solution | Enhanced photosynthesis and increased root and shoot dry weight | [141] |
| Na2SeO3·5H2O 10 μM Direct soil application | Increased the levels of stomatal conductance, chlorophyll and carotene, transpiration rate and net photosynthesis rate | [125] | |
| Se nanoparticles 10 mg/L Foliar Spray | Increased the yield by 21% | [153] | |
| Pepper | Na2SeO3 5 μM Addition to nutrient solution | Increased root development, membrane stability index, chlorophyll concentration, and starch content in leaves | [154] |
| Na2SeO3 3 and 7 μM Direct addition to soil | Plants cultivated in the medium containing 0.25 mM Cd had higher mean productivity, a greater capacity to withstand stress, and a higher yield stability index when the Se doses were added. | [155] | |
| Onion | Na2SeO3 0.5 and 1 kg/ha Foliar spraying | Improvements in both qualitative and physiological markers. Maximum production at 1 kg/ha of foliar Se supplementation | [123] |
| Garlic | Na2SeO4 4, 8 and 16 mg/L Addition to nutrient solution. | Se improved salt tolerance and decreased oxidative damage by boosting the activity of antioxidant enzymes. | [156] |
| Cucumber | Na2SeO3 2 g/L Addition to nutrient solution | Increased root and shoot biomass, as well as chlorophyll content | [157] |
| Mustard greens | Na2SeO4 4 μM/kg Addition to nutrient solution | Improved growth, increased chlorophyll and carotene content, net photosynthesis rate, stomatal conductance, and transpiration rate | [158] |
| Broad Beans | Na2SeO3 1.5 μM Addition to nutrient solution | Decreased MDA content; and H2O2 buildup, increased chlorophyll content shoot elongation and shoot fresh weight | [159] |
| Lemon balm | Na2SeO3.5H2O 0.2 μM Addition to nutrient solution | Enhanced growth | [160] |
| Strawberry | Na2SeO4 nanoparticles, 10 and 20 mg/L Foliar Spray | Increased number of fruit plants−1 by 21.22 and 12.54%, and yield by 21 and 14%, respectively, in two growing seasons | [161] |
| Pomegranate | Na2SeO4 and Se-nanoparticles, 1 and 2 μM Foliar Spray | In two growing seasons, the number of fruits per tree grew by 1.35 and 1.28 times, and the yield grew by 1.17 and 1.16 times. | [162] |
| Cowpea | Na2SeO4 5 and 10 μM Foliar application | Enhanced yield-related indicators, growth, and protein levels | [163] |
| Sunflower | Na2SeO4 5 mg/kg Direct soil application | Increased antioxidant enzyme activity | [124] |
| Tobacco | Na2SeO3 0.1 mg/L Addition to nutrient solution. | Se reduced the toxicity of the high As dosage (5 mg/L) and stimulated the development of the plant by increasing antioxidative stress resistance and decreasing MDA levels. | [164] |
6. Se Biofortification in Livestock
Se Biofortification Effects in Livestock
| Animal | Se Form and Dosage | Se Effects | Reference |
|---|---|---|---|
| Cow | Se yeast supplement | Enhanced antioxidant levels and immunological responses following calving | [15] |
| Se-enriched alfalfa hay | Supplemental selenium increased immunization responses against Escherichia coli during the weaning transition phase and subsequent growth and survival in the feedlot. | [189] | |
| Pig | DL-selenomethionine 2–16 μmol/L | Significant inhibitive effect on Porcine circovirus type 2 replication | [190] |
| SeMet 2–6 μM | Inhibited porcine circovirus type 2 replication and its related oxidative stress | [184] | |
| Se yeast diet | Piglets given selenium yeast showed greater digestibility of DM, crude protein, and crude fat; which impacted the production of inflammatory cytokines, and decreased the quantity of Escherichia coli in feces. | [191] | |
| Chicken | SeMet | Increased immune function and selenoprotein expression, and reduced the inflammation generated by lipopolysaccharides. | [192] |
| 0.3 mg/kg Se yeast 0.3 mg/kg of organic Se from Stenotrophomonas maltophilia (bacterial organic Se, ADS18). | Bacterial selenoprotein or Se-yeast improved the performance index, egg quality features, egg yolk and tissue of Se concentrations and intestinal villus. | [193] | |
| Se Enriched Yeast Na2SeO3 (High—0.30 mg/kg of feed; Low—0.15 mg/kg of feed) | Virus shedding from the cloaca was substantially reduced in all selenium-supplemented groups compared with non-supplemented control groups. | [182] | |
| sodium selenite 10 or 20 μg | Se injection enhanced immune and antioxidant responses | [194] | |
| Probiotics as (P, 0.11 mg Se/kg) Na2SeO3 (SS, 0.41 mg Se/kg) and (SP, 0.41 mg Se/kg) | In groups supplemented with selenium, oocyst shedding and cecal lesion scores were reduced. | [195] | |
| Sheep | Se yeast supplementation >4.9 mg Se/week | Supplementation with Se-yeast enhanced the Se status of sheep and the expression of genes involved in innate immunity in whole blood neutrophils. | [196] |
| Se yeast 0.5–1.0 mg/kg | Drip loss of muscle decreased significantly with an increase in dietary selenium yeast Supplementation. | [197] | |
| Rabbit | Se yeast 0.3 mg Se/kg diet | Positive effect on growth performance of rabbits. Se increased daily gain and the final body weight. Supplementation with Se increased muscle Se content to 559% of the control level. | [198] |
| Sodium selenate solution 10% of Se-fortified olive leaves (2.10 mg/kg) | Meat exhibited better oxidative status and a 5-fold higher Se content compared to that of the other treatments. | [199] |
7. Se Biofortification and Humans
| Country | Se Intake | References |
|---|---|---|
| Russia | 35.5 μg | [222] |
| Brazil | 84.3–105.9 μg | [223,224,225] |
| United States of America | 60–220 μg | [209,224,226,227,228] |
| Turkey | 20–138 μg | [215,229,230,231,232,233,234,235,236,237,238,239] |
| Slovakia | 27–43 μg | [240] |
| Saudi Arabia | 34–121.65 μg | [241,242] |
| Venezuela | 200–350 μg | [21,243] |
| Czech Republic | 10–25 µg | [243] |
| Canada | 98–224 μg | [225,228] |
| England | 12–43 μg | [228] |
| Belgium | 28–61 μg | [224] |
| Germany | 35–47 μg | [224,225] |
| Mexico | 61–73 μg | [224,228] |
| Venezuela | 200–350 μg | [224,228] |
| Australia | 57–87 μg | [209,228] |
| Japan | 104–127 μg | [228] |
| Greece | 110 μg | [228] |
| China | 3–6690 μg | [22,224,243] |
| Poland | 30–40 μg | [244] |
| Finland | 70–80 μg | [71] |
| Spain | 44–50 μg | [117,245] |
| Austria | 48 μg | [21,117] |
| Slovenia | 87 μg | [246] |
| Slovakia | 27–43 μg | [240] |
| Jordan | 59.26 µg | [247] |
| Greenland | 193–5885 μg | [248] |
7.1. Se in Humans
7.1.1. Se Intake
7.1.2. Health Benefits of Se
8. Se Biofortification Challenges
8.1. Influence of Soil Characeristics
8.2. Food Processing Methods
8.3. Toxic Nature of Se
8.4. Government Support
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oldfield, J. Se World Atlas; Se-Tellurium Development Association (STDA): Grimbergen, Belgium, 1999. [Google Scholar]
- Garside, M. Production Volume of Selenium Worldwide in 2020, by Country. 2022. Available online: https://www.statista.com/statistics/1312522/selenium-production-volume-worldwide-by-country/#:~:text=The%20country%20with%20the%20largest,3.33%20thousand%20metric%20tons%20worldwide. (accessed on 13 December 2022).
- Basu, A.; Schilling, K.; Brown, S.T.; Johnson, T.M.; Christensen, J.N.; Hartmann, M.; Reimus, P.W.; Heikoop, J.M.; Woldegabriel, G.; DePaolo, D.J. Se Isotopes as Groundwater Redox Indicators: Detecting Natural Attenuation of Se at an in Situ Recovery U Mine. Environ. Sci. Technol. 2016, 50, 10833–10842. [Google Scholar] [CrossRef]
- Stroud, J.L.; McGrath, S.P.; Zhao, F.J. Selenium speciation in soil extracts using LC-ICP-MS. Int. J. Environ. Anal. Chem. 2012, 92, 222–236. [Google Scholar] [CrossRef]
- Pettine, M.; Gennari, F.; Campanella, L. The reaction of selenium (IV) with ascorbic acid: Its relevance in aqueous and soil systems. Chemosphere 2013, 90, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Reich, H.J.; Hondal, R.J. Why Nature Chose Selenium. ACS Chem. Biol. 2016, 11, 821–841. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.K.; McDonald, T.J.; Sohn, M.; Anquandah, G.A.; Pettine, M.; Zboril, R. Biogeochemistry of selenium. A review. Environ. Chem. Lett. 2015, 13, 49–58. [Google Scholar] [CrossRef]
- Oldfield, J.E.; Muth, O.H.; Schubert, J.R. Selenium and vit. E as related to growth and white muscle disease in lambs. Proc. Soc. Exp. Biol. Med. 1960, 103, 799–800. [Google Scholar] [CrossRef]
- Pilarczyk, B.; Tomza-Marciniak, A.; Pilarczyk, R.; Kuba, J.; Hendzel, D.; Udała, J.; Tarasewicz, Z. Eggs as a source of selenium in the human diet. J. Food Compos. Anal. 2019, 78, 19–23. [Google Scholar] [CrossRef]
- Schwarz, K.; Foltz, C.M. Selenium as an Integral Part of Factor 3 against Dietary Necrotic Liver Degeneration. J. Am. Chem. Soc. 1957, 79, 3292–3293. [Google Scholar] [CrossRef]
- Collery, P. Strategies for the development of selenium-based anticancer drugs. J. Trace Elements Med. Biol. 2018, 50, 498–507. [Google Scholar] [CrossRef]
- Hesketh, J. Nutrigenomics and selenium: Gene expression patterns, physiological targets, and genetics. Annu. Rev. Nutr. 2008, 28, 157–177. [Google Scholar] [CrossRef]
- Saito, Y. Selenium Transport Mechanism via Selenoprotein P—Its Physiological Role and Related Diseases. Front. Nutr. 2021, 8, 685517. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guo, X.; Yan, S.; Zhang, B.; Shi, B. Mechanism Underlying the Protective Effect of Selenium on NO-Induced Oxidative Damage in Bovine Mammary Epithelial Cells. Biol. Trace Element Res. 2019, 191, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Bobe, G.; Vorachek, W.R.; Hugejiletu; Gorman, M.E.; Mosher, W.D.; Pirelli, G.J. Effects of Feeding Selenium-Enriched Alfalfa Hay on Immunity and Health of Weaned Beef Calves. Biol. Trace Element Res. 2013, 156, 96–110. [Google Scholar] [CrossRef]
- Hartikainen, H. Biogeochemistry of selenium and its impact on food chain quality and human health. J. Trace Elements Med. Biol. 2005, 18, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. Selenium intake, status, and health: A complex relationship. Hormones 2019, 19, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Minich, W.B. Selenium Metabolism and Biosynthesis of Selenoproteins in the Human Body. Biochemistry 2022, 87, S168–S177. [Google Scholar] [CrossRef]
- Martinez, S.S.; Huang, Y.; Acuna, L.; Laverde, E.; Trujillo, D.; Barbieri, M.A.; Baum, M.K. Role of selenium in viral infections with a major focus on SARS-CoV-2. Int. J. Mol. Sci. 2022, 23, 280. [Google Scholar] [CrossRef]
- Adadi, P.; Barakova, N.V.; Muravyov, K.Y.; Krivoshapkina, E.F. Designing selenium functional foods and beverages: A review. Food Res. Int. 2019, 120, 708–725. [Google Scholar] [CrossRef]
- Combs, G.F., Jr. Selenium in global food systems. Br. J. Nutr. 2001, 85, 517–547. [Google Scholar] [CrossRef]
- Zhang, B.; Wei, Y.; Yan, S.; Shi, H.; Nie, Y.; Zou, G.; Zhang, X.; Luo, L. Characterization of selenium accumulation of different rice genotypes in Chinese natural seleniferous soil. Plant Soil Environ. 2019, 65, 15–20. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.; LeDuc, D.L. Phytoremediation of selenium using transgenic plants. Curr. Opin. Biotechnol. 2009, 20, 207–212. [Google Scholar] [CrossRef]
- Pilon-Smits, E.A.; Quinn, C.F. Selenium metabolism in plants. In Cell Biology of Metals and Nutrients; Springer: Berlin/Heidelberg, Germany, 2010; pp. 225–241. [Google Scholar]
- Andrews, E.; Hartley, W.; Grant, A. Selenium-responsive diseases of animals in New Zealand. N. Z. Vet.- J. 1968, 16, 3–17. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.-M.E.; Shehata, A.M.; Mohamed, N.G.; Elbaz, A.M.; Ibrahim, N.S. Synergistic effect of Spirulina platensis and selenium nanoparticles on growth performance, serum metabolites, immune responses, and antioxidant capacity of heat-stressed broiler chickens. Biol. Trace Element Res. 2021, 200, 768–779. [Google Scholar] [CrossRef] [PubMed]
- Libera, K.; Konieczny, K.; Witkowska, K.; Żurek, K.; Szumacher-Strabel, M.; Cieslak, A.; Smulski, S. The Association between Selected Dietary Minerals and Mastitis in Dairy Cows—A Review. Animals 2021, 11, 2330. [Google Scholar] [CrossRef] [PubMed]
- Rashnoo, M.; Rahmati, Z.; Azarfar, A.; Fadayifar, A. The effects of maternal supplementation of selenium and iodine via slow-release blouses in late pregnancy on milk production of goats and performance of their kids. Ital. J. Anim. Sci. 2020, 19, 502–513. [Google Scholar] [CrossRef]
- Skrajnowska, D.; Jagielska, A.; Ruszczyńska, A.; Idkowiak, J.; Bobrowska-Korczak, B. Effect of Copper and Selenium Supplementation on the Level of Elements in Rats’ Femurs under Neoplastic Conditions. Nutrients 2022, 14, 1285. [Google Scholar] [CrossRef]
- Safonov, V. Comparison of LPO-AOS Indices and Biochemical Composition of Animal Blood in Biogeochemical Provinces with Different Levels of Selenium. Biol. Trace Element Res. 2021, 200, 2055–2061. [Google Scholar] [CrossRef]
- Hemmati, M.; Delkhosh, B.; Rad, A.H.S.; Mohammadi, G.N. Effect of the Application of Foliar Selenium on Canola Cultivars as Influenced by Different Irrigation Regimes. J. Agric. Sci. 2019, 25, 309–318. [Google Scholar] [CrossRef]
- Kleiber, T.; Krzesiński, W.; Przygocka-Cyna, K.; Spiżewski, T. Alleviation Effect of Selenium on Manganese Stress of Plants. Ecol. Chem. Eng. S 2018, 25, 143–152. [Google Scholar] [CrossRef]
- Asante-Badu, B.; Kgorutla, L.; Li, S.; Danso, P.; Xue, Z.; Qiang, G. Phytoremediation of organic and inorganic compounds in a natural and an agricultural environment: A review. Appl. Ecol. Environ. Res. 2020, 18, 6875–6904. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in Human Health and Disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.M.; Rivero, V.C.; Ballesta, R.J.; Rivero, M.V.C. Selenium Distribution in Topsoils and Plants of a Semi-arid Mediterranean Environment. Environ. Geochem. Health 2005, 27, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.; Zhu, R.; Yuan, L.; Zhang, W.; Zang, H.; Jiao, Y.; Yin, X. The influence of sea animals on selenium distribution in tundra soils and lake sediments in maritime Antarctica. Chemosphere 2021, 291, 132748. [Google Scholar] [CrossRef]
- Feinberg, A.; Stenke, A.; Peter, T.; Hinckley, E.L.S.; Driscoll, C.T.; Winkel, L.H. Reductions in the deposition of sulfur and selenium to agricultural soils pose risk of future nutrient deficiencies. Commun. Earth Environ. 2021, 2, 101. [Google Scholar] [CrossRef]
- Ye, W.; Yuan, L.; Zhu, R.; Yin, X.; Bañuelos, G. Selenium volatilization from tundra soils in maritime Antarctica. Environ. Int. 2021, 146, 106189. [Google Scholar] [CrossRef] [PubMed]
- Hoet, P. Sélénium et ses Composés; Elsevier Masson SAS: Paris, France, 2013. [Google Scholar]
- Dhillon, K.S.; Dhillon, S.K.; Singh, B. Genesis of seleniferous soils and associated animal and human health problems. Adv. Agron. 2018, 154, 1–80. [Google Scholar] [CrossRef]
- Emmanuelle, B.; Virginie, M.; Fabienne, S.; Isabelle, I.; Martine, P.-G.; Bernard, L.; Sylvie, R. Selenium exposure in subjects living in areas with high selenium concentrated drinking water: Results of a French integrated exposure assessment survey. Environ. Int. 2012, 40, 155–161. [Google Scholar] [CrossRef]
- Vinceti, M.; Crespi, C.M.; Bonvicini, F.; Malagoli, C.; Ferrante, M.; Marmiroli, S.; Stranges, S. The need for a reassessment of the safe upper limit of selenium in drinking water. Sci. Total. Environ. 2013, 443, 633–642. [Google Scholar] [CrossRef]
- Santos, S.; Ungureanu, G.; Boaventura, R.; Botelho, C. Selenium contaminated waters: An overview of analytical methods, treatment options and recent advances in sorption methods. Sci. Total. Environ. 2015, 521–522, 246–260. [Google Scholar] [CrossRef]
- Haug, A.; Graham, R.D.; Christophersen, O.A.; Lyons, G.H. How to use the world’s scarce selenium resources efficiently to increase the selenium concentration in food. Microb. Ecol. Health Dis. 2007, 19, 209–228. [Google Scholar]
- Natasha; Shahid, M.; Niazi, N.K.; Khalid, S.; Murtaza, B.; Bibi, I.; Rashid, M.I. A critical review of selenium biogeochemical behavior in soil-plant system with an inference to human health. Environ. Pollut. 2018, 234, 915–934. [Google Scholar] [CrossRef]
- Dungan, R.S.; Frankenberger, W.T. Microbial Transformations of Selenium and the Bioremediation of Seleniferous Environments. Bioremediation J. 1999, 3, 171–188. [Google Scholar] [CrossRef]
- Besser, J.M.; Canfield, T.J.; La Point, T.W. Bioaccumulation of organic and inorganic selenium in a laboratory food chain. Environ. Toxicol. Chem. Int. J. 1993, 12, 57–72. [Google Scholar] [CrossRef]
- Elrashidi, M.A.; Adriano, D.C.; Lindsay, W.L. Solubility, speciation, and transformations of selenium in soils. Selenium Agric. Environ. 1989, 23, 51–63. [Google Scholar]
- Freeman, J.L.; Bañuelos, G.S. Selection of Salt and Boron Tolerant Selenium Hyperaccumulator Stanleya pinnata Genotypes and Characterization of Se Phytoremediation from Agricultural Drainage Sediments. Environ. Sci. Technol. 2011, 45, 9703–9710. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Matraszek, R.; Pogorzelec, M. The dual effects of two inorganic selenium forms on the growth, selected physiological parameters and macronutrients accumulation in cucumber plants. Acta Physiol. Plant. 2015, 37, 41. [Google Scholar] [CrossRef]
- Mikkelsen, R.L.; Page, A.L.; Bingham, F.T.; Jacobs, L.W. Factors Affecting Selenium Accumulation by Agricultural Crops. Selenium Agric. Environ. 2015, 23, 65–94. [Google Scholar] [CrossRef]
- Fernandez-Martinez, A.; Charlet, L. Selenium environmental cycling and bioavailability: A structural chemist point of view. Rev. Environ. Sci. Bio/Technol. 2009, 8, 81–110. [Google Scholar] [CrossRef]
- Masscheleyn, P.H.; Delaune, R.D.; Patrick, W.H. Arsenic and Selenium Chemistry as Affected by Sediment Redox Potential and pH. J. Environ. Qual. 1991, 20, 522–527. [Google Scholar] [CrossRef]
- McNeal, J.M.; Balistrieri, L.S.; Jacobs, L.W. Geochemistry and Occurrence of Selenium: An Overview. Selenium Agric. Environ. 2015, 23, 1–13. [Google Scholar] [CrossRef]
- Kikkert, J.; Berkelaar, E. Plant Uptake and Translocation of Inorganic and Organic Forms of Selenium. Arch. Environ. Contam. Toxicol. 2013, 65, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Dinh, Q.T.; Cui, Z.; Huang, J.; Tran, T.A.T.; Wang, D.; Yang, W.; Zhou, F.; Wang, M.; Yu, D.; Liang, D. Selenium distribution in the Chinese environment and its relationship with human health: A review. Environ. Int. 2018, 112, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xing, G.; Tang, S.; Pang, Y.; Yi, Q.; Huang, Q.; Huang, X.; Huang, J.; Li, P.; Fu, H. Improving soil selenium availability as a strategy to promote selenium uptake by high-Se rice cultivar. Environ. Exp. Bot. 2019, 163, 45–54. [Google Scholar] [CrossRef]
- Kumar, A.; Prasad, K.S. Role of nano-selenium in health and environment. J. Biotechnol. 2020, 325, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Kápolna, E.; Hillestrøm, P.R.; Laursen, K.H.; Husted, S.; Larsen, E.H. Effect of foliar application of selenium on its uptake and speciation in carrot. Food Chem. 2009, 115, 1357–1363. [Google Scholar] [CrossRef]
- Qin, S.; Gao, J.; Huang, K. Effects of different selenium sources on tissue selenium concentrations, blood GSH-Px activities and plasma interleukin levels in finishing lambs. Biol. Trace Elem. Res. 2007, 116, 91–102. [Google Scholar] [CrossRef]
- Kruzhel, B.; Bakowska, M.; Vovk, S.; Nowakowska, E.; Sergei, P. Selenium in the diet of ruminants. Acta Sci. Polonorum. Zootech. 2014, 13, 5–16. [Google Scholar]
- Lyons, M.P.; Papazyan, T.T.; Surai, P.F. Selenium in Food Chain and Animal Nutrition: Lessons from Nature -Review-. Asian-Australas. J. Anim. Sci. 2007, 20, 1135–1155. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B.; Hasanuzzaman, M.; Matraszek-Gawron, R. Mechanisms of Selenium-Induced Enhancement of Abiotic Stress Tolerance in Plants. In Plant Nutrients and Abiotic Stress Tolerance; Springer: Singapore, 2018; pp. 269–295. [Google Scholar]
- Puccinelli, M.; Malorgio, F.; Pezzarossa, B. Selenium Enrichment of Horticultural Crops. Molecules 2017, 22, 933. [Google Scholar] [CrossRef]
- Wang, P.; Lombi, E.; Zhao, F.-J.; Kopittke, P.M. Nanotechnology: A New Opportunity in Plant Sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef]
- Wang, M.; Ali, F.; Wang, M.; Dinh, Q.T.; Zhou, F.; Bañuelos, G.S.; Liang, D. Understanding boosting selenium accumulation in Wheat (Triticum aestivum L.) following foliar selenium application at different stages, forms, and doses. Environ. Sci. Pollut. Res. 2020, 27, 717–728. [Google Scholar] [CrossRef] [PubMed]
- Lidon, F.C.; Oliveira, K.; Ribeiro, M.M.; Pelica, J.; Pataco, I.; Ramalho, J.C.; Leitão, A.E.; Almeida, A.S.; Campos, P.S.; Ribeiro-Barros, A.I.; et al. Selenium biofortification of rice grains and implications on macronutrients quality. J. Cereal Sci. 2018, 81, 22–29. [Google Scholar] [CrossRef]
- Lidon, F.C.; Oliveira, K.; Galhano, C.; Guerra, M.; Ribeiro, M.M.; Pelica, J.; Pataco, I.; Ramalho, J.C.; Leitão, A.E.; Almeida, A.S.; et al. Selenium biofortification of rice through foliar application with selenite and selenate. Exp. Agric. 2019, 55, 528–542. [Google Scholar] [CrossRef]
- Pannico, A.; El-Nakhel, C.; Kyriacou, M.C.; Giordano, M.; Stazi, S.R.; De Pascale, S.; Rouphael, Y. Combating Micronutrient Deficiency and Enhancing Food Functional Quality Through Selenium Fortification of Select Lettuce Genotypes Grown in a Closed Soilless System. Front. Plant Sci. 2019, 10, 1495. [Google Scholar] [CrossRef] [PubMed]
- Alfthan, G.; Eurola, M.; Ekholm, P.; Venäläinen, E.-R.; Root, T.; Korkalainen, K.; Hartikainen, H.; Salminen, P.; Hietaniemi, V.; Aspila, P.; et al. Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: From deficiency to optimal selenium status of the population. J. Trace Elem. Med. Biol. 2015, 31, 142–147. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Stoddard, F.L.; Hartikainen, H.; Seppänen, M.M. Plant species and growing season weather influence the efficiency of selenium biofortification. Nutr. Cycl. Agroecosystems 2019, 114, 111–124. [Google Scholar] [CrossRef]
- Gupta, M.; Gupta, S. An Overview of Selenium Uptake, Metabolism, and Toxicity in Plants. Front. Plant Sci. 2017, 7, 2074. [Google Scholar] [CrossRef]
- Dongli, L.; Liang, D.; Qin, S.; Feng, P.; Wu, X. Effects of selenite and selenate application on growth and shoot selenium accumulation of pak choi (Brassica chinensis L.) during successive planting conditions. Environ. Sci. Pollut. Res. 2015, 22, 11076–11086. [Google Scholar] [CrossRef]
- White, P.J. Selenium accumulation by plants. Ann. Bot. 2015, 117, 217–235. [Google Scholar] [CrossRef]
- Wu, Z.; Banuelos, G.S.; Lin, Z.Q.; Liu, Y.; Yuan, L.; Yin, X.; Li, M. Biofortification and phytoremediation of selenium in China. Front. Plant Sci. 2015, 6, 136. [Google Scholar] [CrossRef]
- Wan, Y.; Yu, Y.; Wang, Q.; Qiao, Y.; Li, H. Cadmium uptake dynamics and translocation in rice seedling: Influence of different forms of selenium. Ecotoxicol. Environ. Saf. 2016, 133, 127–134. [Google Scholar] [CrossRef]
- Winkel, L.H.E.; Vriens, B.; Jones, G.D.; Schneider, L.S.; Pilon-Smits, E.; Bañuelos, G.S. Selenium Cycling Across Soil-Plant-Atmosphere Interfaces: A Critical Review. Nutrients 2015, 7, 4199–4239. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hu, B.; Li, W.; Che, R.; Deng, K.; Li, H.; Yu, F.; Ling, H.; Li, Y.; Chu, C. OsPT2, a phosphate transporter, is involved in the active uptake of selenite in rice. New Phytol. 2014, 201, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- da Silva, D.F.; Cipriano, P.E.; de Souza, R.R.; Júnior, M.S.; Faquin, V.; de Souza Silva, M.L.; Guilherme, L.R.G. Biofortification with selenium and implications in the absorption of macronutrients in Raphanus sativus L. J. Food Compos. Anal. 2020, 86, 103382. [Google Scholar] [CrossRef]
- Zhou, F.; Peng, Q.; Wang, M.; Liu, N.; Dinh, Q.T.; Zhai, H.; Xue, M.; Liang, D. Influence of processing methods and exogenous selenium species on the content and in vitro bioaccessibility of selenium in Pleurotus eryngii. Food Chem. 2020, 338, 127661. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, A.P.; Naozuka, J. Preliminary results on the feasibility of producing selenium-enriched pink (Pleurotus djamor) and white (Pleurotus ostreatus) oyster mushrooms: Bioaccumulation, bioaccessibility, and Se-proteins distribution. Microchem. J. 2019, 145, 1143–1150. [Google Scholar] [CrossRef]
- Barret, M.; Morrissey, J.P.; O’Gara, F. Functional genomics analysis of plant growth-promoting rhizobacterial traits involved in rhizosphere competence. Biol. Fertil. Soils 2011, 47, 729–743. [Google Scholar] [CrossRef]
- Sarwar, N.; Imran, M.; Shaheen, M.R.; Ishaque, W.; Kamran, M.A.; Matloob, A.; Rehim, A.; Hussain, S. Phytoremediation strategies for soils contaminated with heavy metals: Modifications and future perspectives. Chemosphere 2017, 171, 710–721. [Google Scholar] [CrossRef]
- Fordyce, F. Selenium Geochemistry and Health. AMBIO 2007, 36, 94–97. [Google Scholar] [CrossRef]
- Acuña, J.J.; Jorquera, M.A.; Barra, P.J.; Crowley, D.E.; Mora, M.D.L.L. Selenobacteria selected from the rhizosphere as a potential tool for Se biofortification of wheat crops. Biol. Fertil. Soils 2012, 49, 175–185. [Google Scholar] [CrossRef]
- Yasin, M.; El Mehdawi, A.F.; Jahn, C.E.; Anwar, A.; Turner, M.F.S.; Faisal, M.; Pilon-Smits, E.A.H. Seleniferous soils as a source for production of selenium-enriched foods and potential of bacteria to enhance plant selenium uptake. Plant Soil 2014, 386, 385–394. [Google Scholar] [CrossRef]
- Golubkina, N.; Zamana, S.; Seredin, T.; Poluboyarinov, P.; Sokolov, S.; Baranova, H.; Krivenkov, L.; Pietrantonio, L.; Caruso, G. Effect of Selenium Biofortification and Beneficial Microorganism Inoculation on Yield, Quality and Antioxidant Properties of Shallot Bulbs. Plants 2019, 8, 102. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, D.; Huang, X.; Wang, S.; Qiu, R.; Zhang, Z.; Ming, J. Effect of Enterobacter sp. EG16 on Selenium biofortification and speciation in pak choi (Brassica rapa ssp. chinensis). Sci. Hortic. 2023, 310, 111723. [Google Scholar] [CrossRef]
- Husen, A.; Siddiqi, K.S. Phytosynthesis of nanoparticles: Concept, controversy and application. Nanoscale Res. Lett. 2014, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Oremland, R.S.; Herbel, M.J.; Blum, J.S.; Langley, S.; Beveridge, T.J.; Ajayan, P.M.; Sutto, T.; Ellis, A.V.; Curran, S. Structural and Spectral Features of Selenium Nanospheres Produced by Se-Respiring Bacteria. Appl. Environ. Microbiol. 2004, 70, 52–60. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S.; Gientka, I.; Bzducha-Wróbel, A. Accumulation and metabolism of selenium by yeast cells. Appl. Microbiol. Biotechnol. 2015, 99, 5373–5382. [Google Scholar] [CrossRef]
- Gharieb, M.; Gadd, G. Role of glutathione in detoxification of metal(loid)s by Saccharomyces cerevisiae. Biometals 2004, 17, 183–188. [Google Scholar] [CrossRef]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- White, P.J.; Broadley, M.R. Biofortification of crops with seven mineral elements often lacking in human diets–iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009, 182, 49–84. [Google Scholar] [CrossRef]
- Zhu, Y.-G.; Pilon-Smits, E.A.H.; Zhao, F.-J.; Williams, P.N.; Meharg, A.A. Selenium in higher plants: Understanding mechanisms for biofortification and phytoremediation. Trends Plant Sci. 2009, 14, 436–442. [Google Scholar] [CrossRef]
- Ates, D.; Sever, T.; Aldemir, S.; Yagmur, B.; Temel, H.Y.; Kaya, H.B.; Tanyolac, B. Identification QTLs controlling genes for Se uptake in lentil seeds. PLoS ONE 2016, 11, e0149210. [Google Scholar]
- Wang, P.; Wang, H.; Liu, Q.; Tian, X.; Shi, Y.; Zhang, X. QTL mapping of selenium content using a RIL population in wheat. PLoS ONE 2017, 12, e0184351. [Google Scholar] [CrossRef] [PubMed]
- Schiavon, M.; Nardi, S.; dalla Vecchia, F.; Ertani, A. Selenium biofortification in the 21st century: Status and challenges for healthy human nutrition. Plant Soil 2020, 453, 245–270. [Google Scholar] [CrossRef] [PubMed]
- Garg, M.; Sharma, N.; Sharma, S.; Kapoor, P.; Kumar, A.; Chunduri, V.; Arora, P. Biofortified Crops Generated by Breeding, Agronomy, and Transgenic Approaches Are Improving Lives of Millions of People around the World. Front. Nutr. 2018, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- LeDuc, D.L.; AbdelSamie, M.; Móntes-Bayon, M.; Wu, C.P.; Reisinger, S.J.; Terry, N. Overexpressing both ATP sulfurylase and selenocysteine methyltransferase enhances selenium phytoremediation traits in Indian mustard. Environ. Pollut. 2006, 144, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ding, S.; Zhang, A.; Hong, K.; Jiang, H.; Yang, S.; Ruan, B.; Zhang, B.; Dong, G.; Guo, L.; et al. Development of nutritious rice with high zinc/selenium and low cadmium in grains through QTL pyramiding. J. Integr. Plant Biol. 2020, 62, 349–359. [Google Scholar] [CrossRef]
- Liang, Y.; Farooq, M.U.; Zeng, R.; Tang, Z.; Zhang, Y.; Zheng, T.; Zhu, J. Breeding of selenium rich red glutinous rice, protein extraction and analysis of the distribution of selenium in grain. Int. J. Agric. Biol. 2018, 20, 1005–1011. [Google Scholar]
- Mao, H.; Wang, J.; Wang, Z.; Zan, Y.; Lyons, G.; Zou, C. Using agronomic biofortification to boost zinc, selenium, and iodine concentrations of food crops grown on the loess plateau in China. J. Soil Sci. Plant Nutr. 2014, 14, 459–470. [Google Scholar] [CrossRef]
- Yuan, L.; Yin, X.; Zhu, Y.; Li, F.; Huang, Y.; Liu, Y.; Lin, Z. Selenium in plants and soils, and selenosis in Enshi, China: Implications for selenium biofortification. Phytoremediation Biofortification Two Sides One Coin 2012, 7–31. [Google Scholar] [CrossRef]
- Rayman, M.P. The use of high-selenium yeast to raise selenium status: How does it measure up? Br. J. Nutr. 2004, 92, 557–573. [Google Scholar] [CrossRef]
- Jeong, D.; Lee, K.H. Bimodal actions of selenium essential for antioxidant and toxic pro-oxidant activities: The selenium paradox (Review). Mol. Med. Rep. 2011, 5, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Alarcon, M.; Cabrera-Vique, C. Selenium in food and the human body: A review. Sci. Total. Environ. 2008, 400, 115–141. [Google Scholar] [CrossRef]
- Alfthan, G.; Aspila, P.; Ekholm, P.; Eurola, M.; Hartikainen, H.; Hero, H.; Hietaniemi, V.; Root, T.; Salminen, P.; Venäläinen, E.R.; et al. Nationwide supplementation of sodium selenate to commercial fertilizers: History and 25-year results from the Finnish selenium monitoring programme. Combat. Micronutr. Defic. Food-Based Approaches 2011, 312–337. [Google Scholar] [CrossRef]
- Hu, T.; Hui, G.; Li, H.; Guo, Y. Selenium biofortification in Hericium erinaceus (Lion’s Mane mushroom) and its in vitro bioaccessibility. Food Chem. 2020, 331, 127287. [Google Scholar] [CrossRef] [PubMed]
- Yläranta, T. Increasing the Selenium Content of Cereal and Grass Crops in Finland; Yliopistopaino: Cygnaeuksenkatu, Finland, 1985. [Google Scholar]
- Ligowe, I.; Young, S.; Ander, E.; Kabambe, V.; Chilimba, A.; Bailey, E.; Lark, R.; Nalivata, P. Selenium biofortification of crops on a Malawi Alfisol under conservation agriculture. Geoderma 2020, 369, 114315. [Google Scholar] [CrossRef]
- Alfthan, G. Longitudinal study on the selenium status of healthy adults in Finland during 1975–1984. Nutr. Res. 1988, 8, 467–476. [Google Scholar] [CrossRef]
- Bañuelos, G.S.; Lin, Z.Q.; Broadley, M. Selenium biofortification. Selenium Plants Mol. Physiol. Ecol. Evol. Asp. 2017, 11, 231–255. [Google Scholar]
- He, Y.; Xiang, Y.; Zhou, Y.; Yang, Y.; Zhang, J.; Huang, H.; Shang, C.; Luo, L.; Gao, J.; Tang, L. Selenium contamination, consequences and remediation techniques in water and soils: A review. Environ. Res. 2018, 164, 288–301. [Google Scholar] [CrossRef]
- Hurst, R.; Siyame, E.W.P.; Young, S.D.; Chilimba, A.D.C.; Joy, E.J.M.; Black, C.R.; Ander, E.L.; Watts, M.J.; Chilima, B.; Gondwe, J.; et al. Soil-type influences human selenium status and underlies widespread selenium deficiency risks in Malawi. Sci. Rep. 2013, 3, srep01425. [Google Scholar] [CrossRef]
- Mehdi, Y.; Hornick, J.-L.; Istasse, L.; Dufrasne, I. Selenium in the Environment, Metabolism and Involvement in Body Functions. Molecules 2013, 18, 3292–3311. [Google Scholar] [CrossRef]
- Bitterli, C.; Bañuelos, G.S.; Schulin, R. Use of transfer factors to characterize uptake of selenium by plants. J. Geochem. Explor. 2010, 107, 206–216. [Google Scholar] [CrossRef]
- Ei, H.H.; Zheng, T.; Farooq, M.U.; Zeng, R.; Su, Y.; Zhang, Y.; Zhu, J. Impact of selenium, zinc and their interaction on key enzymes, grain yield, selenium, zinc concentrations, and seedling vigor of biofortified rice. Environ. Sci. Pollut. Res. 2020, 27, 16940–16949. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.B.; Raza, A.; Hawrylak-Nowak, B.; Matraszek-Gawron, R.; Al Mahmud, J.; Fujita, M. Selenium in plants: Boon or bane? Environ. Exp. Bot. 2020, 178, 104170. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exp. Bot. 2013, 87, 58–68. [Google Scholar] [CrossRef]
- Mroczek-Zdyrska, M.; Wójcik, M. The Influence of Selenium on Root Growth and Oxidative Stress Induced by Lead in Vicia faba L. minor Plants. Biol. Trace Element Res. 2011, 147, 320–328. [Google Scholar] [CrossRef]
- Ashraf, M.A.; Akbar, A.; Parveen, A.; Rasheed, R.; Hussain, I.; Iqbal, M. Phenological application of selenium differentially improves growth, oxidative defense and ion homeostasis in maize under salinity stress. Plant Physiol. Biochem. 2018, 123, 268–280. [Google Scholar] [CrossRef]
- Bybordi, A.; Saadat, S.; Zargaripour, P. The effect of zeolite, selenium and silicon on qualitative and quantitative traits of onion grown under salinity conditions. Arch. Agron. Soil Sci. 2017, 64, 520–530. [Google Scholar] [CrossRef]
- Habibi, G. Physiological, photochemical and ionic responses of sunflower seedlings to exogenous selenium supply under salt stress. Acta Physiol. Plant. 2017, 39, 213. [Google Scholar] [CrossRef]
- Alyemeni, M.N.; Ahanger, M.A.; Wijaya, L.; Alam, P.; Bhardwaj, R.; Ahmad, P. Selenium mitigates cadmium-induced oxidative stress in tomato (Solanum lycopersicum L.) plants by modulating chlorophyll fluorescence, osmolyte accumulation, and antioxidant system. Protoplasma 2017, 255, 459–469. [Google Scholar] [CrossRef]
- Iqbal, M.; Hussain, I.; Liaqat, H.; Ashraf, M.A.; Rasheed, R.; Rehman, A.U. Exogenously applied selenium reduces oxidative stress and induces heat tolerance in spring wheat. Plant Physiol. Biochem. 2015, 94, 95–103. [Google Scholar] [CrossRef]
- Pukacka, S.; Ratajczak, E.; Kalemba, E. The protective role of selenium in recalcitrant Acer saccharium L. seeds subjected to desiccation. J. Plant Physiol. 2011, 168, 220–225. [Google Scholar] [CrossRef]
- Karimi, R.; Ghabooli, M.; Rahimi, J.; Amerian, M. Effects of foliar selenium application on some physiological and phytochemical parameters of Vitis vinifera L. cv. Sultana under salt stress. J. Plant Nutr. 2020, 43, 2226–2242. [Google Scholar] [CrossRef]
- Khan, S.; Waqas, M.; Ding, F.; Shamshad, I.; Arp, H.P.H.; Li, G. The influence of various biochars on the bioaccessibility and bioaccumulation of PAHs and potentially toxic elements to turnips (Brassica rapa L.). J. Hazard. Mater. 2015, 300, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Moynihan, M.; Peterson, K.E.; Cantoral, A.; Song, P.X.K.; Jones, A.; Solano-González, M.; Meeker, J.D.; Basu, N.; Tellez-Rojo, M.M. Dietary predictors of urinary cadmium among pregnant women and children. Sci. Total Environ. 2017, 575, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, N.; Ibitoye, E.; Jimoh, A.; Sani, Z. Assessment of heavy metals in chicken feeds available in Sokoto, Nigeria. Sokoto J. Vet.- Sci. 1970, 13, 17–21. [Google Scholar] [CrossRef]
- Deng, S.; Li, P.; Li, Y.; Ran, Z.; Peng, Y.; Yang, S.; Yu, J. Alleviating Cd translocation and accumulation in soil–rice systems: Combination of foliar spraying of nano-Si or nano-Se and soil application of nano-humus. Soil Use Manag. 2021, 37, 319–329. [Google Scholar] [CrossRef]
- Li, H.; Liu, X.; Wassie, M.; Chen, L. Selenium supplementation alleviates cadmium-induced damages in tall fescue through modulating antioxidant system, photosynthesis efficiency, and gene expression. Environ. Sci. Pollut. Res. 2020, 27, 9490–9502. [Google Scholar] [CrossRef]
- Huang, B.; Xin, J.; Dai, H.; Zhou, W. Effects of Interaction between Cadmium (Cd) and Selenium (Se) on Grain Yield and Cd and Se Accumulation in a Hybrid Rice (Oryza sativa) System. J. Agric. Food Chem. 2017, 65, 9537–9546. [Google Scholar] [CrossRef]
- Hu, L.; Wang, X.; Wu, D.; Zhang, B.; Fan, H.; Shen, F.; Liao, Y.; Huang, X.; Gao, G. Effects of organic selenium on absorption and bioaccessibility of arsenic in radish under arsenic stress. Food Chem. 2020, 344, 128614. [Google Scholar] [CrossRef]
- Feng, R.; Zhao, P.; Zhu, Y.; Yang, J.; Wei, X.; Yang, L.; Liu, H.; Rensing, C.; Ding, Y. Application of inorganic selenium to reduce accumulation and toxicity of heavy metals (metalloids) in plants: The main mechanisms, concerns, and risks. Sci. Total. Environ. 2021, 771, 144776. [Google Scholar] [CrossRef]
- Guo, Y.; Mao, K.; Cao, H.; Ali, W.; Lei, D.; Teng, D.; Chang, C.; Yang, X.; Yang, Q.; Niazi, N.K.; et al. Exogenous selenium (cadmium) inhibits the absorption and transportation of cadmium (selenium) in rice. Environ. Pollut. 2021, 268, 115829. [Google Scholar] [CrossRef]
- Huang, C.; Qin, N.; Sun, L.; Yu, M.; Hu, W.; Qi, Z. Selenium Improves Physiological Parameters and Alleviates Oxidative Stress in Strawberry Seedlings under Low-Temperature Stress. Int. J. Mol. Sci. 2018, 19, 1913. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Alam, M.; Fujita, M. Modulation of Antioxidant Machinery and the Methylglyoxal Detoxification System in Selenium-Supplemented Brassica napus Seedlings Confers Tolerance to High Temperature Stress. Biol. Trace Element Res. 2014, 161, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Hawrylak-Nowak, B.; Dresler, S.; Rubinowska, K.; Matraszek-Gawron, R.; Woch, W.; Hasanuzzaman, M. Selenium biofortification enhances the growth and alters the physiological response of lamb’s lettuce grown under high temperature stress. Plant Physiol. Biochem. 2018, 127, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Alves, L.R.; Rossatto, D.R.; Rossi, M.L.; Martinelli, A.P.; Gratão, P.L. Selenium improves photosynthesis and induces ultrastructural changes but does not alleviate cadmium-stress damages in tomato plants. Protoplasma 2019, 257, 597–605. [Google Scholar] [CrossRef]
- Taha, R.; Seleiman, M.; Shami, A.; Alhammad, B.; Mahdi, A. Integrated Application of Selenium and Silicon Enhances Growth and Anatomical Structure, Antioxidant Defense System and Yield of Wheat Grown in Salt-Stressed Soil. Plants 2021, 10, 1040. [Google Scholar] [CrossRef] [PubMed]
- Djanaguiraman, M.; Prasad, P.; Seppanen, M. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol. Biochem. 2010, 48, 999–1007. [Google Scholar] [CrossRef]
- Nawaz, F.; Naeem, M.; Ashraf, M.Y.; Tahir, M.N.; Zulfiqar, B.; Salahuddin, M.; Shabbir, R.N.; Aslam, M. Selenium Supplementation Affects Physiological and Biochemical Processes to Improve Fodder Yield and Quality of Maize (Zea mays L.) under Water Deficit Conditions. Front. Plant Sci. 2016, 7, 1438. [Google Scholar] [CrossRef]
- Huang, A.; Huang, K.; Peng, J.; Huang, S.; Bi, X.; Zhai, R.; Tan, H. Effects of foliar spraying of selenium fertilizer on selenium-enriched content, heavy metal content and yield of sweet corn grain. J. South. Agric. 2019, 50, 40–44. [Google Scholar]
- Jiang, C.; Zu, C.; Lu, D.; Zheng, Q.; Shen, J.; Wang, H.; Li, D. Effect of exogenous selenium supply on photosynthesis, Na+ accumulation and antioxidative capacity of maize (Zea mays L.) under salinity stress. Sci. Rep. 2017, 7, srep42039. [Google Scholar] [CrossRef]
- Yildiztugay, E.; Ozfidan-Konakci, C.; Kucukoduk, M.; Tekis, S.A. The impact of selenium application on enzymatic and non-enzymatic antioxidant systems in Zea mays roots treated with combined osmotic and heat stress. Arch. Agron. Soil Sci. 2017, 63, 261–275. [Google Scholar] [CrossRef]
- Elkelish, A.A.; Soliman, M.H.; Alhaithloul, H.A.; El-Esawi, M.A. Selenium protects wheat seedlings against salt stress-mediated oxidative damage by up-regulating antioxidants and osmolytes metabolism. Plant Physiol. Biochem. 2019, 137, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Sattar, A.; Cheema, M.A.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Nawaz, A.; Abbas, T.; Ali, Q. Physiological and biochemical attributes of bread wheat (Triticum aestivum L.) seedlings are influenced by foliar application of silicon and selenium under water deficit. Acta Physiol. Plant. 2019, 41, 146. [Google Scholar] [CrossRef]
- Chauhan, R.; Awasthi, S.; Tripathi, P.; Mishra, S.; Dwivedi, S.; Niranjan, A.; Mallick, S.; Tripathi, P.; Pande, V.; Tripathi, R.D. Selenite modulates the level of phenolics and nutrient element to alleviate the toxicity of arsenite in rice (Oryza sativa L.). Ecotoxicol. Environ. Saf. 2017, 138, 47–55. [Google Scholar] [CrossRef]
- Singh, R.; Upadhyay, A.; Singh, D. Regulation of oxidative stress and mineral nutrient status by selenium in arsenic treated crop plant Oryza sativa. Ecotoxicol. Environ. Saf. 2017, 148, 105–113. [Google Scholar] [CrossRef]
- Moulick, D.; Ghosh, D.; Santra, S.C. Evaluation of effectiveness of seed priming with selenium in rice during germination under arsenic stress. Plant Physiol. Biochem. 2016, 109, 571–578. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.; Quiterio-Gutiérrez, T.; Cadenas-Pliego, G.; Ortega-Ortiz, H.; Hernández-Fuentes, A.D.; Cabrera de la Fuente, M.; Juárez-Maldonado, A. Impact of selenium and copper nanoparticles on yield, antioxidant system, and fruit quality of tomato plants. Plants 2019, 8, 355. [Google Scholar] [CrossRef] [PubMed]
- Shekari, L.; Kamelmanesh, M.M.; Mozafariyan, M.; Hasanuzzaman, M.; Sadeghi, F. Role of selenium in mitigation of cadmium toxicity in pepper grown in hydroponic condition. J. Plant Nutr. 2016, 40, 761–772. [Google Scholar] [CrossRef]
- Mozafariyan, M.; Shekari, L.; Hawrylak-Nowak, B.; Kamelmanesh, M.M. Protective Role of Selenium on Pepper Exposed to Cadmium Stress During Reproductive Stage. Biol. Trace Element Res. 2014, 160, 97–107. [Google Scholar] [CrossRef]
- Astaneh, R.K.; Bolandnazar, S.; Nahandi, F.Z.; Oustan, S. Effects of selenium on enzymatic changes and productivity of garlic under salinity stress. South Afr. J. Bot. 2019, 121, 447–455. [Google Scholar] [CrossRef]
- Haghighi, M.; Sheibanirad, A.; Pessarakli, M. Effects of selenium as a beneficial element on growth and photosynthetic attributes of greenhouse cucumber. J. Plant Nutr. 2015, 39, 1493–1498. [Google Scholar] [CrossRef]
- Handa, N.; Kohli, S.K.; Thukral, A.K.; Bhardwaj, R.; Alyemeni, M.N.; Wijaya, L.; Ahmad, P. Protective role of selenium against chromium stress involving metabolites and essential elements in Brassica juncea L. seedlings. 3 Biotech 2018, 8, 66. [Google Scholar] [CrossRef] [PubMed]
- Mroczek-Zdyrska, M.; Strubińska, J.; Hanaka, A. Selenium Improves Physiological Parameters and Alleviates Oxidative Stress in Shoots of Lead-Exposed Vicia faba L. minor Plants Grown Under Phosphorus-Deficient Conditions. J. Plant Growth Regul. 2016, 36, 186–199. [Google Scholar] [CrossRef]
- Tavakoli, S.; Enteshari, S.; Yousefifard, M. Investigation of the effect of selenium on growth, antioxidant capacity and secondary metabolites in Melissa officinalis. Iran. J. Plant Physiol. 2020, 10, 3125–3134. [Google Scholar]
- Zahedi, S.M.; Abdelrahman, M.; Hosseini, M.S.; Hoveizeh, N.F.; Tran, L.-S.P. Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ. Pollut. 2019, 253, 246–258. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, S.M.; Hosseini, M.S.; Meybodi, N.D.H.; da Silva, J.A.T. Foliar application of selenium and nano-selenium affects pomegranate (Punica granatum cv. Malase Saveh) fruit yield and quality. South Afr. J. Bot. 2019, 124, 350–358. [Google Scholar] [CrossRef]
- Manaf, H.H. Beneficial effects of exogenous selenium, glycine betaine and seaweed extract on salt stressed cowpea plant. Ann. Agric. Sci. 2016, 61, 41–48. [Google Scholar] [CrossRef]
- Han, D.; Xiong, S.; Tu, S.; Liu, J.; Chen, C. Interactive effects of selenium and arsenic on growth, antioxidant system, arsenic and selenium species of Nicotiana tabacum L. Environ. Exp. Bot. 2015, 117, 12–19. [Google Scholar] [CrossRef]
- Underwood, E.; Suttle, N. The Mineral Nutrition of Livestock, 3rd ed.; CABI: Wallingford, UK, 1999. [Google Scholar]
- Mehdi, Y.; Dufrasne, I. Selenium in Cattle: A Review. Molecules 2016, 21, 545. [Google Scholar] [CrossRef]
- Moir, D.C.; Masters, H.G. Selenium deficiency and hepatosis dietica in pigs. Aust. Vet. J. 1970, 55, 360–366. [Google Scholar] [CrossRef]
- Helmer, C.; Hannemann, R.; Humann-Ziehank, E.; Kleinschmidt, S.; Koelln, M.; Kamphues, J.; Ganter, M. A Case of Concurrent Molybdenosis, Secondary Copper, Cobalt and Selenium Deficiency in a Small Sheep Herd in Northern Germany. Animals 2021, 11, 1864. [Google Scholar] [CrossRef]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Malevu, T.D.; Sochor, J.; Baron, M.; Melcova, M.; Zidkova, J.; et al. A Summary of New Findings on the Biological Effects of Selenium in Selected Animal Species—A Critical Review. Int. J. Mol. Sci. 2017, 18, 2209. [Google Scholar] [CrossRef]
- Sordillo, L.M. Selenium-Dependent Regulation of Oxidative Stress and Immunity in Periparturient Dairy Cattle. Vet.- Med. Int. 2013, 2013, 154045. [Google Scholar] [CrossRef]
- Koller, L.D.; Exon, J.H. The two faces of selenium-deficiency and toxicity--are similar in animals and man. Can. J. Vet.-Res. = Rev. Can. Rech. Vet. 1986, 50, 297–306. [Google Scholar]
- Kumssa, D.; Joy, E.; Broadley, M. Global Trends (1961–2017) in Human Dietary Potassium Supplies. Nutrients 2021, 13, 1369. [Google Scholar] [CrossRef] [PubMed]
- McDowell, L.R. Vitamins in Animal and Human Nutrition, 2nd ed.; Wiley-Blackwell: Oxford, UK, 2000. [Google Scholar] [CrossRef]
- Gissel-Nielsen, G. Comparison of selenium treatments of crops in the field. Biol. Trace Element Res. 1986, 10, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Eliopoulos, G.D.; Eliopoulos, I.-P.D.; Tsioubri, M.; Economou-Eliopoulos, M. Distribution of Selenium in the Soil–Plant–Groundwater System: Factors Controlling Its Bio-Accumulation. Minerals 2020, 10, 795. [Google Scholar] [CrossRef]
- Li, H.; Zhang, J.; Xia, Y.; Pan, W.; Zhou, D. Antagonistic effect of nano-selenium on hepatocyte apoptosis induced by DEHP via PI3K/AKT pathway in chicken liver. Ecotoxicol. Environ. Saf. 2021, 218, 112282. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Zhang, B.; Xu, R.; Wang, Y.; Ding, X.; Li, P. Antioxidant activity in vitro of the selenium-contained protein from the Se-enriched Bifidobacterium animalis 01. Anaerobe 2010, 16, 380–386. [Google Scholar] [CrossRef]
- Zhao, M.; Wen, K.; Xue, Y.; Liu, L.; Geng, T.; Gong, D.; Yu, L. Probing the effects of dietary selenised glucose on the selenium concentration, quality, and antioxidant activity of eggs and production performances of laying hens. Animal 2021, 15, 100374. [Google Scholar] [CrossRef]
- Li, J.-L.; Jiang, C.-Y.; Li, S.; Xu, S.-W. Cadmium induced hepatotoxicity in chickens (Gallus domesticus) and ameliorative effect by selenium. Ecotoxicol. Environ. Saf. 2013, 96, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Hao, P.; Zhu, Y.; Wang, S.; Wan, H.; Chen, P.; Wang, Y.; Cheng, Z.; Liu, Y.; Liu, J. Selenium Administration Alleviates Toxicity of Chromium(VI) in the Chicken Brain. Biol. Trace Element Res. 2016, 178, 127–135. [Google Scholar] [CrossRef]
- Han, X.; Qin, P.; Li, W.; Ma, Q.; Ji, C.; Zhang, J.; Zhao, L. Effect of sodium selenite and selenium yeast on performance, egg quality, antioxidant capacity, and selenium deposition of laying hens. Poult. Sci. 2017, 96, 3973–3980. [Google Scholar] [CrossRef]
- Shojadoost, B.; Kulkarni, R.R.; Yitbarek, A.; Laursen, A.; Taha-Abdelaziz, K.; Alkie, T.N.; Barjesteh, N.; Quinteiro-Filho, W.M.; Smith, T.K.; Sharif, S. Dietary selenium supplementation enhances antiviral immunity in chickens challenged with low pathogenic avian influenza virus subtype H9N2. Vet.- Immunol. Immunopathol. 2018, 207, 62–68. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, L.; Li, Y.; Luo, X.; He, J. Effect of Different Selenium Supplementation Levels on Oxidative Stress, Cytokines, and Immunotoxicity in Chicken Thymus. Biol. Trace Element Res. 2016, 172, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Ren, F.; Hesketh, J.; Shi, X.; Li, J.; Gan, F.; Huang, K. Selenium blocks porcine circovirus type 2 replication promotion induced by oxidative stress by improving GPx1 expression. Free. Radic. Biol. Med. 2012, 53, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Beck, M.A.; Levander, O.A.; Handy, J. Selenium Deficiency and Viral Infection. J. Nutr. 2003, 133, 1463S–1467S. [Google Scholar] [CrossRef]
- Reffett, J.K.; Spears, J.W.; Brown, T.T. Effect of Dietary Selenium and Vitamin E on the Primary and Secondary Immune Response in Lambs Challenged with Parainfluenza Virus. J. Anim. Sci. 1988, 66, 1520–1528. [Google Scholar] [CrossRef]
- Khalili, M.; Chamani, M.; Amanlou, H.; Nikkhah, A.; Sadeghi, A.; Dehkordi, F.K.; Rafiei, M.; Shirani, V. The effect of feeding inorganic and organic selenium sources on the hematological blood parameters, reproduction and health of dairy cows in the transition period. Acta Sci. Anim. Sci. 2019, 42, e45371. [Google Scholar] [CrossRef]
- Sun, L.; Liu, G.; Xu, D.; Wu, Z.; Ma, L.; Victoria, S.-F.M.; Baumgard, L.H.; Bu, D. Milk selenium content and speciation in response to supranutritional selenium yeast supplementation in cows. Anim. Nutr. 2021, 7, 1087–1094. [Google Scholar] [CrossRef]
- Hall, J.A.; Bobe, G.; Vorachek, W.R.; Kasper, K.; Traber, M.G.; Mosher, W.D.; Pirelli, G.J.; Gamroth, M. Effect of Supranutritional Organic Selenium Supplementation on Postpartum Blood Micronutrients, Antioxidants, Metabolites, and Inflammation Biomarkers in Selenium-Replete Dairy Cows. Biol. Trace Element Res. 2014, 161, 272–287. [Google Scholar] [CrossRef]
- Pan, Q.; Huang, K.; He, K.; Lu, F. Effect of different selenium sources and levels on porcine circovirus type 2 replication in vitro. J. Trace Elements Med. Biol. 2008, 22, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Zhang, H.; Liu, Z.; Lei, L.; Feng, Z.; Zhang, D.; Zhao, S. Comparative study of yeast selenium vs. sodium selenite on growth performance, nutrient digestibility, anti-inflammatory and anti-oxidative activity in weaned piglets challenged by Salmonella typhimurium. Innate Immun. 2020, 26, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, W.; Zheng, S.; Zhang, Q.; Xu, S. Selenomethionine relieves inflammation in the chicken trachea caused by LPS though inhibiting the NF-κB pathway. Biol. Trace Elem. Res. 2020, 194, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, A.I.; Mohamed, D.A.; Chwen, L.T.; Akit, H.; Samsudin, A.A. Effect of Selenium Sources on Laying Performance, Egg Quality Characteristics, Intestinal Morphology, Microbial Population and Digesta Volatile Fatty Acids in Laying Hens. Animals 2021, 11, 1681. [Google Scholar] [CrossRef]
- Lee, S.H.; Lillehoj, H.S.; Jang, S.I.; Jeong, M.S.; Xu, S.Z.; Kim, J.B.; Park, H.J.; Kim, H.R.; Lillehoj, E.P.; Bravo, D.M. Effects of in ovo injection with selenium on immune and antioxidant responses during experimental necrotic enteritis in broiler chickens. Poult. Sci. 2014, 93, 1113–1121. [Google Scholar] [CrossRef]
- Mengistu, B.M.; Bitsue, H.K.; Huang, K. The Effects of Selenium-Enriched Probiotics on Growth Performance, Oocysts Shedding, Intestinal Cecal Lesion Scores, Antioxidant Capacity, and mRNA Gene Expression in Chickens Infected with Eimeria tenella. Biol. Trace Element Res. 2020, 199, 278–291. [Google Scholar] [CrossRef]
- Hugejiletu, H.; Bobe, G.; Vorachek, W.R.; Gorman, M.E.; Mosher, W.D.; Pirelli, G.J.; Hall, J.A. Selenium Supplementation Alters Gene Expression Profiles Associated with Innate Immunity in Whole-Blood Neutrophils of Sheep. Biol. Trace Element Res. 2013, 154, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Jia, X.; Li, J.; Li, S.; Zhao, Q.; Zhang, K.; Tang, C.; Yang, Y.; Ma, Q.; Wang, J.; Zhao, Z.; et al. Effects of dietary supplementation with different levels of selenium yeast on growth performance, carcass characteristics, antioxidant capacity, and meat quality of Tan sheep. Livest. Sci. 2021, 255, 104783. [Google Scholar] [CrossRef]
- Ebeid, T.; Zeweil, H.; Basyony, M.; Dosoky, W.; Badry, H. Fortification of rabbit diets with vitamin E or selenium affects growth performance, lipid peroxidation, oxidative status and immune response in growing rabbits. Livest. Sci. 2013, 155, 323–331. [Google Scholar] [CrossRef]
- Mattioli, S.; Dal Bosco, A.; Duarte, J.M.M.; D’Amato, R.; Castellini, C.; Beone, G.M.; Proietti, P. Use of Selenium-enriched olive leaves in the feed of growing rabbits: Effect on oxidative status, mineral profile and Selenium speciation of Longissimus dorsi meat. J. Trace Elem. Med. Biol. 2019, 51, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.D. Assessment of requirements for selenium and adequacy of selenium status: A review. Eur. J. Clin. Nutr. 2004, 58, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Thomson, C.D. Selenium and iodine intakes and status in New Zealand and Australia. Br. J. Nutr. 2004, 91, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Quintela, A.; Milton-Laskibar, I.; Trepiana, J.; Gómez-Zorita, S.; Kajarabille, N.; Léniz, A.; González, M.; Portillo, M.P. Key Aspects in Nutritional Management of COVID-19 Patients. J. Clin. Med. 2020, 9, 2589. [Google Scholar] [CrossRef]
- Varo, P.E.R.T.I.I.; Alfthan, G.; Huttunen, J.K.; Aro, A. Nationwide selenium supplementation in Finland-effects on diet, blood and tissue levels, and health. In Selenium in Biology and Human Health; Springer: New York, NY, USA, 1994; Volume 98, pp. 198–218. [Google Scholar]
- Williams, P.; Islam, S.; Islam, R.; Jahiruddin, M.; Adomako, E.; Soliaman, A.R.M.; Rahman, G.K.M.M.; Lu, Y.; Deacon, C.; Zhu, Y.-G.; et al. Arsenic Limits Trace Mineral Nutrition (Selenium, Zinc, and Nickel) in Bangladesh Rice Grain. Environ. Sci. Technol. 2009, 43, 8430–8436. [Google Scholar] [CrossRef]
- Williams, P.; Lombi, E.; Sun, G.-X.; Scheckel, K.; Zhu, Y.-G.; Feng, X.; Zhu, J.; Carey, A.-M.; Adomako, E.; Lawgali, Y.; et al. Selenium Characterization in the Global Rice Supply Chain. Environ. Sci. Technol. 2009, 43, 6024–6030. [Google Scholar] [CrossRef]
- Waegeneers, N.; Thiry, C.; De Temmerman, L.; Ruttens, A. Predicted dietary intake of selenium by the general adult population in Belgium. Food Addit. Contam. Part A 2013, 30, 278–285. [Google Scholar] [CrossRef]
- Dreher, I.; Schütze, N.; Baur, A.; Hesse, K.; Schneider, D.; Köhrle, J.; Jakob, F. Selenoproteins Are Expressed in Fetal Human Osteoblast-like Cells. Biochem. Biophys. Res. Commun. 1998, 245, 101–107. [Google Scholar] [CrossRef]
- Food, N.B.; Board, N. Institute of Medicine. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium and Carotenoids; National Academy Press: Washington, DC, USA, 2000. [Google Scholar]
- Rayman, M.P.; Infante, H.G.; Sargent, M. Food-chain selenium and human health: Spotlight on speciation. Br. J. Nutr. 2008, 100, 238–253. [Google Scholar] [CrossRef]
- Bossola, M.; Di Stasio, E.; Viola, A.; Leo, A.; Carlomagno, G.; Monteburini, T.; Cenerelli, S.; Santarelli, S.; Boggi, R.; Miggiano, G.; et al. Dietary intake of trace elements, minerals, and vitamins of patients on chronic hemodialysis. Int. Urol. Nephrol. 2014, 46, 809–815. [Google Scholar] [CrossRef]
- Pograjc, L.; Stibilj, V.; Falnoga, I. Impact of Intensive Physical Activity on Selenium Status. Biol. Trace Element Res. 2011, 145, 291–299. [Google Scholar] [CrossRef]
- Valent, F.; Horvat, M.; Mazej, D.; Stibilj, V.; Barbone, F. Maternal Diet and Selenium Concentration in Human Milk From an Italian Population. J. Epidemiology 2011, 21, 285–292. [Google Scholar] [CrossRef]
- Al-Saleh, I.; El-Doush, I.; Billedo, G.; Mohamed, G.E.-D.; Yosef, G. Status of Selenium, Vitamin E, and Vitamin A among Saudi Adults: Potential Links with Common Endemic Diseases. J. Environ. Pathol. Toxicol. Oncol. 2007, 26, 221–243. [Google Scholar] [CrossRef]
- Al-Awadi, F.M.; Srikumar, T.S. Determination of selenium concentration and its chemical forms in the milk of Kuwaiti and non-Kuwaiti lactating mothers. J. Trace Elem. Exp. Med. Off. Publ. Int. Soc. Trace Elem. Res. Hum. 2001, 14, 57–67. [Google Scholar] [CrossRef]
- Özdemir, H.S.; Karadas, F.; Pappas, A.; Cassey, P.; Oto, G.; Tunçer. The Selenium Levels of Mothers and Their Neonates Using Hair, Breast Milk, Meconium, and Maternal and Umbilical Cord Blood in Van Basin. Biol. Trace Element Res. 2008, 122, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Joint, F. Vitamin and Mineral Requirements in Human Nutrition; Diamond Pocket Books (P) Ltd.: New Delhi, India, 2004. [Google Scholar]
- Sunde, R.A.; Paterson, E.; Evenson, J.K.; Barnes, K.M.; Lovegrove, J.A.; Gordon, M.H. Longitudinal selenium status in healthy British adults: Assessment using biochemical and molecular biomarkers. Br. J. Nutr. 2008, 99, S37–S47. [Google Scholar] [CrossRef]
- González, S.; Huerta, J.M.; Fernández, S.; Patterson, Ð.M.; Lasheras, C. Food intake and serum selenium concentration in elderly people. Ann. Nutr. Metab. 2006, 50, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Redondo, S.; de Miguel, B.B.; Gómez-Pavón, J.; Vives, C.C. Non-institutionalized nonagenarians health-related quality of life and nutritional status: Is there a link between them? Nutr. Hosp. 2014, 30, 602–608. [Google Scholar] [PubMed]
- Navia, B.; Ortega, R.M.; Perea, J.M.; Aparicio, A.; López-Sobaler, A.M.; Rodríguez-Rodríguez, E.; Research Group: UCM 920030 (VALORNUT). Selenium status in a group of schoolchildren from the region of M adrid, S pain. J. Hum. Nutr. Diet. 2014, 27, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Rivas, A.; Romero, A.; Mariscal-Arcas, M.; Monteagudo, C.; López, G.; Ocaña-Peinado, F.M.; Olea-Serrano, F. Association between dietary antioxidant quality score (DAQs) and bone mineral density in Spanish women. Nutr. Hosp. 2012, 27, 1886–1893. [Google Scholar] [PubMed]
- Golubkina, N.A. Selenium accumulation by cereals in Russia. Russ. Agric. Sci. 2007, 33, 288–291. [Google Scholar] [CrossRef]
- Retondario, A.; Souza, A.D.M.; Fernandes, R.; Bricarello, L.P.; Alves, M.D.A.; Zeni, L.A.R.; Trindade, E.B.D.M.; Vasconcelos, F.D.A.G.D. Usual intake and dietary sources of Selenium in adolescents: A cross-sectional school-based study. Clin. Nutr. ESPEN 2019, 33, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Dumont, E.; Vanhaecke, F.; Cornelis, R. Selenium speciation from food source to metabolites: A critical review. Anal. Bioanal. Chem. 2006, 385, 1304–1323. [Google Scholar] [CrossRef] [PubMed]
- Rayman, M.P. The importance of selenium to human health. Lancet 2000, 356, 233–241. [Google Scholar] [CrossRef]
- Laclaustra, M.; Navas-Acien, A.; Stranges, S.; Ordovas, J.; Guallar, E. Serum Selenium Concentrations and Diabetes in U.S. Adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Environ. Health Perspect. 2009, 117, 1409–1413. [Google Scholar] [CrossRef]
- Laclaustra, M.; Stranges, S.; Navas-Acien, A.; Ordovas, J.; Guallar, E. Serum selenium and serum lipids in US adults: National Health and Nutrition Examination Survey (NHANES) 2003–2004. Atherosclerosis 2010, 210, 643–648. [Google Scholar] [CrossRef]
- Tinggi, U. Essentiality and toxicity of selenium and its status in Australia: A review. Toxicol. Lett. 2002, 137, 103–110. [Google Scholar] [CrossRef]
- Foster, L.H.; Sumar, S. Selenium in health and disease: A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 211–228. [Google Scholar] [CrossRef]
- Arikan, D.C.; Coskun, A.; Ozer, A.; Kilinc, M.; Atalay, F.; Arikan, T. Plasma Selenium, Zinc, Copper and Lipid Levels in Postmenopausal Turkish Women and Their Relation with Osteoporosis. Biol. Trace Element Res. 2011, 144, 407–417. [Google Scholar] [CrossRef]
- Aydemir, B.; Akdemir, R.; Vatan, M.B.; Cinemre, F.B.; Cinemre, H.; Kiziler, A.R.; Bahtiyar, N.; Buyukokuroglu, M.E.; Gurol, G.; Ogut, S. The Circulating Levels of Selenium, Zinc, Midkine, Some Inflammatory Cytokines, and Angiogenic Factors in Mitral Chordae Tendineae Rupture. Biol. Trace Element Res. 2015, 167, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Bay, A.; Dogan, M.; Bulan, K.; Kaba, S.; Demir, N.; Öner, A.F. A study on the effects of pica and iron-deficiency anemia on oxidative stress, antioxidant capacity and trace elements. Hum. Exp. Toxicol. 2013, 32, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Coskun, A.; Arikan, T.; Kilinc, M.; Arikan, D.C.; Ekerbiçer, H. Plasma selenium levels in Turkish women with polycystic ovary syndrome. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 168, 183–186. [Google Scholar] [CrossRef]
- Erkekoğlu, P.; Aşçı, A.; Ceyhan, M.; Kızılgün, M.; Schweizer, U.; Ataş, C.; Koçer-Giray, B. Selenium levels, selenoenzyme activities and oxidant/antioxidant parameters in H1N1-infected children. Turk. J. Pediatr. 2013, 55, 271–282. [Google Scholar] [PubMed]
- Eroglu, M.; Sahin, S.; Durukan, B.; Ozakpinar, O.B.; Erdinc, N.; Turkgeldi, L.; Sofuoglu, K.; Karateke, A. Blood Serum and Seminal Plasma Selenium, Total Antioxidant Capacity and Coenzyme Q10 Levels in Relation to Semen Parameters in Men with Idiopathic Infertility. Biol. Trace Element Res. 2014, 159, 46–51. [Google Scholar] [CrossRef]
- Hıncal, F. Trace elements in growth: Iodine and selenium status of Turkish children. J. Trace Elements Med. Biol. 2007, 21, 40–43. [Google Scholar] [CrossRef]
- Kilinc, M.; Guven, M.A.; Ezer, M.; Ertas, I.E.; Coskun, A. Evaluation of Serum Selenium Levels in Turkish Women with Gestational Diabetes Mellitus, Glucose Intolerants, and Normal Controls. Biol. Trace Element Res. 2008, 123, 35–40. [Google Scholar] [CrossRef]
- Sakız, D.; Kaya, A.; Kulaksizoglu, M. Serum selenium levels in euthyroid nodular thyroid diseases. Biol. Trace Elem. Res. 2016, 174, 21–26. [Google Scholar] [CrossRef]
- Seven, M.; Basaran, S.Y.; Cengiz, M.; Unal, S.; Yuksel, A. Deficiency of selenium and zinc as a causative factor for idiopathic intractable epilepsy. Epilepsy Res. 2013, 104, 35–39. [Google Scholar] [CrossRef]
- Kadrabová, J.; Mad’arič, A.; Ginter, E. Determination of the daily selenium intake in Slovakia. Biol. Trace Elem. Res. 1998, 61, 277–286. [Google Scholar] [CrossRef]
- Al-Ahmary, K.M. Selenium content in selected foods from the Saudi Arabia market and estimation of the daily intake. Arab. J. Chem. 2009, 2, 95–99. [Google Scholar] [CrossRef]
- Mansour, A.; Ahadi, Z.; Qorbani, M.; Hosseini, S. Association between dietary intake and seasonal variations in postmenopausal women. J. Diabetes Metab. Disord. 2014, 13, 52. [Google Scholar] [CrossRef]
- Kieliszek, M.; Błażejak, S. Current Knowledge on the Importance of Selenium in Food for Living Organisms: A Review. Molecules 2016, 21, 609. [Google Scholar] [CrossRef] [PubMed]
- Wasowicz, W.; Gromadzinska, J.; Rydzynski, K.; Tomczak, J. Selenium status of low-selenium area residents: Polish experience. Toxicol. Lett. 2002, 137, 95–101. [Google Scholar] [CrossRef]
- Surai, P.F. Selenium in Nutrition and Health; Nottingham University Press: Nottingham, UK, 2006; Volume 974. [Google Scholar]
- Smrkolj, P.; Pograjc, L.; Hlastan-Ribič, C.; Stibilj, V. Selenium content in selected Slovenian foodstuffs and estimated daily intakes of selenium. Food Chem. 2005, 90, 691–697. [Google Scholar] [CrossRef]
- Arafa, A.M.; Waly, M.; Jriesat, S.; Al Khafajei, A.; Sallam, S. Dietary and lifestyle characteristics of colorectal cancer in Jordan: A case-control study. Asian Pac. J. Cancer Prev. 2011, 12, 1931–1936. [Google Scholar] [PubMed]
- Hansen, J.C.; Pedersen, H.S. Environmental exposure to heavy metals in North Greenland. Arct. Med. Res. 1986, 41, 21–34. [Google Scholar]
- Li, S.; Xiao, T.; Zheng, B. Medical geology of arsenic, selenium and thallium in China. Sci. Total Environ. 2012, 421, 31–40. [Google Scholar] [CrossRef]
- Zachara, B.A. Selenium and Selenium-Dependent Antioxidants in Chronic Kidney Disease. Adv. Clin. Chem. 2015, 68, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Tan, H.-Y.; Li, S.; Xu, Y.; Guo, W.; Feng, Y. Supplementation of Micronutrient Selenium in Metabolic Diseases: Its Role as an Antioxidant. Oxidative Med. Cell. Longev. 2017, 2017, 7478523. [Google Scholar] [CrossRef]
- Keshan, D.R.G.O.T.C.A.O.M.S.B.; Antiepidemic, S.O.S.P.C.; Antiepidemic, S.O.X.D.S.; Antiepidemic, S.O.M.C.S. Observations on effect of sodium selenite in prevention of Keshan disease. Chin. Med. J. 1979, 92, 471–476. [Google Scholar]
- Papp, L.V.; Holmgren, A.; Khanna, K.K. Selenium and Selenoproteins in Health and Disease. Antioxid. Redox Signal. 2010, 12, 793–795. [Google Scholar] [CrossRef] [PubMed]
- Albert Christophersen, O.; Haug, A. Possible roles of oxidative stress, local circulatory failure and nutrition factors in the pathogenesis of hypervirulent influenza: Implications for therapy and global emergency preparedness. Microb. Ecol. Health Dis. 2005, 17, 189–199. [Google Scholar] [CrossRef]
- Cengiz, B.; Söylemez, F.; Öztürk, E.; Çavdar, A.O. Serum zinc, selenium, copper, and lead levels in women with second-trimester induced abortion resulting from neural tube defects: A preliminary study. Biol. Trace Elem. Res. 2004, 97, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Ruder, E.H.; Hartman, T.J.; Blumberg, J.; Goldman, M.B. Oxidative stress and antioxidants: Exposure and impact on female fertility. Hum. Reprod. Updat. 2008, 14, 345–357. [Google Scholar] [CrossRef]
- Ruder, E.H.; Hartman, T.J.; Goldman, M.B. Impact of oxidative stress on female fertility. Curr. Opin. Obstet. Gynecol. 2009, 21, 219–222. [Google Scholar] [CrossRef]
- Frost, D.V.; Ingvoldstad, D. Ecological aspects of selenium and tellurium in human and animal health. Chem. Scr. 1975, 8, 96–107. [Google Scholar]
- Schrauzer, G.; White, D.; Schneider, C. Cancer mortality correlation studies-III: Statistical associations with dietary selenium intakes. Bioinorg. Chem. 1977, 7, 23–34. [Google Scholar] [CrossRef]
- Hofmarcher, T.; Lindgren, P.; Wilking, N.; Jönsson, B. The cost of cancer in Europe 2018. Eur. J. Cancer 2020, 129, 41–49. [Google Scholar] [CrossRef]
- Ip, C. Lessons from Basic Research in Selenium and Cancer Prevention. J. Nutr. 1998, 128, 1845–1854. [Google Scholar] [CrossRef]
- Hossain, A.; Skalicky, M.; Brestic, M.; Maitra, S.; Sarkar, S.; Ahmad, Z.; Vemuri, H.; Garai, S.; Mondal, M.; Bhatt, R.; et al. Selenium Biofortification: Roles, Mechanisms, Responses and Prospects. Molecules 2021, 26, 881. [Google Scholar] [CrossRef] [PubMed]
- Bentley-Hewitt, K.L.; Chen, R.K.Y.; Lill, R.E.; Hedderley, D.I.; Herath, T.D.; Matich, A.J.; McKenzie, M.J. Consumption of selenium-enriched broccoli increases cytokine production in human peripheral blood mononuclear cells stimulated ex vivo, a preliminary human intervention study. Mol. Nutr. Food Res. 2014, 58, 2350–2357. [Google Scholar] [CrossRef] [PubMed]
- Kuehnelt, D.; Juresa, D.; Francesconi, K.A.; Fakih, M.; Reid, M.E. Selenium metabolites in urine of cancer patients receiving l-selenomethionine at high doses. Toxicol. Appl. Pharmacol. 2007, 220, 211–215. [Google Scholar] [CrossRef]
- Zeng, H.; Combs, G.F. Selenium as an anticancer nutrient: Roles in cell proliferation and tumor cell invasion. J. Nutr. Biochem. 2008, 19, 1–7. [Google Scholar] [CrossRef]
- Stranges, S.; Galletti, F.; Farinaro, E.; D’Elia, L.; Russo, O.; Iacone, R.; Capasso, C.; Carginale, V.; De Luca, V.; Della Valle, E.; et al. Associations of selenium status with cardiometabolic risk factors: An 8-year follow-up analysis of the Olivetti Heart Study. Atherosclerosis 2011, 217, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Alarcon, M.; López-Martínez, M. Essentiality of selenium in the human body: Relationship with different diseases. Sci. Total. Environ. 2000, 249, 347–371. [Google Scholar] [CrossRef]
- Cao, S.; Durrani, F.A.; Rustum, Y.M. Selective Modulation of the Therapeutic Efficacy of Anticancer Drugs by Selenium Containing Compounds against Human Tumor Xenografts. Clin. Cancer Res. 2004, 10, 2561–2569. [Google Scholar] [CrossRef]
- Cao, S.; Durrani, A.F.; Tóth, K.; Rustum, Y.M. Se-methylselenocysteine offers selective protection against toxicity and potentiates the antitumour activity of anticancer drugs in preclinical animal models. Br. J. Cancer 2014, 110, 1733–1743. [Google Scholar] [CrossRef]
- Burbano, X.; Miguez-Burbano, M.J.; McCollister, K.; Zhang, G.; Rodriguez, A.; Ruiz, P.; Shor-Posner, G. Impact of a selenium chemoprevention clinical trial on hospital admissions of HIV-infected participants. HIV Clin. Trials 2002, 3, 483–491. [Google Scholar]
- Hurwitz, B.E.; Klaus, J.R.; Llabre, M.M.; Gonzalez, A.; Lawrence, P.J.; Maher, K.J.; Schneiderman, N. Suppression of human immunodeficiency virus type 1 viral load with selenium supplementation: A randomized controlled trial. Arch. Intern. Med. 2007, 167, 148–154. [Google Scholar] [CrossRef]
- Kupka, R.; Mugusi, F.; Aboud, S.; Msamanga, I.G.; Finkelstein, J.L.; Spiegelman, D.; Fawzi, W.W. Randomized, double-blind, placebo-controlled trial of selenium supplements among HIV-infected pregnant women in Tanzania: Effects on maternal and child outcomes. Am. J. Clin. Nutr. 2008, 87, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Broome, C.S.; McArdle, F.; Kyle, J.A.; Andrews, F.; Lowe, N.M.; Hart, C.A.; Arthur, J.R.; Jackson, M.J. An increase in selenium intake improves immune function and poliovirus handling in adults with marginal selenium status. Am. J. Clin. Nutr. 2004, 80, 154–162. [Google Scholar] [CrossRef]
- Hu, M.; Fang, J.; Zhang, Y.; Wang, X.; Zhong, W.; Zhou, Z. Design and evaluation a kind of functional biomaterial for bone tissue engineering: Selenium/mesoporous bioactive glass nanospheres. J. Colloid Interface Sci. 2020, 579, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.; Liao, L.; Wan, Y.-P.; Li, M.-X.; Chen, S.-H.; Mo, W.-J.; Zhao, Q.-L.; Huang, L.-F.; Zeng, G.-Q. Downregulation of selenium-binding protein 1 is associated with poor prognosis in lung squamous cell carcinoma. World J. Surg. Oncol. 2016, 14, 1–8. [Google Scholar] [CrossRef]
- Klein, E.A.; Thompson, I.; Tangen, C.M.; Lucia, M.S.; Goodman, P.; Minasian, L.M.; Ford, L.G.; Parnes, H.L.; Gaziano, J.M.; Karp, D.D.; et al. Vitamin E and the risk of prostate cancer: Updated results of the Selenium and Vitamin E Cancer Prevention Trial (SELECT). J. Clin. Oncol. 2012, 30, 7. [Google Scholar] [CrossRef]
- Kora, A.J.; Rastogi, L. Bacteriogenic synthesis of selenium nanoparticles by Escherichia coli ATCC 35218 and its structural characterisation. IET Nanobiotechnol. 2016, 11, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.F.; Zhao, Z.Q.; Han, Z.Y.; Huang, L.Q.; Lv, C.H.; Zhang, Z.H.; Liu, X.W. Selenium uptake and fruit quality of pear (Pyrus communis L.) treated with foliar Se application. J. Plant Nutr. Soil Sci. 2019, 182, 637–646. [Google Scholar] [CrossRef]
- Ros, G.H.; van Rotterdam, A.M.D.; Bussink, D.W.; Bindraban, P.S. Selenium fertilization strategies for bio-fortification of food: An agro-ecosystem approach. Plant Soil 2016, 404, 99–112. [Google Scholar] [CrossRef]
- Chen, Q.; Shi, W.; Wang, X. Selenium Speciation and Distribution Characteristics in the Rhizosphere Soil of Rice (Oryza sativa L.) Seedlings. Commun. Soil Sci. Plant Anal. 2010, 41, 1411–1425. [Google Scholar] [CrossRef]
- Zhou, X.-B.; Shi, W.-M.; Zhang, L.-H. Iron plaque outside roots affects selenite uptake by rice seedlings (Oryza sativa L.) grown in solution culture. Plant Soil 2006, 290, 17–28. [Google Scholar] [CrossRef]
- Niazi, N.K.; Bibi, I.; Shahid, M.; Ok, Y.S.; Burton, E.D.; Wang, H.; Shaheen, S.M.; Rinklebe, J.; Lüttge, A. Arsenic removal by perilla leaf biochar in aqueous solutions and groundwater: An integrated spectroscopic and microscopic examination. Environ. Pollut. 2018, 232, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Golob, A.; Gadžo, D.; Stibilj, V.; Djikić, M.; Gavrić, T.; Kreft, I.; Germ, M. Sulphur interferes with selenium accumulation in Tartary buckwheat plants. Plant Physiol. Biochem. 2016, 108, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Naz, F.S.; Yusuf, M.; Khan, T.A.; Fariduddin, Q.; Ahmad, A. Low level of selenium increases the efficacy of 24-epibrassinolide through altered physiological and biochemical traits of Brassica juncea plants. Food Chem. 2015, 185, 441–448. [Google Scholar] [CrossRef]
- Mackowiak, C.L.; Amacher, M.C. Soil Sulfur Amendments Suppress Selenium Uptake by Alfalfa and Western Wheatgrass. J. Environ. Qual. 2008, 37, 772–779. [Google Scholar] [CrossRef]
- Lyons, G.H.; Lewis, J.; Lorimer, M.F.; Holloway, R.E.; Brace, D.M.; Stangoulis, J.C.; Graham, R.D. High-selenium wheat: Agronomic biofortification strategies to improve human nutrition. Food Agric. Environ. 2004, 2, 171–178. [Google Scholar]
- Sors, T.G.; Ellis, D.R.; Salt, D.E. Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth. Res. 2005, 86, 373–389. [Google Scholar] [CrossRef]
- Pierart, A.; Shahid, M.; Séjalon-Delmas, N.; Dumat, C. Antimony bioavailability: Knowledge and research perspectives for sustainable agricultures. J. Hazard. Mater. 2015, 289, 219–234. [Google Scholar] [CrossRef]
- Xing, K.; Zhou, S.; Wu, X.; Zhu, Y.; Kong, J.; Shao, T.; Tao, X. Concentrations and characteristics of selenium in soil samples from Dashan Region, a selenium-enriched area in China. Soil Sci. Plant Nutr. 2015, 61, 889–897. [Google Scholar] [CrossRef]
- Munier-Lamy, C.; Deneux-Mustin, S.; Mustin, C.; Merlet, D.; Berthelin, J.; Leyval, C. Selenium bioavailability and uptake as affected by four different plants in a loamy clay soil with particular attention to mycorrhizae inoculated ryegrass. J. Environ. Radioact. 2007, 97, 148–158. [Google Scholar] [CrossRef]
- Johnsson, L. Selenium uptake by plants as a function of soil type, organic matter content and pH. Plant Soil 1991, 133, 57–64. [Google Scholar] [CrossRef]
- White, P.J. Selenium metabolism in plants. Biochim. Et Biophys. Acta BBA-Gen. Subj. 2018, 1862, 2333–2342. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, L.W. Selenium in Agriculture and the Environment; Soil Science Society of America Special Publication 23; SSSA: Madison, WI, USA, 1989. [Google Scholar]
- Elrashidi, M.A.; Adriano, D.C.; Workman, S.M.; Lindsay, W.L. Chemical equilibria of selenium in soils: A theoretical development: 1. Soil Sci. 1987, 144, 141–152. [Google Scholar] [CrossRef]
- Barker, A.V.; Pilbeam, D.J. (Eds.) Handbook of Plant Nutrition; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Wang, C.; Ji, J.; Zhu, F. Characterizing Se transfer in the soil-crop systems under field condition. Plant Soil 2017, 415, 535–548. [Google Scholar] [CrossRef]
- Borawska, M.H.; Witkowska, A.M.; Hukałowicz, K.; Markiewicz, R. Influence of dietary habits on serum selenium concentration. Ann. Nutr. Metab. 2004, 48, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Liu, Y.; Dong, G.; Wu, H. Effect of boiling and frying on the selenium content, speciation, and in vitro bioaccessibility of selenium-biofortified potato (Solanum tuberosum L.). Food Chem. 2021, 348, 129150. [Google Scholar] [CrossRef]
- Pérez, M.B.; Maniero, M.; Londonio, A.; Smichowski, P.; Wuilloud, R.G. Effects of common cooking heat treatments on selenium content and speciation in garlic. J. Food Compos. Anal. 2018, 70, 54–62. [Google Scholar] [CrossRef]
- Khanam, A.; Platel, K. Influence of domestic processing on the bioaccessibility of selenium from selected food grains and composite meals. J. Food Sci. Technol. 2015, 53, 1634–1639. [Google Scholar] [CrossRef]
- Lu, X.; He, Z.; Lin, Z.; Zhu, Y.; Yuan, L.; Liu, Y.; Yin, X. Effects of Chinese Cooking Methods on the Content and Speciation of Selenium in Selenium Bio-Fortified Cereals and Soybeans. Nutrients 2018, 10, 317. [Google Scholar] [CrossRef]
- Moreda-Piñeiro, J.; Moreda-Piñeiro, A.; Bermejo-Barrera, P. In vivo and in vitro testing for selenium and selenium compounds bioavailability assessment in foodstuff. Crit. Rev. Food Sci. Nutr. 2015, 57, 805–833. [Google Scholar] [CrossRef]
- Wang, C.; Duan, H.-Y.; Teng, J.-W. Assessment of Microwave Cooking on the Bioaccessibility of Cadmium from Various Food Matrices Using an In Vitro Digestion Model. Biol. Trace Element Res. 2014, 160, 276–284. [Google Scholar] [CrossRef]
- Matos, J.; Lourenço, H.M.; Brito, P.; Maulvault, A.L.; Martins, L.L.; Afonso, C. Influence of bioaccessibility of total mercury, methyl-mercury and selenium on the risk/benefit associated to the consumption of raw and cooked blue shark (Prionace glauca). Environ. Res. 2015, 143, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Raisbeck, M.F. Selenosis in ruminants. Vet. Clin. Food Anim. Pract. 2020, 36, 775–789. [Google Scholar] [CrossRef] [PubMed]
- Molnár, Á.; Kolbert, Z.; Kéri, K.; Feigl, G.; Ördög, A.; Szőllősi, R.; Erdei, L. Selenite-induced nitro-oxidative stress processes in Arabidopsis thaliana and Brassica juncea. Ecotoxicol. Environ. Saf. 2018, 148, 664–674. [Google Scholar] [CrossRef]
- Moreno, O.D.G.; Aguilar, F.J.A.; Barrientos, E.Y. Selenium Uptake and Biotransformation and Effect of Selenium Exposure on the Essential and Trace Elements Status: Comparative Evaluation of Four Edible Plants. J. Mex. Chem. Soc. 2018, 62, 247–258. [Google Scholar] [CrossRef]
- Lehotai, N.; Lyubenova, L.; Schröder, P.; Feigl, G.; Ördög, A.; Szilágyi, K.; Kolbert, Z. Nitro-oxidative stress contributes to selenite toxicity in pea (Pisum sativum L). Plant Soil 2016, 400, 107–122. [Google Scholar] [CrossRef]
- Hawrylak-Nowak, B. Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Plant Growth Regul. 2013, 70, 149–157. [Google Scholar] [CrossRef]
- Łabanowska, M.; Filek, M.; Kościelniak, J.; Kurdziel, M.; Kuliś, E.; Hartikainen, H. The effects of short-term selenium stress on Polish and Finnish wheat seedlings—EPR, enzymatic and fluorescence studies. J. Plant Physiol. 2012, 169, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Lehotai, N.; Kolbert, Z.; Pető, A.; Feigl, G.; Ördög, A.; Kumar, D.; Erdei, L. Selenite-induced hormonal and signalling mechanisms during root growth of Arabidopsis thaliana L. J. Exp. Bot. 2012, 63, 5677–5687. [Google Scholar] [CrossRef] [PubMed]
- Saffaryazdi, A.; Lahouti, M.; Ganjeali, A.; Bayat, H. Impact of Selenium Supplementation on Growth and Selenium Accumulation on Spinach (Spinacia oleracea L.) Plants. Not. Sci. Biol. 2012, 4, 95–100. [Google Scholar] [CrossRef]
- Freeman, J.L.; Tamaoki, M.; Stushnoff, C.; Quinn, C.F.; Cappa, J.J.; Devonshire, J.; Fakra, S.C.; Marcus, M.A.; McGrath, S.P.; Van Hoewyk, D.; et al. Molecular Mechanisms of Selenium Tolerance and Hyperaccumulation in Stanleya pinnata. Plant Physiol. 2010, 153, 1630–1652. [Google Scholar] [CrossRef]
- Feng, R.; Wei, C.; Tu, S.; Wu, F. Effects of Se on the uptake of essential elements in Pteris vittata L. Plant Soil 2009, 325, 123–132. [Google Scholar] [CrossRef]
- Ramos, S.J.; Faquin, V.; Almeida, H.J.D.; Ávila, F.W.; Guilherme, L.R.G.; Bastos, C.E.A.; Ávila, P.A. Selenato e selenito na produção, nutrição mineral e biofortificação com selênio em cultivares de alface¹. Rev. Bras. Ciência Solo 2011, 35, 1347–1355. [Google Scholar] [CrossRef]
- Zhang, M.; Tang, S.; Huang, X.; Zhang, F.; Pang, Y.; Huang, Q.; Yi, Q. Selenium uptake, dynamic changes in selenium content and its influence on photosynthesis and chlorophyll fluorescence in rice (Oryza sativa L.). Environ. Exp. Bot. 2014, 107, 39–45. [Google Scholar] [CrossRef]
- Matraszek, R.; Hawrylak-Nowak, B. Macronutrients accumulation in useable parts of lettuce as affected by nickel and selenium concentrations in nutrient solution. Fresenius Environ. Bull. 2009, 18, 1059–1065. [Google Scholar]
- Sarwar, N.; Akhtar, M.; Kamran, M.A.; Imran, M.; Riaz, M.A.; Kamran, K.; Hussain, S. Selenium biofortification in food crops: Key mechanisms and future perspectives. J. Food Compos. Anal. 2020, 93, 103615. [Google Scholar] [CrossRef]
- Bouis, H.E.; Chassy, B.M.; Ochanda, J.O. 2. Genetically modified food crops and their contribution to human nutrition and food quality. Trends Food Sci. Technol. 2003, 14, 191–209. [Google Scholar] [CrossRef]
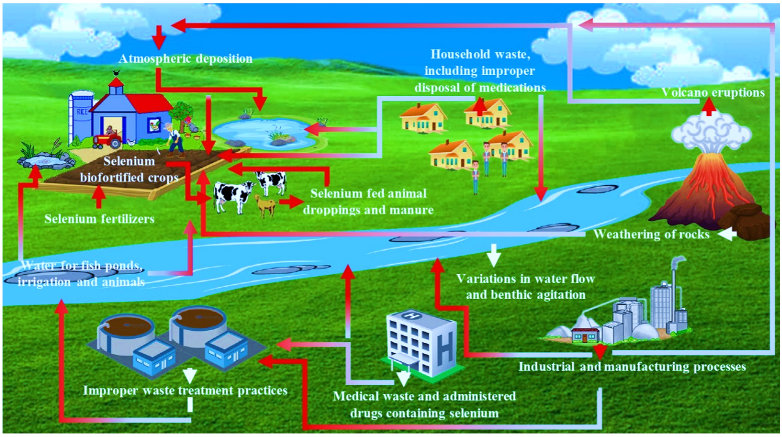
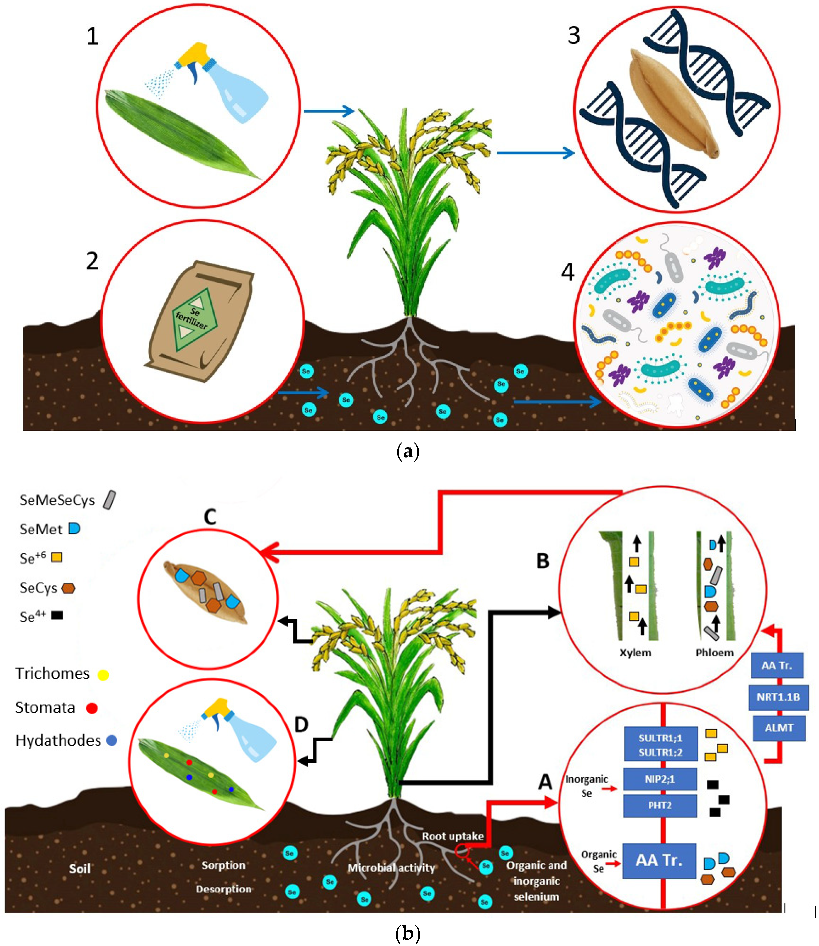
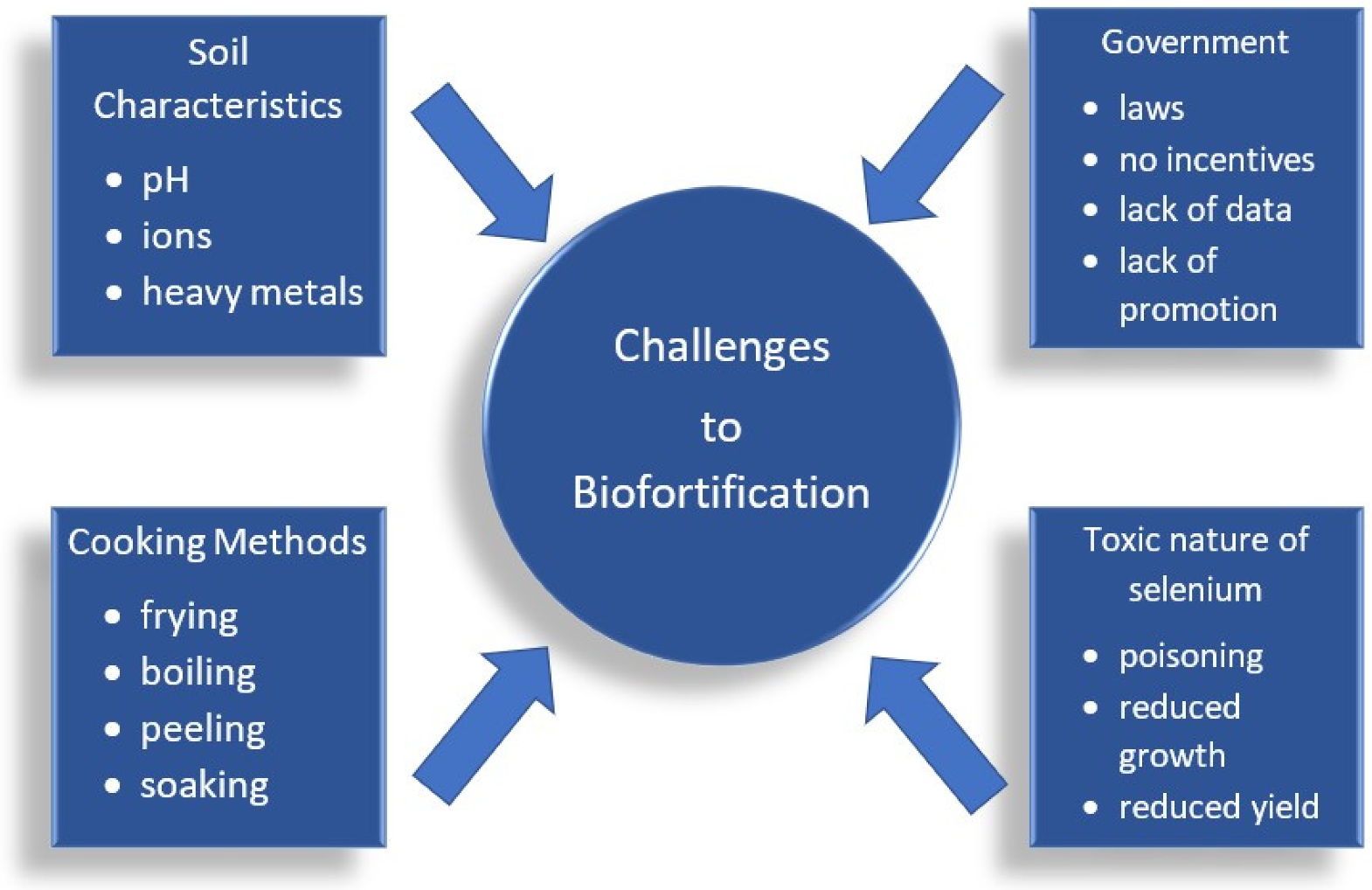
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danso, O.P.; Asante-Badu, B.; Zhang, Z.; Song, J.; Wang, Z.; Yin, X.; Zhu, R. Selenium Biofortification: Strategies, Progress and Challenges. Agriculture 2023, 13, 416. https://doi.org/10.3390/agriculture13020416
Danso OP, Asante-Badu B, Zhang Z, Song J, Wang Z, Yin X, Zhu R. Selenium Biofortification: Strategies, Progress and Challenges. Agriculture. 2023; 13(2):416. https://doi.org/10.3390/agriculture13020416
Chicago/Turabian StyleDanso, Ofori Prince, Bismark Asante-Badu, Zezhou Zhang, Jiaping Song, Zhangmin Wang, Xuebin Yin, and Renbin Zhu. 2023. "Selenium Biofortification: Strategies, Progress and Challenges" Agriculture 13, no. 2: 416. https://doi.org/10.3390/agriculture13020416
APA StyleDanso, O. P., Asante-Badu, B., Zhang, Z., Song, J., Wang, Z., Yin, X., & Zhu, R. (2023). Selenium Biofortification: Strategies, Progress and Challenges. Agriculture, 13(2), 416. https://doi.org/10.3390/agriculture13020416







