Potential of Fruits and Vegetable By-Products as an Alternative Feed Source for Sustainable Ruminant Nutrition and Production: A Review
Abstract
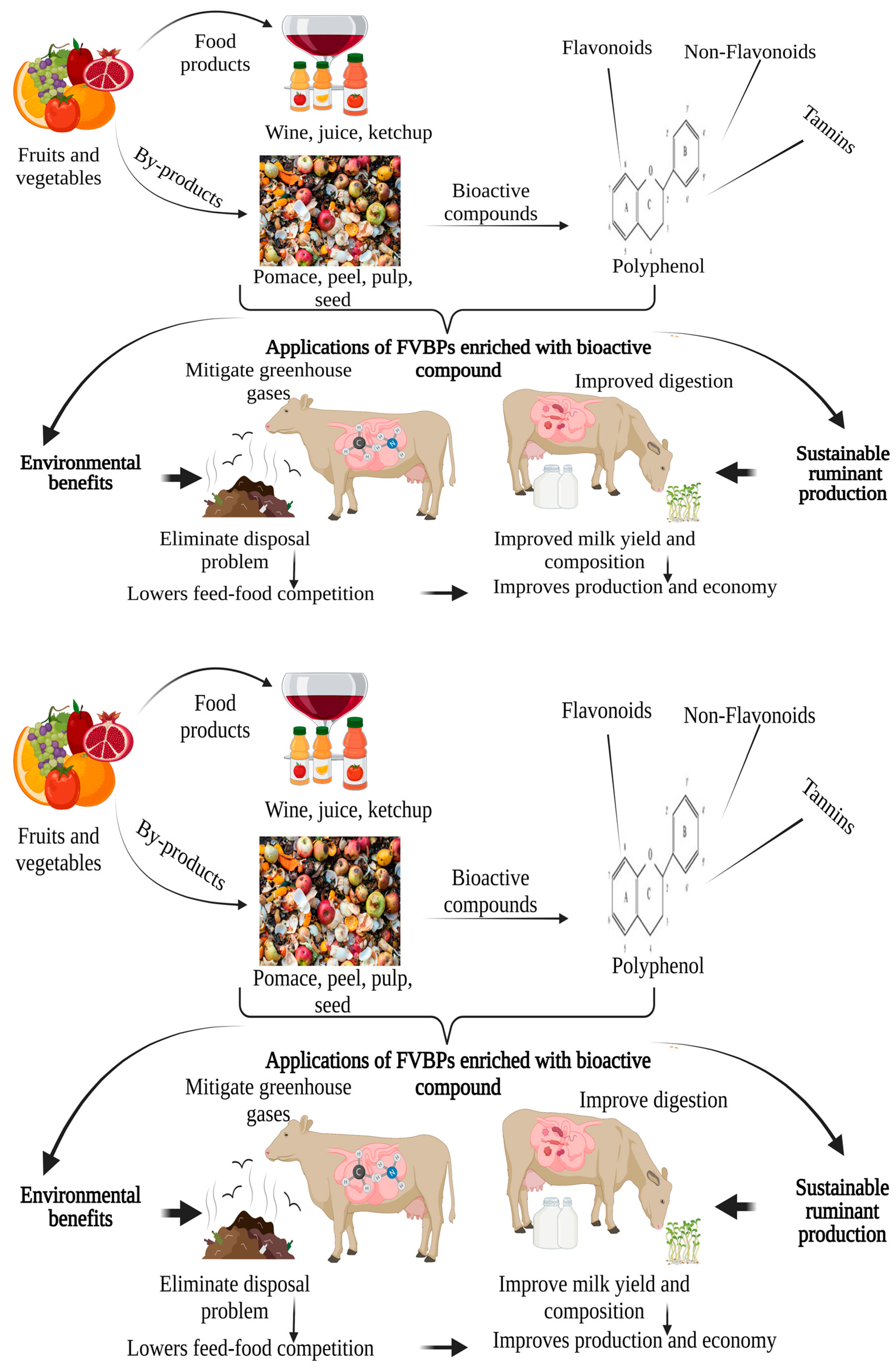
1. Introduction
2. Chemical Composition of FVBPs Used in Ruminant Feeding
| By-Products | DM | OM | CP | EE | NDF | ADF | Ash | References |
|---|---|---|---|---|---|---|---|---|
| Grape pomace | 439 | 918 | 95 | 85 | 474 | 440 | 82 | [44] |
| 950 | - | 119 | 73 | 376 | 317 | 89 | [45] | |
| 525 | 940 | 94 | 52 | 568 | 476 | - | [28] | |
| - | 811 | 138 | 32 | 243 | 193 | - | [29] | |
| Grape marc | 910 | 938 | 113 | 89 | 558 | 465 | - | [46] |
| 934 | - | 111 | 69 | 527 | 389 | 87 | [36] | |
| Grape seed | - | 960 | 116 | 52 | 682 | 584 | - | [1] |
| 974 | - | 93 | 109 | 539 | - | 27 | [30] | |
| Citrus pulp (orange) | 904 | 831 | 77 | 49 | 194 | 128 | 168 | [32] |
| - | 940 | 110 | 25 | 308 | 223 | - | [1] | |
| 937 | - | 50 | 26 | 230 | 162 | 90 | [31] | |
| Citrus pulp (lemon) | - | 957 | 76 | 77 | 247 | 171 | - | [1] |
| 872 | - | 66 | 32 | 209 | 164 | 51 | [47] | |
| 905 | - | 87 | 27 | 175 | 145 | 81 | [48] | |
| Citrus (clementine) | - | 972 | 73 | 20 | 139 | 96 | - | [1] |
| Tomato pomace | - | 952 | 191 | 100 | 552 | 462 | 48 | [37] |
| 851 | 966 | 194 | - | 500 | 340 | - | [49] | |
| 941 | 955 | 217 | 93 | 554 | 422 | - | [38] | |
| 926 | - | 157 | 62 | 616 | 507 | 44 | [36] | |
| Pomegranate pulp | 912 | - | 69 | 26 | 314 | 228 | 36 | [50] |
| 971 | 78 | 413 | [51] | |||||
| Pomegranate seed | 951 | - | 154 | 6 | 680 | 490 | 24 | [33] |
| 880 | 126 | 121 | 496 | 390 | 20 | [52] | ||
| Pomegranate peel | 875 | 943 | 35 | 18 | - | - | 56 | [53] |
| 961 | - | 36 | 6 | 208 | 151 | 54 | [33] | |
| Apple pomace | 973 | 982 | 77 | 18 | 306 | 244 | - | [34] |
| 921 | 981 | 67 | 37 | - | 442 | 354 | [35] | |
| - | 984 | 51 | 60 | 672 | 460 | - | [1] |
3. Bioactive Compounds of FVBPs Used in Ruminant Feeding
| Fruits and Vegetables | FVBPs | Bioactive Compounds | References |
|---|---|---|---|
| Grapes | Pomace | Flavanols, flavanols, anthocyanins, CT, catechin, epicatechin, gallic acids, and proanthocyanidin | [26,36,60,61] |
| Seeds | Anthocyanins, proanthocyanins, ferulic acids, caffeic, gallic acids, CT, and catechin | [26,30,62,63] | |
| Stalk | CT, flavanols, hydroxycinnamates, and flavanols | [64] | |
| Citrus fruits | Peel | Diosmin, narirutin, didymin, sinesetin, gallic acid, p-coumaric, hesperidin, catechins, ferulic acid epicatechins, quercetin, and proanthocyanidin | [23,65,66] |
| Tomato | Pomace | Naringenin, rutin, quercetin, and kaempferol | [36,37] |
| Pomegranate | Seed | Anthocyanins, HT, and flavonoids | [67] |
| Peel | Flavonoids, punicalagin, gallic acid, HT, and CT | [68,69] | |
| Pulp | Tannins | [70] | |
| Apple | Pomace (peels, core, seeds, stems) | Catechins, proanthocyanidins, hydroxycinnamates, flavonols, dihydrochalcones, anthocyanins, quercetin, and glycosides | [26,71] |
4. Effect of Feeding FVBPs on Ruminant’s Nutrition
4.1. Effects on Dry Matter Intake
4.2. Effects on Rumen Digestibility
| By-Products | Inclusion g/kg | Animals | DMD g/kg | OMD g/kg | CPD g/kg | NDFD g/kg | ADFD g/kg | References |
|---|---|---|---|---|---|---|---|---|
| Grape pomace | 762 | lambs | 453 | 510 | 345 | 343 | - | [28] |
| 20 | steers | 625 | 665 | 725 | 622 | 533 | [89] | |
| Citrus pulp | 90 | cows | 741 | - | 759 | 574 | - | [90] |
| 180 | cows | 754 | - | 765 | 576 | - | [90] | |
| 50 | calves | 667 | - | 698 | 546 | 476 | [91] | |
| 100 | calves | 654 | - | 696 | 541 | 462 | [91] | |
| 150 | calves | 653 | - | 691 | 531 | 459 | [91] | |
| 200 | calves | 652 | - | 690 | 525 | 451 | [91] | |
| 100 | lambs | 695 | 716 | 714 | 501 | 472 | [92] | |
| 200 | lambs | 691 | 713 | 706 | 495 | 470 | [92] | |
| 300 | lambs | 681 | 705 | 703 | 488 | 465 | [92] | |
| 400 | lambs | 678 | 704 | 692 | 471 | 461 | [92] | |
| Tomato pomace | 72 | cows | 667 | 680 | - | 397 | - | [93] |
| Pomegranate peel extract | 10 | cows | 566 | - | 601 | 414 | - | [94] |
| 20 | cows | 582 | - | 606 | 416 | - | [94] | |
| 40 | cows | 609 | - | 648 | 458 | - | [94] | |
| Pomegranate marc | 80 | lambs | 732.4 | 754 | 705.6 | 501.8 | 424.9 | [95] |
| 160 | lambs | 701.4 | 723.7 | 617.3 | 445.2 | 293.3 | [95] | |
| Pomegranate peel | 10 | lambs | 671 | 668 | 717 | - | - | [96] |
| 20 | lambs | 664 | 663 | 692 | - | - | [96] | |
| 40 | lambs | 653 | 661 | 683 | - | - | [96] | |
| Ensiled mixed apple and tomato pomace | 150 | cows | 665 | 703.6 | 662.5 | 590 | - | [97] |
| 300 | cows | 668 | 702.1 | 662.4 | 586 | - | [97] | |
| Apple pomace | 50 | cows | 525 | 576 | 638 | 458 | 415 | [98] |
| 100 | cows | 518 | 570 | 595 | 465 | 412 | [98] | |
| 200 | cows | 508 | 554 | 537 | 452 | 402 | [98] | |
| 50 | wethers | 631 | 580 | 746 | 422 | 401 | [79] | |
| 100 | wethers | 625 | 571 | 717 | 424 | 399 | [79] | |
| 200 | wethers | 618 | 566 | 698 | 437 | 409 | [79] |
4.3. Effects on Methane Production
4.4. Effect on Rumen Fermentation Parameters
| FVBPs | Species | Inclusion Level | Main Findings | References |
|---|---|---|---|---|
| Grape pomace | Sheep | 762 g/kg | Reduced NH3 concentration and pH values | [28] |
| Steer | 20 g/kg | Increased NH3 concentration, increased total volatile and propionate, reduced acetate-to-propionate ratio | [89] | |
| Grape seed | Sheep | 300 g/day | Increased NH3, increased rumenic acid and vaccenic acid, reduced linoleic and linolenic acids | [30] |
| Grape marc | Cows | 5 kg/day or 247 g/kg | Reduced NH3 concentration, increased acetic acid, reduced propionic acid, increased acetic-to-propionic ratio | [113] |
| Tomato silage | Goat | 850 g/kg | Reduced acetate-to-propionate ratio | [114] |
| Tomato pomace | Lambs | 50–150 g/kg | Reduced NH3–nitrogen concentration, increased acetate, propionate, butyrate, iso-butyrate and valerate concentrations, and higher total VFA concentration | [115] |
| Ensiled mixed tomato and apple pomace | Cows | 150–200 g/kg | Higher acetic and propionic concentrations, higher acetic-to-propionic ratio and total VFA concentration, lower ruminal pH | [97] |
| Citrus pulp | Ewe | 390 g/kg | Reduced rumen NH3 concentration, increased acetate-to-propionate ratio, reduced butyrate proportion | [116] |
| Ewe | 300 g/kg | Less in vitro NH3 production, low pH, reduced acetate-to-propionate ratio, improved total VFA yield | [117] | |
| Pomegranate peel extract | Cows | 400 ml/cow/day | Decreased NH3–nitrogen concentration, no effect on ruminal pH or the concentration of volatile fatty acids | [118] |
| Cows | 200 g/kg | Reduced NH3 concentration and pH | [119] | |
| Apple pulp | Cows | 250–750 g/kg | No effect on NH3–nitrogen concentration in the rumen or acetate-to-propionate ratio | [35] |
| Apple pomace | Cows | 200 g/kg | Increased acetic acid, decreased propionic concentration, reduced NH3 | [79] |
| Cows | 200 g/kg | Reduced NH3 concentration, and increased acetic acid | [98] |
5. Effect of Feeding FVBPs on Milk Production and Composition
| By-Products | Species | Inclusion Level of by-Products | Milk Yield | Fat | Protein | Lactose | Urea | References |
|---|---|---|---|---|---|---|---|---|
| Grape pomace | Sheep | 100g/day | ↑s | ↓s | ↓s | ns | ns | [36] |
| Sheep | 5 g/100g | ns | ns | ns | ↓s | - | [121] | |
| Sheep | 10 g/100g | ns | ns | ns | ↓s | - | [121] | |
| Cows | 150 g/kg | ns | ns | ns | ↑s | - | [125] | |
| Sheep | 10 g/kg | ns | ns | ns | ns | - | [46] | |
| Grape residue flour | Sheep | 20 g/kg | ns | ↑s | ns | ns | ns | [36] |
| Grape seed | Sheep | 300 g/day | ns | ns | ns | ns | ns | [123] |
| Citrus pulp | Cows | 90 g/kg | ns | ns | ns | ns | ns | [90] |
| Cows | 180 g/kg | ns | ns | ns | ns | ns | [90] | |
| Citrus pulp plus tomato pomace | Buffaloes | 100 g/kg | ↑s | ns | ↑s | ns | - | [24] |
| Tomato pomace | Sheep | 300 g/kg | ns | ns | ns | ↑s | - | [37] |
| Sheep | 100 g/day | ns | ↓s | ↓s | ns | ns | [36] | |
| Goats | 202 g/kg | ns | ↑s | ns | ns | - | [114] | |
| Mixed tomato and apple pomace | Cows | 150 g/kg | ↑s | ns | ns | - | - | [24] |
| Cows | 300 g/kg | ns | ns | ns | - | - | [24] | |
| Pomegranate seed pulp | Goats | 60 g/kg | ns | ↑s | ns | ns | - | [127] |
| Goats | 120 g/kg | ns | ↑s | ns | ↑s | - | [127] | |
| Pomegranate pulp | Sheep | 648 g/kg | ns | ns | ns | ns | ns | [50] |
| Pomegranate peel extract | Cows | 400 mL | ns | ns | ns | ns | - | [118] |
| Cows | 800 mL | ↑s | ns | ns | ns | - | [118] | |
| Cows | 1200 ml | ns | ns | ns | ns | - | [118] | |
| Pomegranate peel | Cows | 20 g/kg | ↑s | ↑s | ↑s | ↓s | - | [53] |
| Cows | 30 g/kg | ns | ns | ns | ns | - | [53] | |
| Cows | 40 g/kg | ↓s | ns | ns | ns | - | [53] | |
| Pomegranate pulp silage | Cows | 75 g/kg | ns | ns | ns | ns | - | [128] |
| Cows | 150 g/kg | ns | ns | ns | ns | - | [128] | |
| Apple pomace | Cows | 250 | ns | ns | ns | ↑s | - | [35] |
| Cows | 500 | ns | ns | ns | ↑s | - | [35] | |
| Cows | 750 | ↓ns | ns | ns | ↑s | - | [35] | |
| Cows | 4 kg/day | ↑s | ↑s | ↑s | - | - | [129] |
6. Merits and Demerits of Using FVBPs in Ruminant Feeding
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- García-Rodríguez, J.; Ranilla, M.J.; France, J.; Alaiz-Moretón, H.; Carro, M.D.; López, S. Chemical Composition, In Vitro Digestibility and Rumen Fermentation Kinetics of Agro-Industrial By-Products. Animals 2019, 9, 861. [Google Scholar] [CrossRef] [PubMed]
- Correddu, F.; Lunesu, M.F.; Buffa, G.; Atzori, A.S.; Nudda, A.; Battacone, G.; Pulina, G. Can Agro-Industrial By-Products Rich in Polyphenols Be Advantageously Used in the Feeding and Nutrition of Dairy Small Ruminants? Animals 2020, 10, 131. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-Industrial Wastes and Their Utilization Using Solid State Fermentation: A Review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Čolović, D.; Rakita, S.; Banjac, V.; Đuragić, O.; Čabarkapa, I. Plant Food By-Products as Feed: Characteristics, Possibilities, Environmental Benefits, and Negative Sides. Food Rev. Int. 2019, 35, 363–389. [Google Scholar] [CrossRef]
- Adhikari, B.K.; Barrington, S.; Martinez, J. Predicted Growth of World Urban Food Waste and Methane Production. Waste Manag. Res. 2006, 24, 421–433. [Google Scholar] [CrossRef] [PubMed]
- McClements, D.J. Future Foods: Is It Possible to Design a Healthier and More Sustainable Food Supply? Nutr. Bull. 2020, 45, 341–354. [Google Scholar] [CrossRef]
- Devendra, C.; Leng, R.A. Feed Resources for Animals in Asia: Issues, Strategies for Use, Intensification and Integration for Increased Productivity. Asian-Aust. J. Anim. Sci. 2011, 24, 303–321. [Google Scholar] [CrossRef]
- Akram, M.Z. Effect of Cabbage and Kohlrabi Leaf Silages on in Vitro and Classical Nutrient Digestibility in Akkaraman Rams as an Alternative Feed Source. Master’s Thesis, Niğde Ömer Halisdemir Üniversitesi/Fen Bilimleri Enstitüsü, Niğde, Turkey, 2020. [Google Scholar]
- Mahesh, M. Crop Residues for Sustainable Livestock Production. Adv. Dairy Res. 2014, 2, 1–2. [Google Scholar] [CrossRef]
- Röös, E.; Bajželj, B.; Smith, P.; Patel, M.; Little, D.; Garnett, T. Greedy or Needy? Land Use and Climate Impacts of Food in 2050 under Different Livestock Futures. Glob. Environ. Change 2017, 47, 1–12. [Google Scholar] [CrossRef]
- Saritha, M.; Arora, A. Lata Biological Pretreatment of Lignocellulosic Substrates for Enhanced Delignification and Enzymatic Digestibility. Indian J. Microbiol. 2012, 52, 122–130. [Google Scholar] [CrossRef]
- Halmemies-Beauchet-Filleau, A.; Rinne, M.; Lamminen, M.; Mapato, C.; Ampapon, T.; Wanapat, M.; Vanhatalo, A. Review: Alternative and Novel Feeds for Ruminants: Nutritive Value, Product Quality and Environmental Aspects. Animal 2018, 12, s295–s309. [Google Scholar] [CrossRef] [PubMed]
- Rakita, S.; Banjac, V.; Djuragic, O.; Cheli, F.; Pinotti, L. Soybean Molasses in Animal Nutrition. Animals 2021, 11, 514. [Google Scholar] [CrossRef]
- Cavallini, D.; Mammi, L.M.E.; Palmonari, A.; García-González, R.; Chapman, J.D.; McLean, D.J.; Formigoni, A. Effect of an Immunomodulatory Feed Additive in Mitigating the Stress Responses in Lactating Dairy Cows to a High Concentrate Diet Challenge. Animals 2022, 12, 2129. [Google Scholar] [CrossRef]
- Vastolo, A.; Calabrò, S.; Cutrignelli, M.I. A Review on the Use of Agro-Industrial CO-Products in Animals’ Diets. Ital. J. Anim. Sci. 2022, 21, 577–594. [Google Scholar] [CrossRef]
- Dilucia, F.; Lacivita, V.; Conte, A.; Del Nobile, M.A. Sustainable Use of Fruit and Vegetable By-Products to Enhance Food Packaging Performance. Foods 2020, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Maheri-Sis, N. Nutritive Value of Some AgroIndustrial By-Products for Ruminants—A Review. World J. Zool. 2008, 3, 40–46. [Google Scholar]
- Branciari, R.; Galarini, R.; Trabalza-Marinucci, M.; Miraglia, D.; Roila, R.; Acuti, G.; Giusepponi, D.; Dal Bosco, A.; Ranucci, D. Effects of Olive Mill Vegetation Water Phenol Metabolites Transferred to Muscle through Animal Diet on Rabbit Meat Microbial Quality. Sustainability 2021, 13, 4522. [Google Scholar] [CrossRef]
- Gerber, P.J.; Steinfeld, H.; Henderson, B.; Mottet, A.; Opio, C.; Dijkman, J.; Falcucci, A.; Tempio, G. Tackling Climate Change through Livestock: A Global Assessment of Emissions and Mitigation Opportunities; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2013. [Google Scholar]
- Henderson, B.; Falcucci, A.; Mottet, A.; Early, L.; Werner, B.; Steinfeld, H.; Gerber, P. Marginal Costs of Abating Greenhouse Gases in the Global Ruminant Livestock Sector. Mitig. Adapt. Strateg. Glob. Change 2017, 22, 199–224. [Google Scholar] [CrossRef]
- Yanza, Y.R.; Szumacher-Strabel, M.; Bryszak, M.; Gao, M.; Kolodziejski, P.; Stochmal, A.; Slusarczyk, S.; Patra, A.K.; Cieslak, A. Coleus Amboinicus (Lour.) Leaves as a Modulator of Ruminal Methanogenesis and Biohydrogenation in Vitro1. J. Anim. Sci. 2018, 96, 4868–4881. [Google Scholar] [CrossRef]
- Moate, P.J.; Williams, S.R.O.; Torok, V.A.; Hannah, M.C.; Ribaux, B.E.; Tavendale, M.H.; Eckard, R.J.; Jacobs, J.L.; Auldist, M.J.; Wales, W.J. Grape Marc Reduces Methane Emissions When Fed to Dairy Cows. J. Dairy Sci. 2014, 97, 5073–5087. [Google Scholar] [CrossRef]
- Tayengwa, T.; Mapiye, C. Citrus and Winery Wastes: Promising Dietary Supplements for Sustainable Ruminant Animal Nutrition, Health, Production, and Meat Quality. Sustainability 2018, 10, 3718. [Google Scholar] [CrossRef]
- Mahmoud Abdel Gawad, A.R.; Ahamed Hanafy, M.; Mohamed Mahmoud, A.E.; Hassan Al-Slibi, Y. Effect of Tomato Pomace, Citrus and Beet Pulp on Productive Performance and Milk Quality of Egyptian Buffaloes. Pak. J. Biol. Sci. 2020, 23, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Devi, S.; Gupta, C.; Jat, S.L.; Parmar, M.S. Crop Residue Recycling for Economic and Environmental Sustainability: The Case of India. Open Agric. 2017, 2, 486–494. [Google Scholar] [CrossRef]
- Salami, S.A.; Luciano, G.; O’Grady, M.N.; Biondi, L.; Newbold, C.J.; Kerry, J.P.; Priolo, A. Sustainability of Feeding Plant By-Products: A Review of the Implications for Ruminant Meat Production. Anim. Feed. Sci. Technol. 2019, 251, 37–55. [Google Scholar] [CrossRef]
- Palmonari, A.; Cavallini, D.; Sniffen, C.J.; Fernandes, L.; Holder, P.; Fusaro, I.; Giammarco, M.; Formigoni, A.; Mammi, L.M.E. In vitro evaluation of sugar digestibility in molasses. Ital. J. Anim. Sci. 2021, 20, 571–577. [Google Scholar] [CrossRef]
- Abarghuei, M.J.; Rouzbehan, Y.; Alipour, D. The Influence of the Grape Pomace on the Ruminal Parameters of Sheep. Livest. Sci. 2010, 132, 73–79. [Google Scholar] [CrossRef]
- Guerra-Rivas, C.; Gallardo, B.; Mantecón, Á.R.; del Álamo-Sanza, M.; Manso, T. Evaluation of Grape Pomace from Red Wine By-Product as Feed for Sheep. J. Sci. Food Agric. 2017, 97, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Correddu, F.; Nudda, A.; Battacone, G.; Boe, R.; Francesconi, A.H.D.; Pulina, G. Effects of Grape Seed Supplementation, Alone or Associated with Linseed, on Ruminal Metabolism in Sarda Dairy Sheep. Anim. Feed. Sci. Technol. 2015, 199, 61–72. [Google Scholar] [CrossRef]
- Castrica, M.; Rebucci, R.; Giromini, C.; Tretola, M.; Cattaneo, D.; Baldi, A. Total Phenolic Content and Antioxidant Capacity of Agri-Food Waste and by-Products. Ital. J. Anim. Sci. 2019, 18, 336–341. [Google Scholar] [CrossRef]
- Fegeros, K.; Zervas, G.; Stamouli, S.; Apostolaki, E. Nutritive Value of Dried Citrus Pulp and Its Effect on Milk Yield and Milk Composition of Lactating Ewes. J. Dairy Sci. 1995, 78, 1116–1121. [Google Scholar] [CrossRef]
- Mirzaei-Aghsaghali, A.; Maheri-Sis, N.; Mansouri, H.; Razeghi, M.E.; Mirza-Aghazadeh, A.; Cheraghi, H.; Aghajanzadeh-Golshani, A. ARPN Journal of Agricultural and Biological Science Evaluating Potential Nutritive Value of Pomegranate Processing By-Products for Ruminants Using In Vitro Gas Production Technique. ARPN J. Agric. Biol. Sci. 2011, 6, 45–51. [Google Scholar]
- Giller, K.; Bossut, L.; Eggerschwiler, L.; Terranova, M. In Vitro Ruminal Fermentation, Methane Production and Nutrient Degradability as Affected by Fruit and Vegetable Pomaces in Differing Concentrations. J. Anim. Physiol. Anim. Nutr. 2021, 106, 957–967. [Google Scholar] [CrossRef]
- Steyn, L.; Meeske, R.; Cruywagen, C.W. The Effect of Replacing Maize with Dried Apple Pomace in the Concentrate on Performance of Jersey Cows Grazing Kikuyu Pasture. Anim. Feed. Sci. Technol. 2018, 239, 85–93. [Google Scholar] [CrossRef]
- Nudda, A.; Buffa, G.; Atzori, A.S.; Cappai, M.G.; Caboni, P.; Fais, G.; Pulina, G. Small Amounts of Agro-Industrial Byproducts in Dairy Ewes Diets Affects Milk Production Traits and Hematological Parameters. Anim. Feed. Sci. Technol. 2019, 251, 76–85. [Google Scholar] [CrossRef]
- Abbeddou, S.; Rischkowsky, B.; Richter, E.K.; Hess, H.D.; Kreuzer, M. Modification of Milk Fatty Acid Composition by Feeding Forages and Agro-Industrial Byproducts from Dry Areas to Awassi Sheep. J. Dairy Sci. 2011, 94, 4657–4668. [Google Scholar] [CrossRef]
- Razzaghi, A.; Naserian, A.A.; Valizadeh, R.; Ebrahimi, S.H.; Khorrami, B.; Malekkhahi, M.; Khiaosa-ard, R. Pomegranate Seed Pulp, Pistachio Hulls, and Tomato Pomace as Replacement of Wheat Bran Increased Milk Conjugated Linoleic Acid Concentrations without Adverse Effects on Ruminal Fermentation and Performance of Saanen Dairy Goats. Anim. Feed. Sci. Technol. 2015, 210, 46–55. [Google Scholar] [CrossRef]
- Sol, C.; Castillejos, L.; López-Vergé, S.; Gasa, J. Prediction of the Digestibility and Energy Contents of Non-Conventional By-Products for Pigs from Their Chemical Composition and in Vitro Digestibility. Anim. Feed. Sci. Technol. 2017, 234, 237–243. [Google Scholar] [CrossRef]
- Abo-Zeid, H.M.; El-Zaiat, H.M.; Morsy, A.S.; Attia, M.F.A.; Abaza, M.A.; Sallam, S.M.A. Effects of Replacing Dietary Maize Grains with Increasing Levels of Sugar Beet Pulp on Rumen Fermentation Constituents and Performance of Growing Buffalo Calves. Anim. Feed. Sci. Technol. 2017, 234, 128–138. [Google Scholar] [CrossRef]
- Lu, S.; Chen, S.; Li, H.; Paengkoum, S.; Taethaisong, N.; Meethip, W.; Surakhunthod, J.; Sinpru, B.; Sroichak, T.; Archa, P.; et al. Sustainable Valorization of Tomato Pomace (Lycopersicon esculentum) in Animal Nutrition: A Review. Animals 2022, 12, 3294. [Google Scholar] [CrossRef]
- Marcos, C.N.; de Evan, T.; Molina-Alcaide, E.; Carro, M.D. Nutritive Value of Tomato Pomace for Ruminants and Its Influence on In Vitro Methane Production. Animals 2019, 9, 343. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, J.; Zhang, Y.; Wang, X.; Wang, R.; Li, J. Effects of tomato pomace fermentation feed on growth performance, milk composition and blood cell parameters for Xinjiang Brown cows. Xinjiang Agric. Sci. 2012, 49, 1546–1551. [Google Scholar]
- Ishida, K.; Kishi, Y.; Oishi, K.; Hirooka, H.; Kumagai, H. Effects of Feeding Polyphenol-Rich Winery Wastes on Digestibility, Nitrogen Utilization, Ruminal Fermentation, Antioxidant Status and Oxidative Stress in Wethers. Anim. Sci. J. 2015, 86, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Rivas, C.; Vieira, C.; Rubio, B.; Martínez, B.; Gallardo, B.; Mantecón, A.R.; Lavín, P.; Manso, T. Effects of Grape Pomace in Growing Lamb Diets Compared with Vitamin E and Grape Seed Extract on Meat Shelf Life. Meat Science 2016, 116, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Tsiplakou, E.; Zervas, G. The Effect of Dietary Inclusion of Olive Tree Leaves and Grape Marc on the Content of Conjugated Linoleic Acid and Vaccenic Acid in the Milk of Dairy Sheep and Goats. J. Dairy Res. 2008, 75, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Paya, H.; Taghizadeh, A.; Lashkari, S.; Shirmohammadi, S. Evaluation of rumen fermentation kinetics of some by-products using in situ and in vitro gas production technique. Slovak J. Anim. Sci. 2012, 45, 127–133. [Google Scholar]
- Lashkari, S.; Taghizadeh, A. Nutrient digestibility and evaluation of protein and carbohydrate fractionation of citrus by-products. J. Anim. Physiol. Anim. Nutr. 2013, 97, 701–709. [Google Scholar] [CrossRef]
- Shdaifat, M.M.; Al-Barakah, F.S.; Kanan, A.Q.; Obeidat, B.S. The Effect of Feeding Agricultural By-Products on Performance of Lactating Awassi Ewes. Small Rumin. Res. 2013, 113, 11–14. [Google Scholar] [CrossRef]
- Valenti, B.; Luciano, G.; Morbidini, L.; Rossetti, U.; Codini, M.; Avondo, M.; Priolo, A.; Bella, M.; Natalello, A.; Pauselli, M. Dietary Pomegranate Pulp: Effect on Ewe Milk Quality during Late Lactation. Animals 2019, 9, 283. [Google Scholar] [CrossRef]
- Eliyahu, D.; Shaani, Y.; Yosef, E.; Ben-Meir, Y.; Nikbachat, M.; Solomon, R.; Mabjeesh, S.J.; Weinberg, Z.G.; Miron, J. Effect of ensiling pomegranate pulp with solid additives on chemical composition, intake and digestibility by sheep. Small Rumin. Res. 2015, 131, 93–98. [Google Scholar] [CrossRef]
- Safari, M.; Ghasemi, E.; Alikhani, M.; Ansari-Mahyari, S. Supplementation effects of pomegranate by-products on oxidative status, metabolic profile, and performance in transition dairy cows. J. Dairy Sci. 2018, 101, 11297–11309. [Google Scholar] [CrossRef]
- Abbas, H.; Abd- EL kader, Y. Effect of Dietary Pomegranate Peel (Punica Granatum) Supplementation on Milk Production and Quality of Labneh of Friesian Dairy Cows. J. Anim. Poult. Prod. 2019, 10, 395–398. [Google Scholar] [CrossRef]
- Quideau, S.; De_eux, D.; Douat-Casassus, C.; Pouységu, L. Plant polyphenols: Chemical properties, biological activities, and synthesis. Angew. Chem. Int. Ed. 2011, 50, 586–621. [Google Scholar] [CrossRef]
- Bravo, L. Polyphenols: Chemistry, Dietary Sources, Metabolism, and Nutritional Significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef]
- Goel, G.; Makkar, H.P.S. Methane Mitigation from Ruminants Using Tannins and Saponins. Trop. Anim. Health Prod. 2012, 44, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Fernández, P.d.F.; Mantecón, Á.R.; Angulo, G.H.; García, F.J.G. Tannins and Ruminant Nutrition: Review. Span. J. Agric. Res. 2004, 2, 191–202. [Google Scholar]
- Kobayashi, Y.; Oh, S.; Myint, H.; Koike, S. Use of Asian Selected Agricultural Byproducts to Modulate Rumen Microbes and Fermentation. J. Anim. Sci. Biotechnol. 2016, 7, 70. [Google Scholar] [CrossRef]
- Kim, E.T.; Guan, L.L.; Lee, S.J.; Lee, S.M.; Lee, S.S.; Lee, I.D.; Lee, S.K.; Lee, S.S. Effects of Flavonoid-Rich Plant Extracts on In Vitro Ruminal Methanogenesis, Microbial Populations and Fermentation Characteristics. Asian-Aust. J. Anim. Sci. 2015, 28, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, F. Extraction of Phenolic Compounds from Red Grape Marc for Use as Food Lipid Antioxidants. Food Chem. 1999, 66, 209–215. [Google Scholar] [CrossRef]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638. [Google Scholar] [CrossRef]
- Spanghero, M.; Salem, A.Z.M.; Robinson, P.H. Chemical Composition, Including Secondary Metabolites, and Rumen Fermentability of Seeds and Pulp of Californian (USA) and Italian Grape Pomaces. Anim. Feed. Sci. Technol. 2009, 152, 243–255. [Google Scholar] [CrossRef]
- Lutterodt, H.; Slavin, M.; Whent, M.; Turner, E.; Yu, L. (Lucy) Fatty Acid Composition, Oxidative Stability, Antioxidant and Antiproliferative Properties of Selected Cold-Pressed Grape Seed Oils and Flours. Food Chem. 2011, 128, 391–399. [Google Scholar] [CrossRef]
- Alonso, Á.M.; Guillén, D.A.; Barroso, C.G.; Puertas, B.; García, A. Determination of Antioxidant Activity of Wine Byproducts and Its Correlation with Polyphenolic Content. J. Agric. Food Chem. 2002, 50, 5832–5836. [Google Scholar] [CrossRef] [PubMed]
- Santana-Méridas, O.; González-Coloma, A.; Sánchez-Vioque, R. Agricultural Residues as a Source of Bioactive Natural Products. Phytochem. Rev. 2012, 11, 447–466. [Google Scholar] [CrossRef]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of Polyphenolic Antioxidants from Orange Peel Waste Using Deep Eutectic Solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Elfalleh, W. Total Phenolic Contents and Antioxidant Activities of Pomegranate Peel, Seed, Leaf and Flower. J. Med. Plants Res. 2012, 6, 4724–4730. [Google Scholar] [CrossRef]
- Ambigaipalan, P.; de Camargo, A.C.; Shahidi, F. Phenolic Compounds of Pomegranate Byproducts (Outer Skin, Mesocarp, Divider Membrane) and Their Antioxidant Activities. J. Agric. Food Chem. 2016, 64, 6584–6604. [Google Scholar] [CrossRef]
- Gullon, B.; Pintado, M.E.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Assessment of Polyphenolic Profile and Antibacterial Activity of Pomegranate Peel (Punica Granatum) Flour Obtained from Co-Product of Juice Extraction. Food Control 2016, 59, 94–98. [Google Scholar] [CrossRef]
- Abarghuei, M.J.; Rouzbehan, Y.; Salem, A.Z.M.; Zamiri, M.J. Nitrogen Balance, Blood Metabolites and Milk Fatty Acid Composition of Dairy Cows Fed Pomegranate-Peel Extract. Livest. Sci. 2014, 164, 72–80. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Rigo, A.; Tonon, D.; Mattivi, F. Quantitation of Polyphenols in Different Apple Varieties. J. Agric. Food Chem. 2004, 52, 6532–6538. [Google Scholar] [CrossRef]
- Villarreal, M.; Cochran, R.C.; Rojas-Bourrillón, A.; Murillo, O.; Muñoz, H.; Poore, M. Effect of Supplementation with Pelleted Citrus Pulp on Digestibility and Intake in Beef Cattle Fed a Tropical Grass-Based Diet (Cynodon Nlemfuensis). Anim. Feed. Sci. Technol. 2006, 125, 163–173. [Google Scholar] [CrossRef]
- Jerónimo, E.; Alfaia, C.M.M.; Alves, S.P.; Dentinho, M.T.P.; Prates, J.A.M.; Vasta, V.; Santos-Silva, J.; Bessa, R.J.B. Effect of Dietary Grape Seed Extract and Cistus Ladanifer L. in Combination with Vegetable Oil Supplementation on Lamb Meat Quality. Meat Sci. 2012, 92, 841–847. [Google Scholar] [CrossRef]
- de Assis, A.J.; Campos, J.M.d.S.; Valadares Filho, S.d.C.; de Queiroz, A.C.; Lana, R.d.P.; Euclydes, R.F.; Mendes Neto, J.; Magalhães, A.L.R.; Mendonça, S.d.S. Citrus Pulp in Diets for Milking Cows. 1. Intake of Nutrients, Milk Production and Composition. R. Bras. Zootec. 2004, 33, 242–250. [Google Scholar] [CrossRef]
- Cribbs, J.T.; Bernhard, B.C.; Young, T.R.; Jennings, M.A.; Burdick Sanchez, N.C.; Carroll, J.A.; Callaway, T.R.; Schmidt, T.B.; Johnson, B.J.; Rathmann, R.J. Dehydrated Citrus Pulp Alters Feedlot Performance of Crossbred Heifers during the Receiving Period and Modulates Serum Metabolite Concentrations before and after an Endotoxin Challenge1. J. Anim. Sci. 2015, 93, 5791–5800. [Google Scholar] [CrossRef]
- Zhao, J.X.; Li, Q.; Zhang, R.X.; Liu, W.Z.; Ren, Y.S.; Zhang, C.X.; Zhang, J.X. Effect of Dietary Grape Pomace on Growth Performance, Meat Quality and Antioxidant Activity in Ram Lambs. Anim. Feed. Sci. Technol. 2018, 236, 76–85. [Google Scholar] [CrossRef]
- Wadhwa, M.; Bakshi, M.P.S.; Makkar, H.P.S. Utilization of Fruit and Vegetable Wastes as Livestock Feed and as Substrates for Generation of Other Value-Added Products. Rap Publ. 2013, 4, 67. [Google Scholar]
- Ghoreishi, S.; Rasoul, P.; Teimouri, A. Effects of Ensiled Apple Pomace on Milk Yield, Milk Composition and DM Intake of Holstein Dairy Cows. J. Anim. Vet. Adv. 2007, 6, 1074–1078. [Google Scholar]
- Fang, J.; Xia, G.; Cao, Y. Effects of Replacing Commercial Material with Apple Pomace on the Fermentation Quality of Total Mixed Ration Silage and Its Digestibility, Nitrogen Balance and Rumen Fermentation in Wethers. Grassl. Sci. 2020, 66, 124–131. [Google Scholar] [CrossRef]
- Belibasakis, N.G. The Effect of Dried Tomato Pomace on Milk Yield and Its Composition, and on Some Blood Plasma Biochemical Components in the Cow. World Rev. Anim. Prod. 1990, 25, 39–42. [Google Scholar]
- Jayanegara, A.; Palupi, E. Condensed Tannin Effects on Nitrogen Digestion in Ruminants: A Meta-Analysis from in Vitro and in Vivo Studies. Media Peternak. 2010, 33, 176. [Google Scholar] [CrossRef]
- Romero, M.J.; Madrid, J.; Hernández, F.; Cerón, J.J. Digestibility and Voluntary Intake of Vine Leaves (Vitis Vinifera L.) by Sheep. Small Rumin. Res. 2000, 38, 191–195. [Google Scholar] [CrossRef]
- Frutos, P.; Hervás, G.; Giráldez, F.J.; Mantecón, A.R. Review. Tannins and Ruminant Nutrition. Span. J. Agric. Res. 2004, 2, 191. [Google Scholar] [CrossRef]
- Sedighi-Vesagh, R.; Naserian, A.A.; Ghaffari, M.H.; Petit, H.V. Effects of Pistachio By-Products on Digestibility, Milk Production, Milk Fatty Acid Profile and Blood Metabolites in Saanen Dairy Goats. J. Anim. Physiol. Anim. Nutr. 2015, 99, 777–787. [Google Scholar] [CrossRef]
- Martín García, A.I.; Moumen, A.; Yáñez Ruiz, D.R.; Molina Alcaide, E. Chemical Composition and Nutrients Availability for Goats and Sheep of Two-Stage Olive Cake and Olive Leaves. Anim. Feed. Sci. Technol. 2003, 107, 61–74. [Google Scholar] [CrossRef]
- Lanza, M.; Scerra, M.; Bognanno, M.; Buccioni, A.; Cilione, C.; Biondi, L.; Priolo, A.; Luciano, G. Fatty Acid Metabolism in Lambs Fed Citrus Pulp1. J. Anim. Sci. 2015, 93, 3179–3188. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Cortés, P.; Guerra-Rivas, C.; Gallardo, B.; Lavín, P.; Mantecón, A.R.; de la Fuente, M.A.; Manso, T. Grape Pomace in Ewes Diet: Effects on Meat Quality and the Fatty Acid Profile of Their Suckling Lambs. Food Res. Int. 2018, 113, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Mapiye, C.; Vahmani, P.; Aalhus, J.L.; Rolland, D.C.; Baron, V.S.; Block, H.C.; Uttaro, B.; Dugan, M.E.R.; McAllister, T.A. Fatty Acid Composition of Beef Steers as Affected by Diet and Fat Depot. S. Afr. J. Anim. Sci. 2015, 45, 386–394. [Google Scholar] [CrossRef]
- Foiklang, S.; Wanapat, M.; Norrapoke, T. Effect of Grape Pomace Powder, Mangosteen Peel Powder and Monensin on Nutrient Digestibility, Rumen Fermentation, Nitrogen Balance and Microbial Protein Synthesis in Dairy Steers. Asian-Aust. J. Anim. Sci. 2016, 29, 1416–1423. [Google Scholar] [CrossRef]
- Santos, G.T.; Lima, L.S.; Schogor, A.L.B.; Romero, J.V.; De Marchi, F.E.; Grande, P.A.; Santos, N.W.; Santos, F.S.; Kazama, R. Citrus Pulp as a Dietary Source of Antioxidants for Lactating Holstein Cows Fed Highly Polyunsaturated Fatty Acid Diets. Asian-Aust. J. Anim. Sci. 2014, 27, 1104–1113. [Google Scholar] [CrossRef]
- Muhammad, Z.J.; Muhammad, S.; Shoukat, A.B.; Muhammad, Q.B.; Fayyaz, A.; Fawwad, A.; Muhammad, S.-u.R.; Muhammad, T. Nutrient Intake, Nitrogen Balance and Growth Performance in Buffalo Calves Fed Citrus Pulp as a Concentrate Source. Afr. J. Agric. Res. 2016, 11, 2562–2568. [Google Scholar] [CrossRef]
- Sharif, M.; Ashraf, M.S.; Mushtaq, N.; Nawaz, H.; Mustafa, M.I.; Ahmad, F.; Younas, M.; Javaid, A. Influence of Varying Levels of Dried Citrus Pulp on Nutrient Intake, Growth Performance and Economic Efficiency in Lambs. J. Appl. Anim. Res. 2018, 46, 264–268. [Google Scholar] [CrossRef]
- Weiss, W.P.; Frobose, D.L.; Koch, M.E. Wet Tomato Pomace Ensiled with Corn Plants for Dairy Cows. J. Dairy Sci. 1997, 80, 2896–2900. [Google Scholar] [CrossRef] [PubMed]
- Jami, E.; Shabtay, A.; Nikbachat, M.; Yosef, E.; Miron, J.; Mizrahi, I. Effects of Adding a Concentrated Pomegranate-Residue Extract to the Ration of Lactating Cows on in Vivo Digestibility and Profile of Rumen Bacterial Population. J. Dairy Sci. 2012, 95, 5996–6005. [Google Scholar] [CrossRef]
- Hatami, A.; Alipour, D.; Hozhabri, F.; Tabatabaei, M. Effect of Different Levels of Pomegranate Marc with or without Polyethylene Glycol on Performance, Nutrients Digestibility and Protozoal Population in Growing Lambs. Anim. Feed. Sci. Technol. 2018, 235, 15–22. [Google Scholar] [CrossRef]
- Sadq, S.M.; Ramzi, D.O.M.; Hamasalim, H.J.; Ahmed, K.A. Growth Performance and Digestibility in Karadi Lambs Receiving Different Levels of Pomegranate Peels. OJAS 2016, 6, 16–23. [Google Scholar] [CrossRef]
- Abdollahzadeh, F.; Pirmohammadi, R.; Farhoomand, P.; Fatehi, F.; Pazhoh, F.F. The Effect of Ensiled Mixed Tomato and Apple Pomace on Holstein Dairy Cow. Ital J Animal Sci 2010, 9, e41. [Google Scholar] [CrossRef]
- Fang, J.; Cao, Y.; Matsuzaki, M.; Suzuki, H. Effects of Apple Pomace Proportion Levels on the Fermentation Quality of Total Mixed Ration Silage and Its Digestibility, Preference and Ruminal Fermentation in Beef Cows. Anim. Sci. J. 2016, 87, 217–223. [Google Scholar] [CrossRef]
- Opio, C.; Gerber, P.; Mottet, A.; Falcucci, A.; Tempio, G.; MacLeod, M.; Vellinga, T.; Henderson, B.; Steinfeld, H. Greenhouse Gas Emissions from Ruminant Supply Chains—A Global Life Cycle Assessment; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Montes, F.; Meinen, R.; Dell, C.; Rotz, A.; Hristov, A.N.; Oh, J.; Waghorn, G.; Gerber, P.J.; Henderson, B.; Makkar, H.P.S.; et al. SPECIAL TOPICS—Mitigation of Methane and Nitrous Oxide Emissions from Animal Operations: II. A Review of Manure Management Mitigation Options1. J. Anim. Sci. 2013, 91, 5070–5094. [Google Scholar] [CrossRef]
- Kamra, D.N.; Pawar, M.; Singh, B. Effect of Plant Secondary Metabolites on Rumen Methanogens and Methane Emissions by Ruminants. In Dietary Phytochemicals and Microbes; Patra, A.K., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2012; pp. 351–370. ISBN 978-94-007-3926-0. [Google Scholar]
- Patra, A.K.; Min, B.-R.; Saxena, J. Dietary Tannins on Microbial Ecology of the Gastrointestinal Tract in Ruminants. In Dietary Phytochemicals and Microbes; Patra, A.K., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 2012; pp. 237–262. ISBN 978-94-007-3926-0. [Google Scholar]
- Vasta, V.; Daghio, M.; Cappucci, A.; Buccioni, A.; Serra, A.; Viti, C.; Mele, M. Invited Review: Plant Polyphenols and Rumen Microbiota Responsible for Fatty Acid Biohydrogenation, Fiber Digestion, and Methane Emission: Experimental Evidence and Methodological Approaches. J. Dairy Sci. 2019, 102, 3781–3804. [Google Scholar] [CrossRef]
- Tavendale, M.H.; Meagher, L.P.; Pacheco, D.; Walker, N.; Attwood, G.T.; Sivakumaran, S. Methane Production from in Vitro Rumen Incubations with Lotus Pedunculatus and Medicago Sativa, and Effects of Extractable Condensed Tannin Fractions on Methanogenesis. Anim. Feed. Sci. Technol. 2005, 123–124, 403–419. [Google Scholar] [CrossRef]
- Rochfort, S.; Parker, A.J.; Dunshea, F.R. Plant Bioactives for Ruminant Health and Productivity. Phytochemistry 2008, 69, 299–322. [Google Scholar] [CrossRef]
- Khiaosa-ard, R.; Metzler-Zebeli, B.U.; Ahmed, S.; Muro-Reyes, A.; Deckardt, K.; Chizzola, R.; Böhm, J.; Zebeli, Q. Fortification of Dried Distillers Grains plus Solubles with Grape Seed Meal in the Diet Modulates Methane Mitigation and Rumen Microbiota in Rusitec. J. Dairy Sci. 2015, 98, 2611–2626. [Google Scholar] [CrossRef]
- Cobellis, G.; Trabalza-Marinucci, M.; Yu, Z. Critical Evaluation of Essential Oils as Rumen Modifiers in Ruminant Nutrition: A Review. Sci. Total Environ. 2016, 545–546, 556–568. [Google Scholar] [CrossRef]
- Ohene-Adjei, S.; Chaves, A.V.; McAllister, T.A.; Benchaar, C.; Teather, R.M.; Forster, R.J. Evidence of Increased Diversity of Methanogenic Archaea with Plant Extract Supplementation. Microb. Ecol. 2008, 56, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Patra, A.K.; Saxena, J. Exploitation of Dietary Tannins to Improve Rumen Metabolism and Ruminant Nutrition. J. Sci. Food Agric. 2011, 91, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Toral, P.G.; Hervás, G.; Bichi, E.; Belenguer, Á.; Frutos, P. Tannins as Feed Additives to Modulate Ruminal Biohydrogenation: Effects on Animal Performance, Milk Fatty Acid Composition and Ruminal Fermentation in Dairy Ewes Fed a Diet Containing Sunflower Oil. Anim. Feed. Sci. Technol. 2011, 164, 199–206. [Google Scholar] [CrossRef]
- Bodas, R.; Prieto, N.; García-González, R.; Andrés, S.; Giráldez, F.J.; López, S. Manipulation of Rumen Fermentation and Methane Production with Plant Secondary Metabolites. Anim. Feed. Sci. Technol. 2012, 176, 78–93. [Google Scholar] [CrossRef]
- Francisco, A.; Alves, S.P.; Portugal, P.V.; Dentinho, M.T.; Jerónimo, E.; Sengo, S.; Almeida, J.; Bressan, M.C.; Pires, V.M.R.; Alfaia, C.M.; et al. Effects of Dietary Inclusion of Citrus Pulp and Rockrose Soft Stems and Leaves on Lamb Meat Quality and Fatty Acid Composition. Animal 2018, 12, 872–881. [Google Scholar] [CrossRef]
- Moate, P.J.; Jacobs, J.L.; Hixson, J.L.; Deighton, M.H.; Hannah, M.C.; Morris, G.L.; Ribaux, B.E.; Wales, W.J.; Williams, S.R.O. Effects of Feeding Either Red or White Grape Marc on Milk Production and Methane Emissions from Early-Lactation Dairy Cows. Animals 2020, 10, 976. [Google Scholar] [CrossRef] [PubMed]
- Arco-Pérez, A.; Ramos-Morales, E.; Yáñez-Ruiz, D.R.; Abecia, L.; Martín-García, A.I. Nutritive Evaluation and Milk Quality of Including of Tomato or Olive By-Products Silages with Sunflower Oil in the Diet of Dairy Goats. Anim. Feed. Sci. Technol. 2017, 232, 57–70. [Google Scholar] [CrossRef]
- Omer, H.A.A.; Abdel-Magid, S.S. Incorporation of dried tomato pomace in growing sheep rations. Glob. Vet. 2015, 14, 1–6. [Google Scholar]
- Piquer, O.; Ródenas, L.; Casado, C.; Blas, E.; Pascual, J.J. Whole Citrus Fruits as an Alternative to Wheat Grain or Citrus Pulp in Sheep Diet: Effect on the Evolution of Ruminal Parameters. Small Rumin. Res. 2009, 83, 14–21. [Google Scholar] [CrossRef]
- Sparkes, J.L.; Chaves, A.V.; Fung, Y.T.E.; van Ekris, I.; Bush, R.D. Effects of Replacing Lucerne (Medicago Sativa L.) Hay with Fresh Citrus Pulp on Ruminal Fermentation and Ewe Performance. Asian-Aust. J. Anim. Sci. 2009, 23, 197–204. [Google Scholar] [CrossRef]
- Abarghuei, M.J.; Rouzbehan, Y.; Salem, A.Z.M.; Zamiri, M.J. Nutrient Digestion, Ruminal Fermentation and Performance of Dairy Cows Fed Pomegranate Peel Extract. Livest. Sci. 2013, 157, 452–461. [Google Scholar] [CrossRef]
- Elmorsy, A.M.; Shoukry, M.M.; Soliman, S.M.; Soliman, M.M. Influence of Using Pomegranate Peel Silage in Rations of Dairy Cows on Their Productive Performance; In Review: Houston, TX, USA, 2022. [Google Scholar]
- Romero-Huelva, M.; Martín-García, A.I.; Nogales, R.; Molina-Alcaide, E. The Effects of Feed Blocks Containing Tomato and Cucumber By-Products on in Vitro Ruminal Fermentation, Microbiota, and Methane Production. J. Anim. Feed Sci. 2013, 22, 229–237. [Google Scholar] [CrossRef]
- Manso, T.; Gallardo, B.; Salvá, A.; Guerra-Rivas, C.; Mantecón, A.R.; Lavín, P.; de la Fuente, M.A. Influence of Dietary Grape Pomace Combined with Linseed Oil on Fatty Acid Profile and Milk Composition. J. Dairy Sci. 2016, 99, 1111–1120. [Google Scholar] [CrossRef]
- Alba, D.F.; Campigotto, G.; Cazarotto, C.J.; dos Santos, D.S.; Gebert, R.R.; Reis, J.H.; Souza, C.F.; Baldissera, M.D.; Gindri, A.L.; Kempka, A.P.; et al. Use of Grape Residue Flour in Lactating Dairy Sheep in Heat Stress: Effects on Health, Milk Production and Quality. J. Therm. Biol. 2019, 82, 197–205. [Google Scholar] [CrossRef]
- Nudda, A.; Correddu, F.; Marzano, A.; Battacone, G.; Nicolussi, P.; Bonelli, P.; Pulina, G. Effects of Diets Containing Grape Seed, Linseed, or Both on Milk Production Traits, Liver and Kidney Activities, and Immunity of Lactating Dairy Ewes. J. Dairy Sci. 2015, 98, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Abbeddou, S.; Rischkowsky, B.; Hilali, M.E.-D.; Haylani, M.; Hess, H.D.; Kreuzer, M. Supplementing Diets of Awassi Ewes with Olive Cake and Tomato Pomace: On-Farm Recovery of Effects on Yield, Composition and Fatty Acid Profile of the Milk. Trop. Anim. Health Prod. 2015, 47, 145–152. [Google Scholar] [CrossRef]
- Chedea, V.S.; Pelmus, R.S.; Lazar, C.; Pistol, G.C.; Calin, L.G.; Toma, S.M.; Dragomir, C.; Taranu, I. Effects of a Diet Containing Dried Grape Pomace on Blood Metabolites and Milk Composition of Dairy Cows. J. Sci. Food Agric. 2017, 97, 2516–2523. [Google Scholar] [CrossRef]
- Ebeid, H.M.; Gawad, R.M.A.; Mahmoud, A.E.M. Influence of Ration Containing Tomato Pomace Silage on Performance of Lactating Buffaloes and Milk Quality. Asian J. Anim. Vet. Adv. 2015, 10, 14–24. [Google Scholar] [CrossRef]
- Modaresi, J.; Fathi Nasri, M.H.; Rashidi, L.; Dayani, O.; Kebreab, E. Short Communication: Effects of Supplementation with Pomegranate Seed Pulp on Concentrations of Conjugated Linoleic Acid and Punicic Acid in Goat Milk. J. Dairy Sci. 2011, 94, 4075–4080. [Google Scholar] [CrossRef]
- Kotsampasi, Β.; Christodoulou, C.; Tsiplakou, E.; Mavrommatis, A.; Mitsiopoulou, C.; Karaiskou, C.; Dotas, V.; Robinson, P.H.; Bampidis, V.A.; Christodoulou, V.; et al. Effects of Dietary Pomegranate Pulp Silage Supplementation on Milk Yield and Composition, Milk Fatty Acid Profile and Blood Plasma Antioxidant Status of Lactating Dairy Cows. Anim. Feed. Sci. Technol. 2017, 234, 228–236. [Google Scholar] [CrossRef]
- Edwards, N.J.; Parker, W.J. Apple promace as a supplement to pasture for dairy cows in late lactation. In Proceedings-New Zealand Society of Animal Production; New Zealand Society of Animal Prod Publication: Wellington, New Zealand, 1995; Volume 55, p. 67. [Google Scholar]
- Manju Wadhwa, M.W.; Bakshi, M.P.S.; Makkar, H.P.S. Waste to Worth: Fruit Wastes and by-Products as Animal Feed. CABI Rev. 2015, 2015, 1–26. [Google Scholar] [CrossRef]
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant Activity of Citrus Fruits. Food Chem. 2016, 196, 885–896. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, I.; Cavallini, D.; Giammarco, M.; Manetta, A.C.; Martuscelli, M.; Mammi, L.M.E.; Lanzoni, L.; Formigoni, A.; Vignola, G. Oxidative Status of Marchigiana Beef Enriched in n-3 Fatty Acids and Vitamin E, Treated with a Blend of Oregano and Rosemary Essential Oils. Front. Vet. Sci. 2021, 8, 662079. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, I.; Cavallini, D.; Giammarco, M.; Serio, A.; Mammi, L.M.E.; de Matos Vettori, J.; Lanzoni, L.; Formigoni, A.; Vignola, G. Effect of Diet and Essential Oils on the Fatty Acid Composition, Oxidative Stability and Microbiological Profile of Marchigiana Burgers. Antioxidants 2022, 11, 827. [Google Scholar] [CrossRef] [PubMed]
- Mordenti, A.L.; Giaretta, E.; Campidonico, L.; Parazza, P.; Formigoni, A. A Review Regarding the Use of Molasses in Animal Nutrition. Animals 2021, 11, 115. [Google Scholar] [CrossRef]
- Mordenti, A.L.; Brogna, N.; Formigoni, A. The link between feeding dairy cows and Parmigiano-Reggiano cheese production area. Prof. Anim. Sci. 2017, 33, 520–529. [Google Scholar] [CrossRef]
- Colombino, E.; Ferrocino, I.; Biasato, I.; Cocolin, L.S.; Prieto-Botella, D.; Zduńczyk, Z.; Jankowski, J.; Milala, J.; Kosmala, M.; Fotschki, B.; et al. Dried fruit pomace inclusion in poultry diet: Growth performance, intestinal morphology and physiology. J. Anim. Sci. Biotechnol. 2020, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Walker, P. Food Residuals: Waste Product, By-Product, or Coproduct. In Food Waste to Animal Feed; John Wiley & Sons, Ltd: Hoboken, NJ, USA, 2000; pp. 17–30. ISBN 978-0-470-29021-7. [Google Scholar]
- García, A.J.; Esteban, M.B.; Márquez, M.C.; Ramos, P. Biodegradable Municipal Solid Waste: Characterization and Potential Use as Animal Feedstuffs. Waste Manag. 2005, 25, 780–787. [Google Scholar] [CrossRef]
- Bistanji, G.; Hamadeh, S.; Hassan, S.H.; Tami, F.; Tannous, R. The potential of agro-industrial byproducts as feeds for livestock in Lebanon. Livest. Res. Rural. Dev. 2000, 12, 1–6. [Google Scholar]
- Sharma, K.; Mahato, N.; Cho, M.H.; Lee, Y.R. Converting Citrus Wastes into Value-Added Products: Economic and Environmently Friendly Approaches. Nutrition 2017, 34, 29–46. [Google Scholar] [CrossRef]
- Chikwanha, O.C.; Raffrenato, E.; Opara, U.L.; Fawole, O.A.; Setati, M.E.; Muchenje, V.; Mapiye, C. Impact of Dehydration on Retention of Bioactive Profile and Biological Activities of Different Grape (Vitis Vinifera L.) Pomace Varieties. Anim. Feed. Sci. Technol. 2018, 244, 116–127. [Google Scholar] [CrossRef]
- Sargın, H.; Denek, N. Effect of adding different levels of dried molasses sugar beet pulp on the silage quality and in vitro digestibility of wet tomato pomace silage. Harran Univ. J. Fac. Vet. Med. 2017, 6, 84–89. [Google Scholar] [CrossRef]
- Mammi, L.M.E.; Buonaiuto, G.; Ghiaccio, F.; Cavallini, D.; Palmonari, A.; Fusaro, I.; Massa, V.; Giorgino, A.; Formigoni, A. Combined Inclusion of Former Foodstuff and Distiller Grains in Dairy Cows Ration: Effect on Milk Production, Rumen Environment and Fiber Digestibility. Animals 2022, 12, 3519. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalal, H.; Giammarco, M.; Lanzoni, L.; Akram, M.Z.; Mammi, L.M.E.; Vignola, G.; Chincarini, M.; Formigoni, A.; Fusaro, I. Potential of Fruits and Vegetable By-Products as an Alternative Feed Source for Sustainable Ruminant Nutrition and Production: A Review. Agriculture 2023, 13, 286. https://doi.org/10.3390/agriculture13020286
Jalal H, Giammarco M, Lanzoni L, Akram MZ, Mammi LME, Vignola G, Chincarini M, Formigoni A, Fusaro I. Potential of Fruits and Vegetable By-Products as an Alternative Feed Source for Sustainable Ruminant Nutrition and Production: A Review. Agriculture. 2023; 13(2):286. https://doi.org/10.3390/agriculture13020286
Chicago/Turabian StyleJalal, Hassan, Melania Giammarco, Lydia Lanzoni, Muhammad Zeeshan Akram, Ludovica M. E. Mammi, Giorgio Vignola, Matteo Chincarini, Andrea Formigoni, and Isa Fusaro. 2023. "Potential of Fruits and Vegetable By-Products as an Alternative Feed Source for Sustainable Ruminant Nutrition and Production: A Review" Agriculture 13, no. 2: 286. https://doi.org/10.3390/agriculture13020286
APA StyleJalal, H., Giammarco, M., Lanzoni, L., Akram, M. Z., Mammi, L. M. E., Vignola, G., Chincarini, M., Formigoni, A., & Fusaro, I. (2023). Potential of Fruits and Vegetable By-Products as an Alternative Feed Source for Sustainable Ruminant Nutrition and Production: A Review. Agriculture, 13(2), 286. https://doi.org/10.3390/agriculture13020286








