Abstract
The Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae) is an insect defoliator of the potato, Solanum tuberosum L. This species thrives in agricultural environments because of its flexible and complex life history, as well as its ability to evolve insecticide resistance. As a result, it has become a widely distributed agricultural pest. Ledprona (trade name Calantha) is a recently developed novel double-stranded RNA (dsRNA) insecticide that controls populations of Colorado potato beetle through RNA interference (RNAi). Previous studies have demonstrated the efficacy of ledprona through laboratory, greenhouse, and field studies. Colorado potato beetles from geographically distinct populations are known to vary in their response to insecticides, including experimental compounds based on RNAi. We tested the mortality and foliage consumption of beetles from different areas in the US treated with ledprona and found significant variation in both parameters. The beetles originating from New York were significantly less susceptible to ledprona in leaf disc assays compared to other populations. However, currently there is no evidence of reduced performance of ledprona against that population under field conditions, possibly because intoxicated beetles cannot withstand multiple stressors present in the field. The results of this study confirmed that the ledprona efficacy differs among geographically distinct populations, which may have implications for managing Colorado potato beetles.
1. Introduction
The cultivated potato, Solanum tuberosum L., is an important global food crop that is preceded only by rice, wheat, and corn in terms of consumption. Reductions in the quantity and quality of its tubers can seriously jeopardize human food security [1]. The Colorado potato beetle, Leptinotarsa decemlineata (Say), has been a very damaging insect pest of the potato for more than 100 years. Its range has expanded dramatically since the species’ first description, and it has become a common agricultural pest in most potato-growing areas of the world.
The Colorado potato beetle has a complex and flexible life history that has allowed the species to expand its geographic distribution and thrive in agricultural environments [2,3]. Furthermore, it is rather notorious for its ability to rapidly evolve resistance to a wide variety of insecticides [4,5]. In newly colonized areas, populations of Colorado potato beetle often experience the founder effect, resulting in reduced genetic diversity compared to the population of their origin [5]. Gene flow and genetic dissimilarities among North American populations of this species are not fully understood. However, previous studies have demonstrated that Colorado potato beetle populations are often both geographically and genetically distinct (see [5] for a review). This isolation has led to variation in insecticide resistance [6,7,8] and, in some cases, in overall fitness [9,10]. Understanding geographic variation in insecticide sensitivity can help with customizing management protocols to achieve good crop protection and delay the evolution of insecticide resistance in each location.
RNA interference (RNAi) is a novel pest control technology that aims to knock down or silence the function of a target gene through the ingestion of double-stranded RNA (dsRNA) molecules into a target organism [11]. Ledprona is a dsRNA insecticide for foliar application that is being developed for suppressing Colorado potato beetle in potatoes. It compromises the synthesis of proteasome proteins by targeting its subunit beta 5 messenger RNA [12]. Reducing the synthesis of proteasome proteins likely results in the loss of the beetle’s ability to remove damaged proteins, which, in turn, causes the increase in poly-ubiquitinated protein in its cells. The intoxicated beetles stop feeding and eventually expire from this toxic buildup. Previously published results have demonstrated that ledprona provides good control against Colorado potato beetles both by increasing beetle mortality and by decreasing feeding damage [12,13,14].
RNAi efficacy has been demonstrated to differ among geographically isolated insect populations [15,16]. In particular, Mehlhorn et al. [16] have shown significant differences in responsiveness to dsRNA among geographically isolated populations of Colorado potato beetles in Europe. In the present study, we sought to compare the effects of ledprona on feeding and mortality in populations of the same species originating from different areas in the United States of America (US).
2. Materials and Methods
2.1. 2020 Experiment
During the 2020 field season, Colorado potato beetle eggs were obtained from untreated potato plots on research farms in New York, Oregon, Maine, Washington, and Wisconsin and sent to the University of Maine campus at Orono. Between 150 and 200 egg masses were collected from each location. Upon receipt, they were maintained until hatching in environmental chambers (Series 33 Controlled Environment Chamber, Percival Scientific Inc., Perry, IA, USA) at 24 ± 1 °C and 16 L: 8 D photoperiod. The larvae were then moved onto potted potato plants kept in wooden cages with mesh sides and roofs [17] and raised to the second instar. Each colony was kept in its own cage isolated from the other colonies. Ten second instars from each colony were collected and placed into Petri dishes (90 by 15 mm) lined with a damp paper towel on the bottom and kept in an environmental chamber at 24 ± 1 °C.
The larvae were then provided with a cut potato leaf treated with distilled water, a low concentration (2.40 × 10−7 g/L), or a high concentration (4.75 × 10−5 g/L) of ledprona. The treatments followed the previously published results of a variable dose bioassay conducted with second instars from the Presque Isle potato beetle colony [12]. The low concentration was the lethal concentration required to kill 50% of larvae (LC50) after eight days and the high concentration was the lethal concentration required to kill 90% of larvae (LC90) after eight days. Petri dish arrangement followed randomized complete block design. The experiment was replicated five times.
2.2. 2021 Experiment
During the 2021 field season, the experiment was repeated with minor modifications. Adult beetles were collected from untreated field plots on research farms in New York, Oregon, Maine, Virginia, and Minnesota, and sent to the University of Maine. Approximately 100 beetles were obtained from each location. The adults were then kept on caged potato plants as described above ensuring that each colony was independent from the other colonies. All the egg masses that they laid were collected and incubated in environmental chambers at 24 ± 1 °C with a 16 L: 8 D photoperiod. The hatched larvae from each colony were kept isolated from the other colonies, reared to second instar, and moved to Petri dishes following the protocol used in 2020.
During 2021, the second instars were provided with a cut potato leaf treated with distilled water, a low concentration (5.56 × 10−7 g/L), or a high concentration (6.62 × 10−1 g/L) of ledprona. The new concentrations were the LC50 and LC90 after nine days based on the results of a variable dose bioassay performed on second instars from the Colorado potato beetle colony maintained at the University of Maine [13]. The Petri dishes were arranged in a randomized complete block design. The experiment was replicated five times.
2.3. Data Collection
The leaf area eaten by the larvae in each Petri dish was measured daily by a leaf area meter (LI-3100, LI-COR Inc, Lincoln, NE, USA). The consumed or deteriorated foliage was replaced daily by freshly treated potato leaves. The rate of leaf consumption per surviving beetle was calculated as the area of leaf consumed that day divided by the number of beetles alive on the same day.
The number of dead beetles was counted daily. The beetles were recorded as dead if they did not react after being probed with a soft brush or forceps. Raw mortality was then corrected for background control mortality using Abbott’s formula [18].
2.4. Data Analyses
RStudio [19] was used to analyze all the collected data. The data normality was checked by the Shapiro–Wilk test [20]. This test is highly sensitive, while ANOVA is usually sufficiently robust to handle slight deviations from normality. Therefore, a = 0.01 was used as a cut-off value. The data that did not follow the normal distribution were transformed before analysis using aligned ranks [21]. ANOVA was used to analyze the data on total leaf area consumption over nine days, with treatment as the main factor. Repeated-measures ANOVA was used to analyze the data on daily measurements including leaf area consumed per beetle, control mortality, and Abbott’s corrected mortality [21,22], with treatment and time as the main effects. Post hoc comparisons of the estimated marginal means followed all ANOVAs [23].
3. Results
3.1. 2020 Experiment
The larvae from New York and Maine experienced significantly less background mortality on the leaves treated with distilled water than the other three populations (Table 1 and Table 2). Still, control mortality was acceptably low across all tested populations despite the variation. When the larvae were treated with the low dose of ledprona, the average daily Abbott’s corrected mortality ranged from 7 to 12% and did not significantly differ among the populations (Table 1). Following exposure to the high dose of ledprona, on the other hand, the population effect became significant (Table 2), with the lowest (21%) number of beetles dying in the New York population and the highest (82%) in the Wisconsin population (Table 1). Larval mortality increased as the time progressed (Figure 1; Table 2). When treated with the high concentration of ledprona, the New York larvae were the slowest to die, while Maine and Wisconsin had the fastest mortality (Figure 1; Table 2).

Table 1.
Proportion of Colorado potato beetle larvae dying after feeding on excised potato foliage treated with distilled water and two concentrations of ledprona in 2020 experiments (mean ± SEM). The data were averaged over the nine-day duration of the experiment. The corresponding ANOVA results are shown in Table 2. Means followed by the same letters were not significantly different from each other (estimated marginal means, p < 0.05).

Table 2.
ANOVA statistics for mortality and foliage consumption by Colorado potato beetle larvae in 2020 experiments.
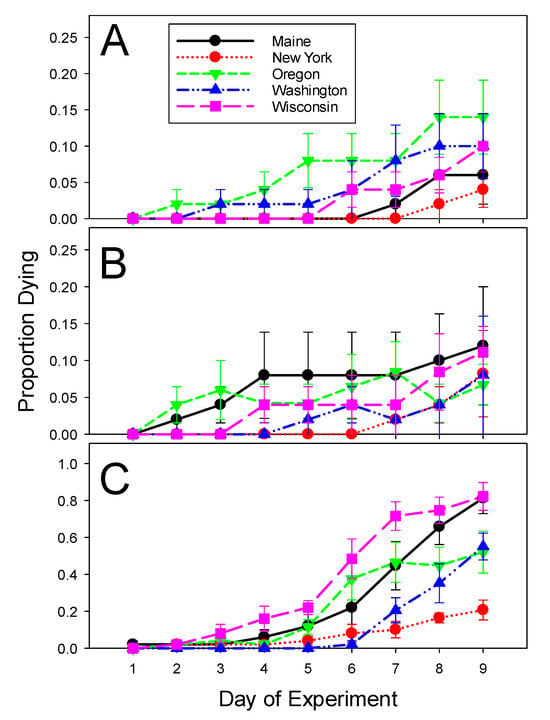
Figure 1.
Mortality of Colorado potato beetles from geographically isolated populations in 2020 experiments. (A) Mean daily mortality on control potato leaves treated with distilled water. (B) Mean daily mortality on potato leaves treated with the low concentration (2.40 × 10−7 g/L) of ledprona and corrected using Abbott’s formula. (C) Mean daily mortality on potato leaves treated with the high concentration (4.75 × 10−5 g/L) of ledprona and corrected using Abbott’s formula. Error bars denote standard error about the mean.
The total leaf area consumed during the nine-day bioassay was significantly smaller for larvae from Wisconsin and Washington than for larvae from Maine when fed on leaves treated with distilled water (Table 2 and Table 3A). There was no significant difference among locations for the larvae exposed to the low dose of ledprona, with ca. 226 cm2 consumed per dish originally containing ten larvae. The high dose of ledprona, on the other hand, had a significant impact on the total foliage consumption, with Wisconsin larvae being most affected and New York larvae being the least affected (Table 2 and Table 3A).

Table 3.
Consumption of excised potato foliage (mean cm2 eaten ± SEM) treated with distilled water and two concentrations of ledprona by Colorado potato beetle larvae in 2020 experiments. The corresponding ANOVA results are shown in Table 2. Means followed by the same letters were not significantly different from each other (estimated marginal means, p < 0.05).
The daily leaf area consumed per larva was significantly lower for Wisconsin and Washington populations than for Maine, Oregon, and New York populations in the distilled water control. When treated with the low dose of ledprona, the larvae from Washington ate less foliage than larvae from the other four populations. When treated with the high concentration of ledprona, the larvae from Maine and Wisconsin consumed less foliage compared to the larvae from the other three populations (Table 2 and Table 3B). Daily foliage consumption increased as time progressed, and the larvae grew, except that the populations from Maine and Wisconsin fed very little throughout the experiment when treated with the high dose of ledprona. The increase in feeding was also slightly higher on the last two days of the experiment for the Maine and Oregon populations in distilled water control (Figure 2; Table 2).
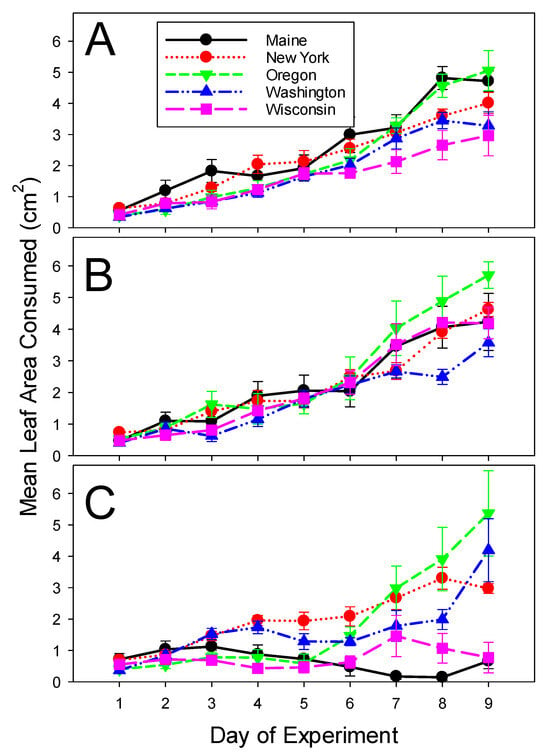
Figure 2.
Per beetle consumption of potato leaves by Colorado potato beetles from geographically isolated populations in 2020 experiments. (A) Control leaves treated with distilled water. (B) Leaves treated with the low concentration (2.40 × 10−7 g/L) of ledprona, and (C) Leaves treated with the high concentration (4.75 × 10−5 g/L) of ledprona. Error bars denote standard error about the mean.
3.2. 2021 Experiment
The background mortality of beetles collected in 2021 and treated with distilled water was the lowest (4%) in larvae from New York and the highest (16%) in larvae from Maine and Oregon (Table 4 and Table 5; Figure 3A). Despite the significant variation detected, the control mortality was acceptably low in larvae from all five locations. Fewer New York larvae (16%) died compared to any other populations (68–98%) when exposed to the low dose of ledprona, as evidenced by comparing their Abbott’s corrected mortalities. There was also significant variation among other populations, but their pairwise comparisons revealed several overlaps (Table 4 and Table 5; Figure 3B). The high dose of ledprona provided complete mortality of all populations except New York (only 58% mortality) after nine days (Table 4 and Table 5; Figure 3C). As in 2020, mortality increased throughout the experiment. The Maine, Minnesota, Oregon, and Virginia populations were all approaching 100% mortality after exposure to the high dose of ledprona by five days after the start of the experiment, while the New York larvae were the slowest to die when feeding on ledprona-treated leaves (Figure 3; Table 5).

Table 4.
Proportion of Colorado potato beetle larvae dying after feeding on excised potato foliage treated with distilled water and two concentrations of ledprona in 2021 experiments (mean ± SEM). The data were averaged over the nine-day duration of the experiment. The corresponding ANOVA results are shown in Table 5. Means followed by the same letters were not significantly different from each other (estimated marginal means, p < 0.05).

Table 5.
ANOVA statistics for mortality and foliage consumption by Colorado potato beetle larvae in 2021 experiments.
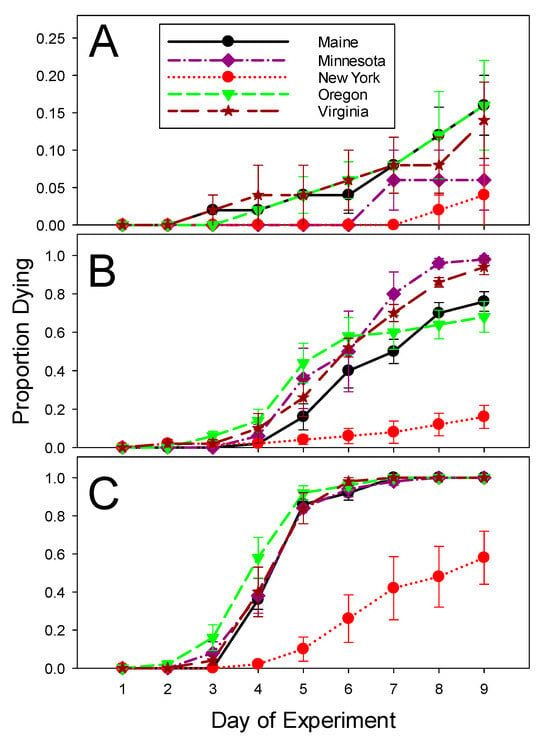
Figure 3.
Mortality of Colorado potato beetles from geographically isolated populations in 2021 experiments. (A) Mean daily mortality on control potato leaves treated with distilled water. (B) Mean daily mortality on potato leaves treated with the low concentration (5.56 × 10−7 g/L) of ledprona and corrected using Abbott’s formula. (C) Mean daily mortality on potato leaves treated with the high concentration (6.62 × 10−1 g/L) of ledprona and corrected using Abbott’s formula. Error bars denote standard error about the mean.
The total leaf area consumed across the nine-day bioassay significantly differed among locations for larvae fed leaves treated with distilled water, with larvae from Minnesota and New York eating more foliage than larvae from the other three populations (Table 5 and Table 6A). In the presence of the low concentration of ledprona, larvae from New York ate more compared to the larvae from all other populations except Oregon (Table 5 and Table 6A). Similarly, the New York population remained the most voracious on the potato leaves treated with the high concentration of ledprona (Table 5 and Table 6A).

Table 6.
Consumption of excised potato foliage (mean cm2 eaten ± SEM) treated with distilled water and two concentrations of ledprona by Colorado potato beetle larvae in 2021 experiments. The corresponding ANOVA results are shown in Table 5. Means followed by the same letters were not significantly different from each other (estimated marginal means, p < 0.05).
The untreated larvae from Minnesota and New York had the highest daily leaf consumption per individual, followed by larvae from Virginia, and then, by larvae from Maine and Oregon (Table 5 and Table 6B). The low dose of ledprona had a stronger effect on the feeding by surviving larvae from Minnesota, Maine, and Virginia than on the feeding by surviving larvae from New York and Oregon (Table 5 and Table 6B). While the daily leaf consumption by the surviving larvae from the New York population was reduced by the high concentration of ledprona, it was relatively more tolerant to exposure to the high concentration of ledprona than larvae from other locations (Table 5 and Table 6B).
Similar to 2020, there was a general trend towards higher foliage consumption from the first to the last day of the experiment in control treatments in the absence of ledprona. A similar foliage consumption trend was observed for the larvae from New York and Oregon populations ingesting the low dose of ledprona. Only New York larvae increased feeding when ingesting the high dose, but the amount of consumed foliage plateaued at a steady state of much reduced feeding compared to the water control (Figure 4; Table 5).
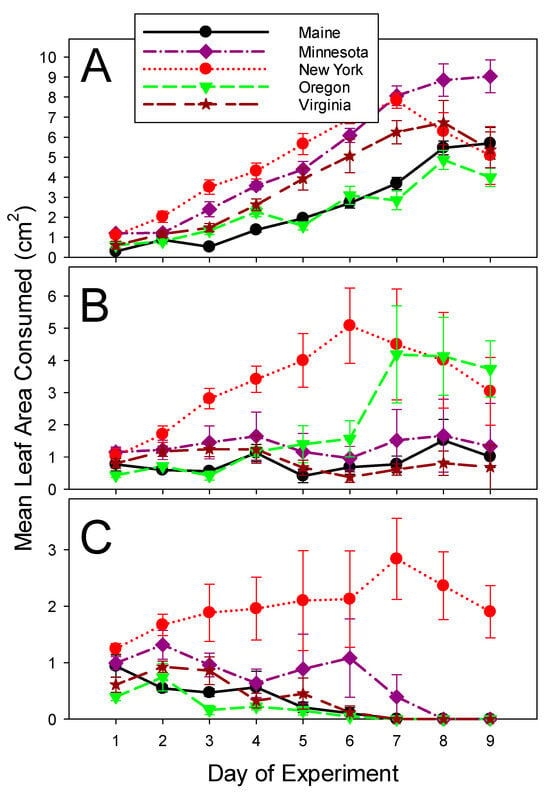
Figure 4.
Per beetle consumption of potato leaves by Colorado potato beetles from geographically isolated populations in 2021 experiments. (A) Control leaves treated with distilled water. (B) Leaves treated with the low concentration (5.56 × 10−7 g/L) of ledprona, and (C) Leaves treated with the high concentration (6.62 × 10−1 g/L) of ledprona. Error bars denote standard error about the mean.
4. Discussion
Ledprona was toxic to the Colorado potato beetles collected from different geographic areas and subsequently tested in this study. Although there was statistically significant variation in the extent of that sensitivity, only the New York population distinctly stood out as having lower susceptibility. The New York larvae also had higher survivorship and better appetite than several other populations in the absence of ledprona. Being overall more fit or healthier than the other populations may have at least partially contributed to their lower susceptibility to RNAi. Although rare, tolerance to environmental stressors through superior general fitness has been previously reported. For example, Groden and Casagrande [9] were able to select a Colorado potato beetle strain that laid 1.7 times more eggs compared to the unselected strain when fed on cultivated S. tuberosum. The fecundity of the selected strain was significantly reduced on an unfavorable wild host Solanum berthaultii Hawkes. Despite that reduction, however, it was still not significantly different from that of the unselected strain on S. tuberosum.
A study performed with the Colorado potato beetle populations collected from several geographically isolated locations across Europe also identified one population with distinctly reduced response to dsRNA targeting actin [16]. In both studies, most differences were relatively small while variation was often high. However, in the present study, there was a detectable natural variation in sensitivity to ledprona in populations without prior history of exposure to this biological pesticide.
In both years, we observed differences in mortality and foliage consumption among the Colorado potato beetle populations, even in the absence of ledprona. Chen et al. [10] also found geographic variation in several fitness parameters of the Colorado potato beetle that they measured in the laboratory. Moreover, Baker et al. [10] reported different rates of cannibalism in geographically isolated Colorado potato beetle populations. Nevertheless, interpopulation differences remained detectable for the beetles feeding on the foliage treated with ledprona when treatment mortality was corrected for control mortality using Abbott’s formula and when foliage consumption was adjusted for the number of surviving beetles. Therefore, these differences could not be entirely explained by background variation in performance on untreated foliage.
The Colorado potato beetle population from upstate New York was consistently less affected by ledprona in both years. The difference in ledprona susceptibility was more pronounced during the 2021, bioassay wherein the larvae from all populations had a much stronger response to the higher concentration of ledprona. The average mortality of New York larvae fed on foliage treated with the high concentration of ledprona was 25% in 2020 and 50% in 2021. For all other populations, pretty much all beetles died over the nine-day bioassay when exposed to the same concentrations as New York larvae (Figure 3B).
Insecticide susceptibility is known to differ among geographically and genetically distinct populations of Colorado potato beetles [5,8], as well as among the populations of other insect species. For example, Main et al. [24] reported differences in insecticide resistance in Anopheles coluzzii Coetzee and Wilkerson due to genetic variation, in particular, the expression of P450 genes. Similarly, Soleño et al. [25] showed significant variation in the median lethal dose of an organophosphate among geographically distinct populations of Cydia pomonella (L.).
Even excluding the larvae from New York, the other populations tested in this study still varied in their responses to ledprona in both years. Feeding on potato leaves treated with the high concentrations of ledprona in 2021 resulted in high mortality for larvae from the four assessed populations. At the same time, feeding on leaves treated with the lower concentration for nine days killed almost all larvae from Minnesota and Virginia but not larvae from Maine and Oregon. The beetles from Minnesota and Virginia may have been more susceptible to ledprona. These populations were only assessed in 2021, having replaced the Washington and Wisconsin populations in the assay. The Oregon population was somewhat less susceptible to low and high concentrations of ledprona in both 2020 and 2021 compared to the other tested populations, except the one from New York.
The trends in leaf consumption generally followed the trends in mortality, with a decrease in feeding preceding an increase in mortality. However, similar proportions of larvae from Oregon and Maine died when ingesting the low concentration of ledprona in 2021, but their per larva feeding rates were very distinct from each other. Additionally, larvae from New York and Oregon consumed similar amounts of foliage treated with the low concentration of ledprona per larva, even though their mortalities were significantly different in the same treatment. Along the same lines, the Oregon and New York larvae fed on leaves treated with the high concentration of ledprona had similar per larva feeding rates but significantly different corrected mortality. In the field, suppressed feeding reduces the damage to potato crops. Moreover, it is likely to further debilitate the affected beetles and increase their susceptibility to natural enemies and other environmental influences.
For the larvae feeding on potato foliage treated with the low dose of ledprona, leaf consumption spiked on days 7, 8, and 9 for Wisconsin beetles in 2020 (Figure 2B) and for Oregon beetles in 2021 (Figure 4B). No such spikes were noticeable for the same populations fed on control leaves (Figure 2A and Figure 4A, respectively) or on leaves treated with the high dose of ledprona (Figure 2C and Figure 4C, respectively). It is possible that there was a hermetic effect of a low dose of ledprona that stimulated beetle voracity. Hormesis in response to pesticides is fairly common in insects (e.g., [26,27]), including the Colorado potato beetle [28]. If this is indeed the case for ledprona, it highlights the importance of using high field rates for protecting potato crops from Colorado potto beetle damage.
The variation in responses to ledprona among populations of Colorado potato beetle may manifest itself in physiologically distinct ways for mortality and leaf consumption. The surviving beetles in some populations may be more likely to continue feeding on the foliage treated with ledprona than surviving beetles in other populations. The same may apply to other sublethal responses to RNAi. Significant differences in RNAi susceptibility were reported for populations of the migratory locust, Locusta migratoria (L.), with distinct phylogenetic origins. Resistance was dominant and probably polygenic. However, the expression levels of nine genes known to be associated with RNAi in other species were not correlated with the observed differences in susceptibility [15]. Similarly, the migratory history and phylogenetic origin of Colorado potato beetle populations may affect their insecticide resistance and susceptibility to RNAi. Colorado potato beetles are known to produce dsRNAases in their gut that can rapidly degrade ingested dsRNA molecules [11]. However, the exact mechanisms underlying the differences observed in this study still need to be determined.
The significant variation in ledprona sensitivity among beetle populations highlights the importance of monitoring insecticide efficacy locally. Applying insecticides at a full field use rate (high enough dose to kill the individuals that are heterozygous at the resistant allele) is one of the key components of a successful resistance management approach for the Colorado potato beetle. In addition, ledprona (or other RNAi based insecticides) must be used in a rotation with other modes of action in order to ensure the sustained utility of this technology given the genetic variability that exists amongst CPB populations [4,29].
Although the New York larvae displayed relatively lower susceptibility to ledprona at the doses used across two years of laboratory studies, they still suffered decreased feeding and increased mortality compared to their control. Furthermore, the New York population was still controlled in field studies (B.M., unpublished data). Field use rate is higher than the laboratory rates tested in this study. Furthermore, multiple stressors, such as natural enemies, wind, rain, etc., are present in the field but not under laboratory conditions. Their combined action may substantially increase the mortality of the intoxicated beetles. This indicates that ledprona is a new excellent option for controlling geographically distinct Colorado potato beetle populations.
Author Contributions
All authors conceptualized the study and contributed to its experimental design. S.P., A.A. and B.M. designed the experiments. S.P. conducted the experiments, curated the data, and performed its statistical analyses. S.P. and A.A. wrote the first draft of the manuscript. B.M., T.B.R., E.B. and K.N. commented on the first draft of the manuscript and edited its text. All authors have read and agreed to the published version of the manuscript.
Funding
GreenLight Biosciences and the USDA National Institute of Food and Agriculture, Hatch Project Number ME0-32125 through the Maine Agricultural & Forest Experiment Station.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Publicly available datasets were analyzed in this study. These data can be found here: [http://andreialyokhin.com/research_data/Pallis_et_al_data.xlsx].
Acknowledgments
We thank Audrie French and Roman Wlodkowski for technical assistance.
Conflicts of Interest
Authors B.M., T.B.R., E.B. and K.N. were employed by GreenLight Biosciences. The authors declare that this study received funding from GreenLight Biosciences. The funder had the following involvement with the study: the authors employed by the funder contributed to the study as described above.
References
- Camire, M.E.; Kubow, S.; Donnelly, D.J. Potatoes and Human Health. Crit. Rev. Food Sci. Nutr. 2009, 49, 823–840. [Google Scholar] [CrossRef] [PubMed]
- Biever, K.D.; Chauvin, R.L. Prolonged Dormancy in a Pacific Northwest Population of the Colorado Potato Beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae). Can. Entomol. 1990, 122, 175–177. [Google Scholar] [CrossRef]
- Weber, D. Colorado Beetle: Pest on the Move. Pestic. Outlook 2003, 14, 256–259. [Google Scholar] [CrossRef]
- Alyokhin, A.; Baker, M.; Mota-Sanchez, D.; Dively, G.; Grafius, E. Colorado Potato Beetle Resistance to Insecticides. Am. J. Potato Res. 2008, 85, 395–413. [Google Scholar] [CrossRef]
- Alyokhin, A.; Benkovskaya, G.; Udalov, M. Colorado Potato Beetle. In Insect Pests of Potato; Alyokhin, A., Rondon, S.I., Gao, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 29–43. [Google Scholar]
- Crossley, M.S.; Rondon, S.I.; Schoville, S.D. A Comparison of Resistance to Imidacloprid in Colorado Potato Beetle (Leptinotarsa decemlineata Say) Populations Collected in the Northwest and Midwest U.S. Am. J. Potato Res. 2018, 95, 495–503. [Google Scholar] [CrossRef]
- Dively, G.P.; Crossley, M.S.; Schoville, S.D.; Steinhauer, N.; Hawthorne, D.J. Regional Differences in Gene Regulation May Underlie Patterns of Sensitivity to Novel Insecticides in Leptinotarsa decemlineata. Pest Manag. Sci. 2020, 76, 4278–4285. [Google Scholar] [CrossRef] [PubMed]
- Klein, C.; Baker, M.; Alyokhin, A.; Mota-Sanchez, D. Geographic Variation in Dominance of Spinosad Resistance in Colorado Potato Beetles (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2021, 114, 320–325. [Google Scholar] [CrossRef]
- Groden, E.; Casagrande, R.A. Population Dynamics of the Colorado Potato Beetle, Leptinotarsa decemlineata (Coleoptera: Chrysomelidae), on Solanum Berthaultii. J. Econ. Entomol. 1986, 79, 91–97. [Google Scholar] [CrossRef]
- Chen, J.; Alyokhin, A.; Mota-Sanchez, D.; Baker, M.; Whalon, M. Variation in Fitness among Geographically Isolated Colorado Potato Beetle (Coleoptera: Chrysomelidae) Populations. Ann. Entomol. Soc. Am. 2014, 107, 128–135. [Google Scholar] [CrossRef]
- Ma, M.; He, W.; Xu, S.; Xu, L.; Zhang, J. RNA Interference in Colorado Potato Beetle (Leptinotarsa decemlineata): A Potential Strategy for Pest Control. J. Integr. Agric. 2020, 19, 428–437. [Google Scholar] [CrossRef]
- Rodrigues, T.B.; Mishra, S.K.; Sridharan, K.; Barnes, E.R.; Alyokhin, A.; Tuttle, R.; Kokulapalan, W.; Garby, D.; Skizim, N.J.; Tang, Y.; et al. First Sprayable Double-Stranded RNA-Based Biopesticide Product Targets Proteasome Subunit Beta Type-5 in Colorado Potato Beetle (Leptinotarsa decemlineata). Front. Plant Sci. 2021, 12, 728652. [Google Scholar] [CrossRef] [PubMed]
- Pallis, S.; Alyokhin, A.; Manley, B.; Rodrigues, T.B.; Buzza, A.; Barnes, E.; Narva, K. Toxicity of a Novel dsRNA-Based Insecticide to the Colorado Potato Beetle in Laboratory and Field Trials. Pest Manag. Sci. 2022, 78, 3836–3848. [Google Scholar] [CrossRef] [PubMed]
- Pallis, S.; Alyokhin, A.; Manley, B.; Rodrigues, T.; Barnes, E.; Narva, K. Effects of Low Doses of a Novel dsRNA-Based Biopesticide (Calantha) on the Colorado Potato Beetle. J. Econ. Entomol. 2023, 116, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, R.; Tanaka, S.; Jouraku, A.; Shiotsuki, T. Geographic Variation in RNAi Sensitivity in the Migratory Locust. Gene 2017, 605, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Mehlhorn, S.G.; Geibel, S.; Bucher, G.; Nauen, R. Profiling of RNAi Sensitivity after Foliar dsRNA Exposure in Different European Populations of Colorado Potato Beetle Reveals a Robust Response with Minor Variability. Pestic. Biochem. Physiol. 2020, 166, 104569. [Google Scholar] [CrossRef] [PubMed]
- Galimberti, A.; Alyokhin, A. Lethal and Sublethal Effects of Mineral Oil on Potato Pests. J. Econ. Entomol. 2018, 111, 1261–1267. [Google Scholar] [CrossRef] [PubMed]
- Abbott, W.S. A Method of Computing the Effectiveness of an Insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Royston, J.P. Algorithm AS 181: The W Test for Normality. J. R. Stat. Soc. Ser. C Appl. Stat. 1982, 31, 176–180. [Google Scholar] [CrossRef]
- Kay, M.; Elkin, L.; Higgins, J.; Wobbrock, J. ARTool: Aligned Rank Transform for Nonparametric Factorial ANOVAs; University of Washington: Seattle, WA, USA, 2021. [Google Scholar]
- Wobbrock, J.O.; Findlater, L.; Gergle, D.; Higgins, J.J. The Aligned Rank Transform for Nonparametric Factorial Analyses Using Only Anova Procedures. In Proceedings of the SIGCHI Conference on Human Factors in Computing Systems, New York, NY, USA, 7 May 2011; Association for Computing Machinery: New York, NY, USA, 2011; pp. 143–146. [Google Scholar]
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Main, B.J.; Everitt, A.; Cornel, A.J.; Hormozdiari, F.; Lanzaro, G.C. Genetic Variation Associated with Increased Insecticide Resistance in the Malaria Mosquito, Anopheles coluzzii. Parasit. Vectors 2018, 11, 225. [Google Scholar] [CrossRef]
- Soleño, J.; Anguiano, O.L.; Cichón, L.B.; Garrido, S.A.; Montagna, C.M. Geographic Variability in Response to Azinphos-Methyl in Field-Collected Populations of Cydia pomonella (Lepidoptera: Tortricidae) from Argentina. Pest Manag. Sci. 2012, 68, 1451–1457. [Google Scholar] [CrossRef]
- Miksanek, J.R.; Tuda, M. Endosymbiont-Mediated Resistance to Entomotoxic Nanoparticles and Sex-Specific Responses in a Seed Beetle. J. Pest Sci. 2023, 96, 1257–1270. [Google Scholar] [CrossRef]
- Miksanek, J.R.; Adarkwah, C.; Tuda, M. Low Concentrations of Selenium Nanoparticles Enhance the Performance of a Generalist Parasitoid and Its Host, with No Net Effect on Host Suppression. Pest Manag. Sci. 2023; accepted author manuscript. [Google Scholar] [CrossRef]
- Alyokhin, A.; Chen, Y.H.; Udalov, M.; Benkovskaya, G.; Lindström, L. Evolutionary Considerations in Potato Pest Management. In Insect Pests of Potato, 2nd ed.; Alyokhin, A., Rondon, S.I., Gao, Y., Eds.; Academic Press: Oxford, UK, 2022; pp. 429–450. ISBN 978-0-12-821237-0. [Google Scholar]
- Alyokhin, A.; Mota-Sanchez, D.; Baker, M.; Snyder, W.E.; Menasha, S.; Whalon, M.; Dively, G.; Moarsi, W.F. The Red Queen in a Potato Field: Integrated Pest Management versus Chemical Dependency in Colorado Potato Beetle Control. Pest Manag. Sci. 2015, 71, 343–356. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).