Growing Patterns of the Branca Chicken Breed—Concentrate vs. Maize-Based Diet
Abstract
1. Introduction
2. Materials and Methods
2.1. Bird Husbandry and Data Collection
2.2. Growth Functions Studied
2.3. Statistical Procedure
- The regression model exhibits linearity in both the coefficients and residuals. Guaranteed by the equations of the models.
- The error terms have a mean of zero. Confirmed with a one-sample t-test.
- The independent variable ‘age’ does not correlate with the residuals. Verified through Spearman’s correlation test.
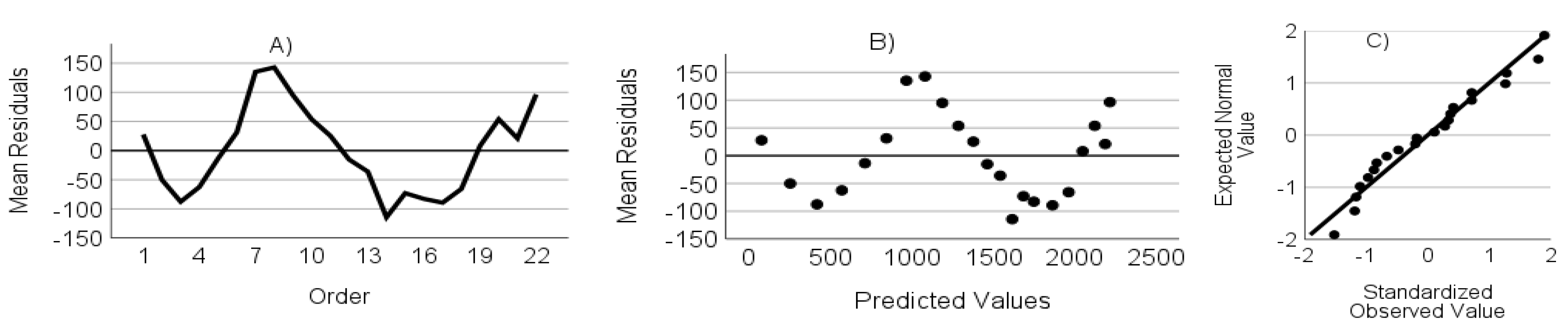
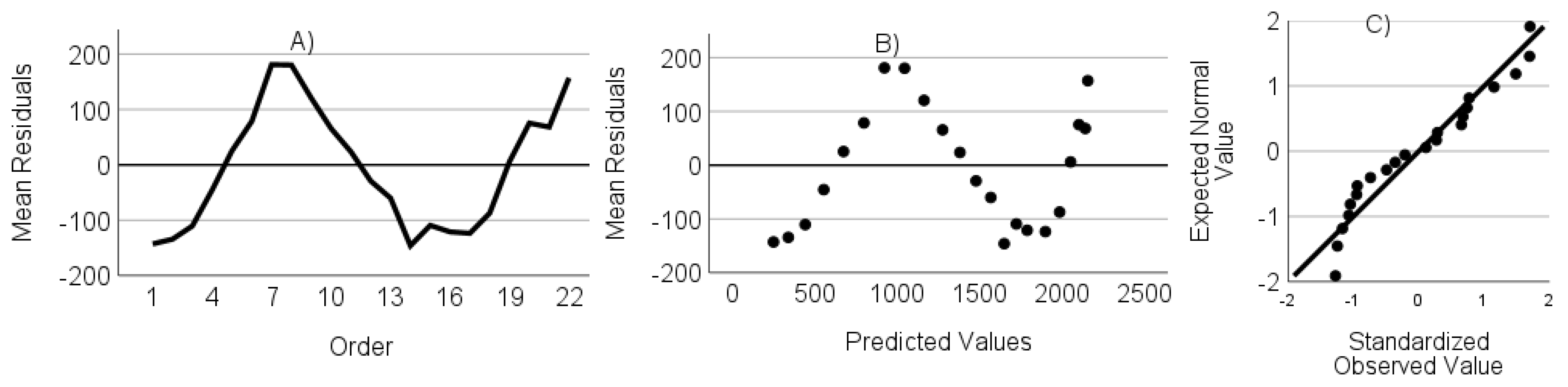
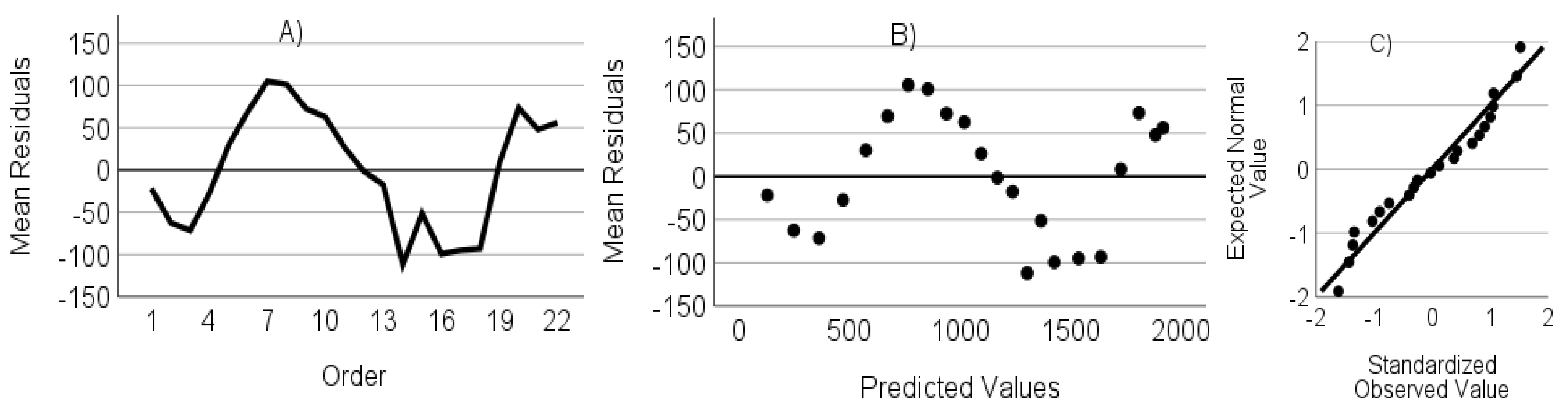
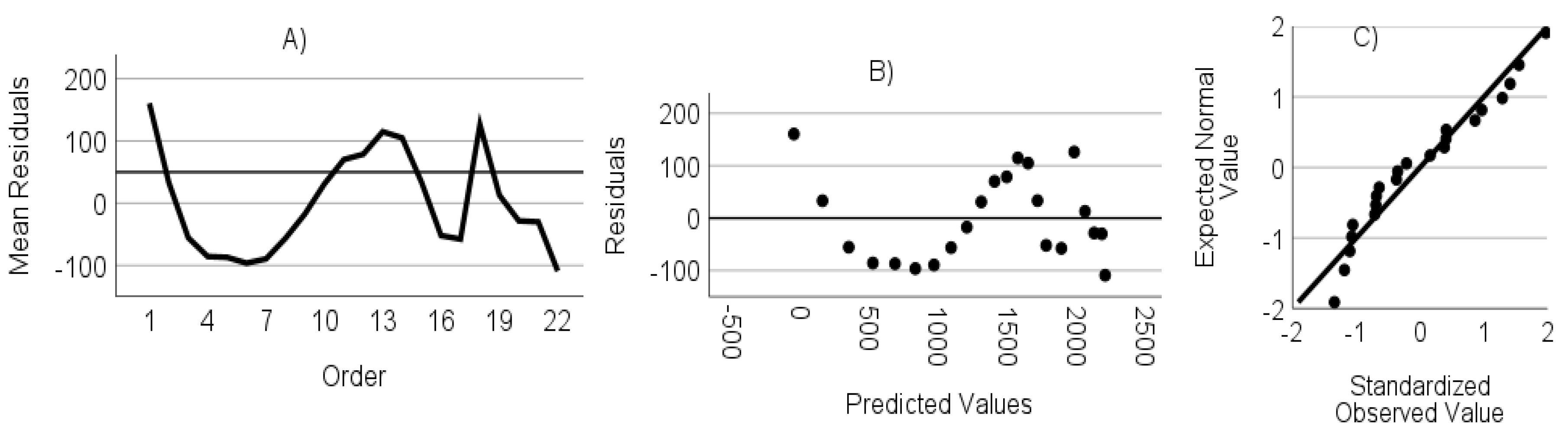
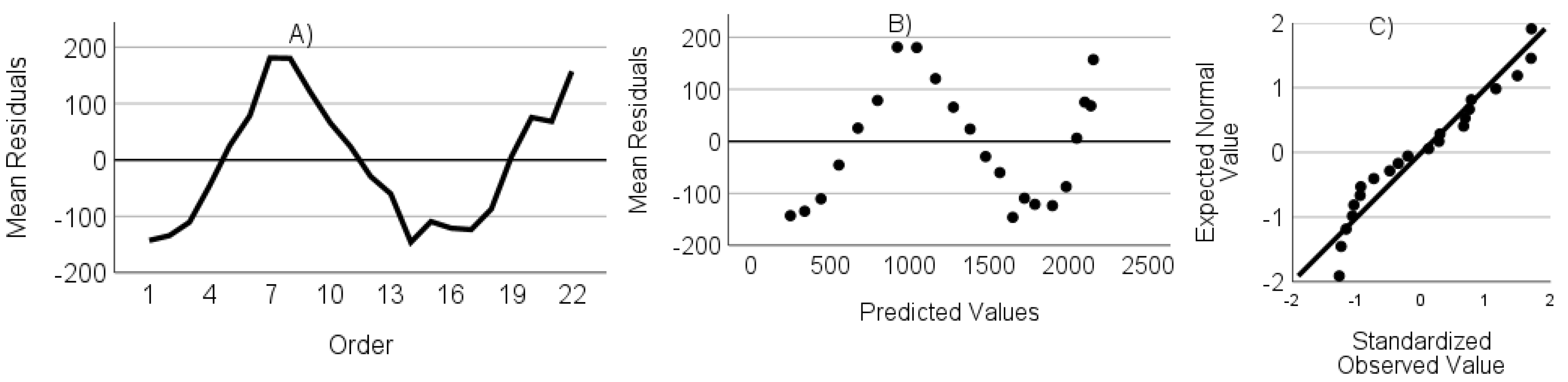
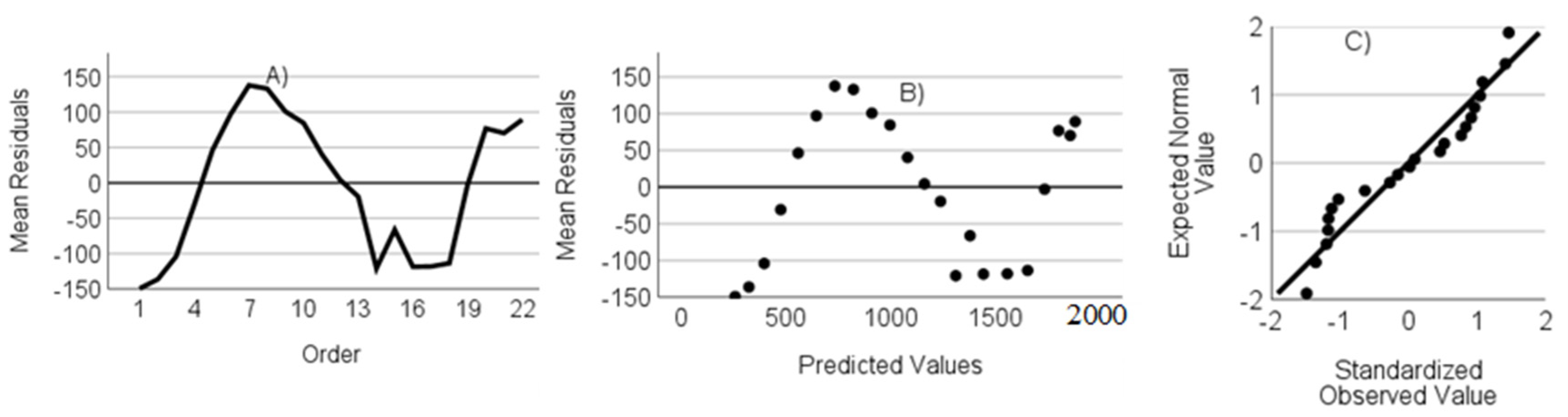
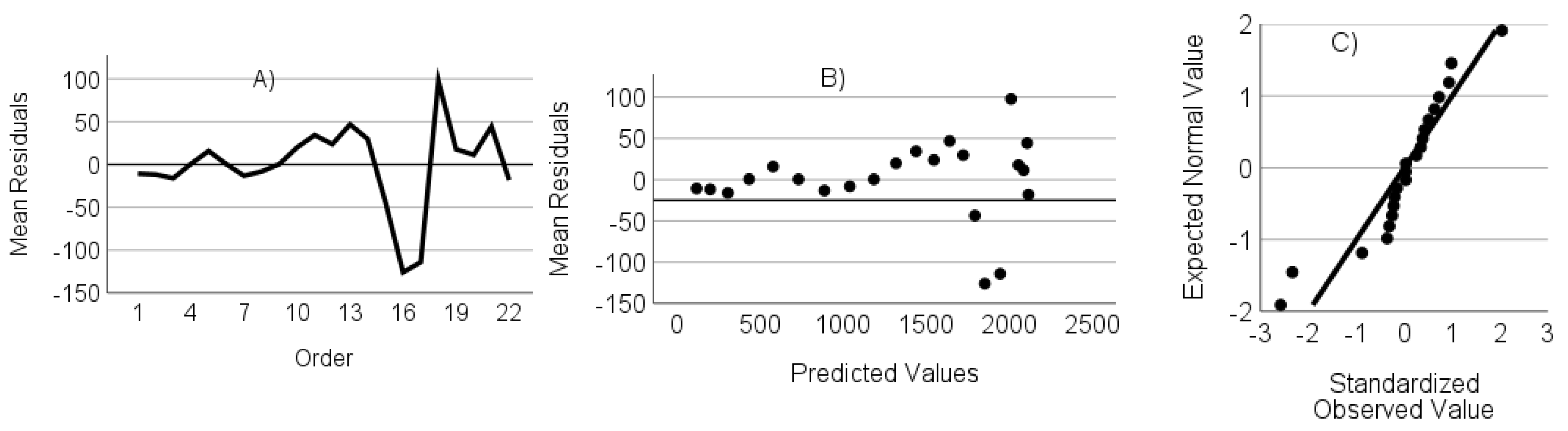
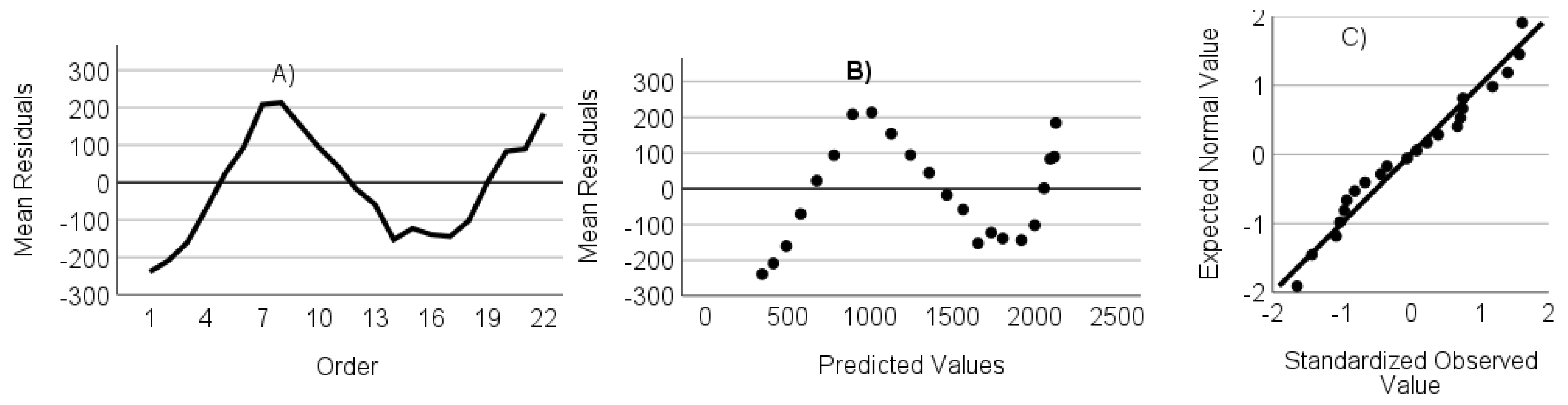
- The residuals are not autocorrelated. Verified through the randomness of an ordered residual plot.
- The residuals do not show heteroscedasticity. Assessed via predicted values versus the residuals plot.
- Lack of correlation between independent variables. Ensured by the presence of a single independent variable across all models (age).
- The residuals have a normal distribution. Assessed through a standardized residuals Q-Q plot.
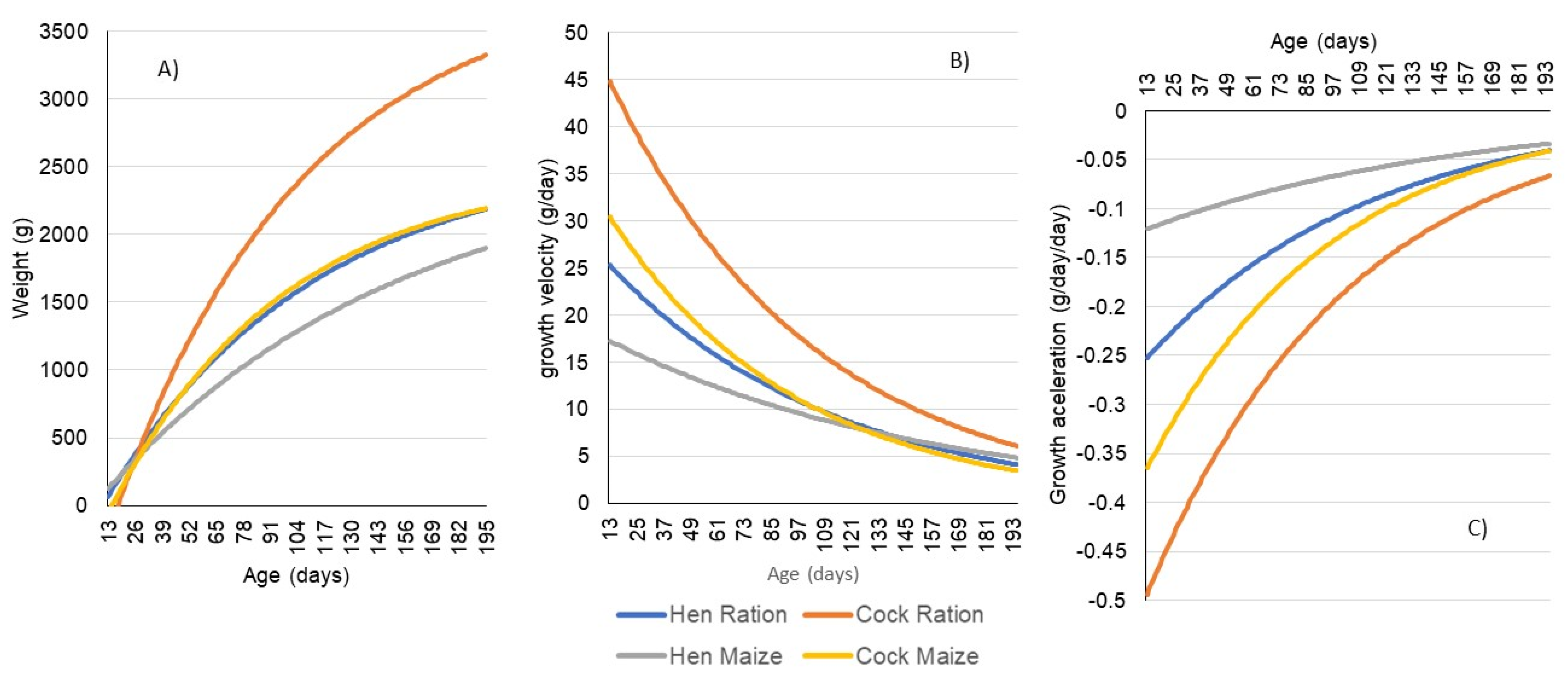
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A




References
- Fumihito, A.; Miyake, T.; Takada, M.; Shingu, R.; Endo, T.; Gojobori, T.; Kondo, N.; Ohno, S. Monophyletic Origin and Unique Dispersal Patterns of Domestic Fowls. Proc. Natl. Acad. Sci. USA 1996, 93, 6792–6795. [Google Scholar] [CrossRef] [PubMed]
- Gobvu, V.; Ncube, S.; Caron, A.; Mugabe, P.H. Community-Based Performance Indicators for Monitoring and Evaluating Livestock Interventions. Trop. Anim. Health Prod. 2021, 53, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Francis, R.C. Domesticated: Evolution in a Man-Made World; WW Norton & Company: New York, NY, USA, 2015; ISBN 0393246515. [Google Scholar]
- Sponenberg, D.P.; Beranger, J.; Martin, A. Managing Breeds for a Secure Future: Strategies for Breeders and Breed Associations; 5m Books Ltd.: Essex, UK, 2017. [Google Scholar]
- da Costa Soares, M.L. Caracterização Fenotípica e Genotípica Das Raças Autóctones de Galináceos Portugueses: Pedrês Portuguesa, Preta Lusitânica e Amarela; Instituto de Ciências Biomédicas Abel Salazar: Porto, Portugal, 2015. [Google Scholar]
- Verrier, E.; Naves, M.; Tixier-Boichard, M.; Bernigaud, R. Values of Local Breeds for Niche Productions and/or Adaptation to Specific Environments. In Proceedings of the Second International Workshop: “Governance of Biodiversity as a Global Public Good: Bioprospection, Intellectual Property Rights and Traditional Knowledge”, Louvain-la-Neuve, France, 5–6 February 2004; pp. 1–8. [Google Scholar]
- Besbes, B.; Tixier-Boichard, M.; Hoffmann, I.; Jain, G.L. Future Trends for Poultry Genetic Resources. In Proceedings of the International Conference of Poultry in the 21st Century: Avian Influenza and Beyond, Bangkok, Thailand, 5–7 November 2007; pp. 5–7. [Google Scholar]
- Sponenberg, D.P.; Martin, A.; Couch, C.; Beranger, J. Conservation Strategies for Local Breed Biodiversity. Diversity 2019, 11, 177. [Google Scholar] [CrossRef]
- Mata-Estrada, A.; González-Cerón, F.; Pro-Martínez, A.; Torres-Hernández, G.; Bautista-Ortega, J.; Becerril-Pérez, C.M.; Vargas-Galicia, A.J.; Sosa-Montes, E. Comparison of Four Nonlinear Growth Models in Creole Chickens of Mexico. Poult. Sci. 2020, 99, 1995–2000. [Google Scholar] [CrossRef] [PubMed]
- Mata, F.; Mwakifuna, B. Comparative Mortality and Predation in Relation to Egg Production Traits of Rhode Island Red, Black Australorp and Hyblack Laying Hens in Scavenging Production Systems of Rural Malawi. Br. Poult. Sci. 2012, 53, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Lordelo, M.; Cid, J.; Cordovil, C.M.D.S.; Alves, S.P.; Bessa, R.J.B.; Carolino, I. A Comparison between the Quality of Eggs from Indigenous Chicken Breeds and That from Commercial Layers. Poult. Sci. 2020, 99, 1768–1776. [Google Scholar] [CrossRef]
- Meira, M.; Afonso, I.M.; Cruz, R.; Lopes, J.C.; Martins, R.S.; Domingues, J.; Ribeiro, V.; Dantas, R.; Casal, S.; Brito, N. V Carcass Yields and Meat Composition of Roosters of the Portuguese Autochthonous Poultry Breeds: “Branca”, “Amarela”, “Pedrês Portuguesa”, and “Preta Lusitânica”. Foods 2023, 12, 4020. [Google Scholar] [CrossRef]
- Meira, M.; Afonso, I.M.; Casal, S.; Lopes, J.C.; Domingues, J.; Ribeiro, V.; Dantas, R.; Leite, J.V.; Brito, N.V. Carcass and Meat Quality Traits of Males and Females of the “Branca” Portuguese Autochthonous Chicken Breed. Animals 2022, 12, 2640. [Google Scholar] [CrossRef]
- Soares, L.C.; Lopes, J.C.; Brito, N.V.; Carvalheira, J. Growth and Carcass Traits of Three Portuguese Autochthonous Chicken Breeds: Amarela, Preta Lusitânica and Pedrês Portuguesa. Ital. J. Anim. Sci. 2015, 14, 3566. [Google Scholar] [CrossRef][Green Version]
- Davidian, M. Nonlinear Models for Repeated Measurement Data; Routledge: New York, NY, USA, 2017. [Google Scholar]
- Faraji Arough, H.; Rokouei, M.; Maghsoudi, A.; Mehri, M. Evaluation of Non-Linear Growth Curves Models for Native Slow-Growing Khazak Chickens. Poult. Sci. J. 2019, 7, 25–32. [Google Scholar]
- Nogueira, B.R.F.; Reis, M.P.; Carvalho, A.C.; Mendoza, E.A.C.; Oliveira, B.L.; Silva, V.A.; Bertechini, A.G. Performance, Growth Curves and Carcass Yield of Four Strains of Broiler Chicken. Braz. J. Poult. Sci. 2019, 21, eRBCA-2018. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Nguyen, C.X.; Luu, M.Q.; Nguyen, A.T.; Bui, D.H.; Pham, D.K.; Do, D.N. Mathematical Models to Describe the Growth Curves of Vietnamese Ri Chicken. Braz. J. Biol. 2021, 83. [Google Scholar] [CrossRef] [PubMed]
- Ridho, M.; Putra, W.P.B.; Sola-Ojo, F.E. The Growth Curve of Gompertz and Logistic Models in Body Weight of Ecotype Fulani Chickens (Gallus Domesticus). IOP Conf. Ser. Earth Environ. Sci. 2021, 637, 012098. [Google Scholar] [CrossRef]
- Brody, S. Bioenergetics and Growth, with Special Reference to the Efficiency Complex in Domestic Animals; Hafner Publishing Company, Inc.: New York, NY, USA, 1945. [Google Scholar]
- Verhulst, P.-F. Notice Sur La Loi Que La Population Suit Dans Son Accroissement. Corresp. Math. Et Phys. 1838, 10, 113–129. [Google Scholar]
- Winsor, C.P. The Gompertz Curve as a Growth Curve. Proc. Natl. Acad. Sci. USA 1932, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Crispim, A.C.; Kelly, M.J.; Guimarães, S.E.F.; e Silva, F.F.; Fortes, M.R.S.; Wenceslau, R.R.; Moore, S. Multi-Trait GWAS and New Candidate Genes Annotation for Growth Curve Parameters in Brahman Cattle. PLoS ONE 2015, 10, e0139906. [Google Scholar] [CrossRef] [PubMed]
- Frost, J. Regression Analysis: An Intuitive Guide for Using and Interpreting Linear Models; Statistics By Jim Publishing: 2019; ISBN 1735431184. Available online: https://www.researchgate.net/profile/Pradeep-Paraman/post/SPSS-PLUM_Shall_I_introduce_binary_variables_as_covariates/attachment/6005a2242b12470001e4b378/AS%3A981343471149059%401610981924682/download/SampleRegressionAnalysisAnIntuitiveGuide.pdf (accessed on 3 November 2023).
- Brito, N.V.; Lopes, J.C.; Ribeiro, V.; Dantas, R.; Leite, J. V Biometric Characterization of the Portuguese Autochthonous Hens Breeds. Animals 2021, 11, 498. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F. Sex Differences in Metabolic Homeostasis, Diabetes, and Obesity. Biol. Sex Differ. 2015, 6, 1–9. [Google Scholar] [CrossRef]
- Lee, K.-C.; Kil, D.Y.; Sul, W.J. Cecal Microbiome Divergence of Broiler Chickens by Sex and Body Weight. J. Microbiol. 2017, 55, 939–945. [Google Scholar] [CrossRef]
- Cui, L.; Zhang, X.; Cheng, R.; Ansari, A.R.; Elokil, A.A.; Hu, Y.; Chen, Y.; Nafady, A.A.; Liu, H. Sex Differences in Growth Performance Are Related to Cecal Microbiota in Chicken. Microb. Pathog. 2021, 150, 104710. [Google Scholar] [CrossRef]
- Taylor, J.; Walk, C.; Misiura, M.; Sorbara, J.-O.B.; Giannenas, I.; Kyriazakis, I. Quantifying the Effect of Coccidiosis on Broiler Performance and Infection Outcomes in the Presence and Absence of Control Methods. Poult. Sci. 2022, 101, 101746. [Google Scholar] [CrossRef] [PubMed]
- De Marchi, M.; Cassandro, M.; Lunardi, E.; Baldan, G.; Siegel, P.B. Carcass Characteristics and Qualitative Meat Traits of the Padovana Breed of Chicken. Int. J. Poult. Sci. 2005, 4, 233–238. [Google Scholar]
- Bongiorno, V.; Schiavone, A.; Renna, M.; Sartore, S.; Soglia, D.; Sacchi, P.; Gariglio, M.; Castillo, A.; Mugnai, C.; Forte, C. Carcass Yields and Meat Composition of Male and Female Italian Slow-Growing Chicken Breeds: Bianca Di Saluzzo and Bionda Piemontese. Animals 2022, 12, 406. [Google Scholar] [CrossRef] [PubMed]

| Model | Equation parameterization |
|---|---|
| Brody | |
| Gompertz | |
| Logistic |
| Functions | a | SE | 95%CI | b | SE | 95%CI | c | SE | 95%CI | |
|---|---|---|---|---|---|---|---|---|---|---|
| Hens ration-fed | Brody | 2596.52 | 130.2 | 2324.01; 2869.03 | 1.109 | 0.034 | 1.037; 1.181 | 0.010 | 0.001 | 0.008; 0.013 |
| Gompertz | 2251.85 | 93.57 | 2056.01; 2447.69 | 2.903 | 0.310 | 2.254; 3.552 | 0.021 | 0.002 | 0.016; 0.026 | |
| Logistic | 2165.13 | 91.38 | 1973.88; 2356.38 | 7.878 | 1.705 | 4.309; 1.447 | 0.031 | 0.004 | 0.023; 0.039 | |
| Functions | a | SE | 95%CI | b | SE | 95%CI | c | SE | 95%CI | |
| Cocks ration-fed | Brody | 3873.71 | 240.9 | 3369.57; 4377.85 | 1.215 | 0.053 | 1.103; 1.322 | 0.011 | 0.001 | 0.008; 0.014 |
| Gompertz | 3229.30 | 27.10 | 3172.58; 3286.02 | 5.240 | 0.223 | 4.773; 5.706 | 0.030 | 0.001 | 0.028; 0.031 | |
| Logistic | 3111.77 | 28.55 | 3052.02; 3171.53 | 24.057 | 2.300 | 19.243; 28.871 | 0.046 | 0.002 | 0.043; 0.050 | |
| Functions | a | SE | 95%CI | b | SE | 95%CI | c | SE | 95%CI | |
| Hens maize-fed | Brody | 2588.74 | 234.6 | 2090.75; 3079.72 | 1.043 | 0.028 | 0.984; 1.102 | 0.007 | 0.001 | 0.005; 0.010 |
| Gompertz | 2088.10 | 131.2 | 1813.57; 2362.63 | 2.604 | 0.234 | 2.113; 3.094 | 0.017 | 0.002 | 0.012; 0.021 | |
| Logistic | 1968.27 | 112.53 | 1732.74; 2203.80 | 6.924 | 1.248 | 4.312; 9.535 | 0.025 | 0.003 | 0.018; 0.032 | |
| Functions | a | SE | 95%CI | b | SE | 95%CI | c | SE | 95%CI | |
| Cocks maize-fed | Brody | 2479.02 | 106.8 | 2255.58; 2702.45 | 1.196 | 0.042 | 1.108; 1.285 | 0.012 | 0.001 | 0.010; 0.015 |
| Gompertz | 2151.00 | 30.0 | 2088.04; 2213.40 | 4.167 | 0.256 | 3.631; 4.702 | 0.028 | 0.001 | 0.026; 0.031 | |
| Logistic | 2502.33 | 99.97 | 2295.19; 2709.46 | 11.147 | 0.875 | 9.315; 12.979 | 0.002 | 0.0001 | 0.0017; 0.0021 |
| Males | Ration Fed | Maize Fed | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RMS | r2 | Cp | ρEt | RMS | r2 | Cp | ρEt | |||
| Brody | 2758 | 0.98 | 1 | 0 | 0.039 NS | 7302 | 0.99 | 1 | 0 | −0.039 NS |
| Gompertz | 3162 | 0.99 | 1 | 0 | −0.134 NS | 2593 | 0.99 | 1 | 0 | 0.178 NS |
| Logistic | 3862 | 0.99 | 1 | 0 | 0.239 NS | 3125 | 0.99 | 1 | 0 | 0.161 NS |
| Females | Ration Fed | Maize Fed | ||||||||
| RMS | r2 | Cp | ρEt | RMS | r2 | Cp | ρEt | |||
| Brody | 6265 | 0.99 | 1 | 0 | −0.045 NS | 5316 | 0.98 | 1 | 0 | −0.030 NS |
| Gompertz | 13,384 | 0.97 | 1 | 0 | 0.141 NS | 10,451 | 0.97 | 1 | 0 | 0.095 NS |
| Logistic | 21,366 | 0.95 | 1 | 0 | 0.212 NS | 15,287 | 0.95 | 1 | 0 | 0.153 NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soares, L.; Mata, F.; Cerqueira, J.L.; Araújo, J. Growing Patterns of the Branca Chicken Breed—Concentrate vs. Maize-Based Diet. Agriculture 2023, 13, 2282. https://doi.org/10.3390/agriculture13122282
Soares L, Mata F, Cerqueira JL, Araújo J. Growing Patterns of the Branca Chicken Breed—Concentrate vs. Maize-Based Diet. Agriculture. 2023; 13(12):2282. https://doi.org/10.3390/agriculture13122282
Chicago/Turabian StyleSoares, Laura, Fernando Mata, Joaquim L. Cerqueira, and José Araújo. 2023. "Growing Patterns of the Branca Chicken Breed—Concentrate vs. Maize-Based Diet" Agriculture 13, no. 12: 2282. https://doi.org/10.3390/agriculture13122282
APA StyleSoares, L., Mata, F., Cerqueira, J. L., & Araújo, J. (2023). Growing Patterns of the Branca Chicken Breed—Concentrate vs. Maize-Based Diet. Agriculture, 13(12), 2282. https://doi.org/10.3390/agriculture13122282








