The Influence of Ozonation Carried Out during Vegetation on the Content of Selected Bioactive Phytochemicals and the Microbiological Load of Tubers of Raphanus sativus var. sativus
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
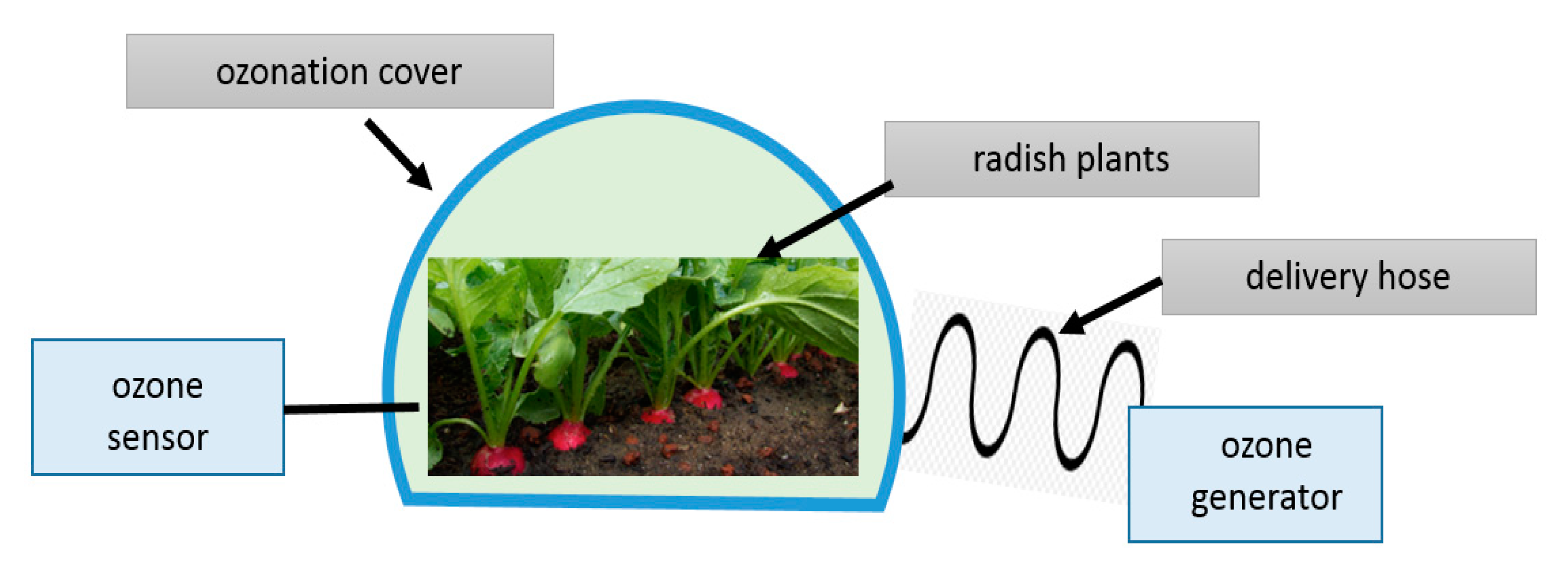
2.1.1. Plant Materials in a Field Experiment
- -
- Control—no variable factors were used.
- -
- 1 ppm for 1 min.
- -
- 1 ppm for 5 min.
- -
- 5 ppm for 1 min.
- -
- 5 ppm for 5 min.
2.1.2. Growth Conditions
2.2. Ozonation of Radish Plants
2.3. Mechanical Properties—Compression Test
2.4. Measurement of Water Content
2.5. Antioxidant Potential, Polyphenol Content, and Vitamin C
2.6. Microbiological Analyses
2.7. Statistical Analysis
3. Results and Discussion
3.1. Measurement of Water Content during Storage
3.2. Measurement of Mechanical Properties
3.3. Ascorbic Acid Content, Antioxidant Activity, and Total Phenolic Content
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Rodrigues, R.R.; Pizetta, S.C.; Teixeira, A.G.; Reis, E.F.; Hott, M.O. Produção de rabanete em diferentes disponibilidades de água no solo. Enciclopédia Biosf. 2013, 9, 2121–2130. [Google Scholar] [CrossRef]
- Saha, S.; Paul, S.A.; Afroz, A.; Dey, A.; Chatterjee, A.; Khanra, R. Raphanus sativus—A review of its traditional uses, phytochemistry, and pharmacology. Asian J. Pharm. Clin. Res. 2023, 16, 7–12. [Google Scholar] [CrossRef]
- Jorgeana, A.; Sobrinho, O.; Cantanhede, E.; Schalcher, A.; Silva, V.; Santos, L. Agronomic performance of Raphanus sativus L. cultivars grown under different spacings. Rev. Cienc. Agrícolas 2020, 37, 19–26. [Google Scholar] [CrossRef]
- Banihani, S.A. Radish (Raphanus sativus) and Diabetes. Nutrients 2017, 9, 1014. [Google Scholar] [CrossRef]
- Husnain, R.T.; Amjad, H.; Ziaf, K.; Sahi, S.T. Planting geometry effects on seed yield and quality of two radish cultivars. Pak. J. Agric. Sci. 2020, 57, 637–643. [Google Scholar]
- Linhares, P.C.F.; Pereira, M.F.S.; Oliveira, B.S.; Henriques, G.P.S.A.; Maracajá, P.B. Produtividade de rabanete em sistema orgânico de produção. Rev. Verde Agroecol. Desenvolv. Sustent. 2010, 5, 94–101. [Google Scholar]
- Ito, H.; Horie, A.A. Chromatographic method for separating and identifying intact 4-methylthio-3-butenyl glucosinolate in Japanese radish (Raphanus sativus L.). Jpn. Agric. Res. Q. 2008, 42, 109–114. [Google Scholar] [CrossRef][Green Version]
- Poudel, K.; Karki, S.; Sah Teli, M.; Mandal, J. Evaluation of radish (Raphanus sativus L.) genotypes in Eastern mid-hills condition of Nepal. World News Nat. Sci. 2018, 19, 155–159. [Google Scholar]
- Raphanus sativus (Cultivated R. sativus): Go Botany. Native Plant Trust. Available online: https://gobotany.nativeplanttrust.org/species/raphanus/sativus (accessed on 10 October 2023).
- Raphanus sativus; n.d. Useful Tropical Plants. Available online: https://tropical.theferns.info/viewtropical.php?id=Raphanus+sativus (accessed on 10 October 2023).
- Pan, L.; Gao, J.; Han, Y.; Shi, Y.; Tang, X.; Pu, L.; Lai, X.; Dongzhu, R.; Zhang, J.; Xiangmao, Q.; et al. The Treatment of Cholecystitis and Cholelithiasis by Tibetan Medicine. Evid. Based Complement. Altern. Med. 2021, 30, 9502609. [Google Scholar] [CrossRef]
- Gutiérrez, R.M.; Perez, R.L. Raphanus sativus (Radish): Their chemistry and biology. Sci. World J. 2004, 13, 811–837. [Google Scholar] [CrossRef]
- Gamba, M.; Asllanaj, E.; Raguindin, P.F.; Glisic, M.; Franco, O.H.; Minder, B. Nutritional and phytochemical characterization of radish (Raphanus sativus): A systematic review. Trends Food Sci. Technol. 2021, 113, 205–218. [Google Scholar] [CrossRef]
- Barillari, J.; Iori, R.; Papi, A.; Orlandi, M.; Bartolini, G.; Gabbanini, S. Kaiware Daikon (Raphanus sativus L.) extract: A naturally multipotent chemopreventive agent. J. Agric. Food Chem. 2008, 56, 7823–7830. [Google Scholar] [CrossRef] [PubMed]
- Głodowska, M.; Gałązka, A. Unsustainable agriculture and its environmental consequences. Zesz. Probl. Postępów Nauk. Rol. 2018, 592, 3–13. [Google Scholar]
- Tandzi, L.; Mutengwa, C. Factors Affecting Yield of Crops. Agron. Clim. Chang. Food Secur. 2020, 3–15. [Google Scholar] [CrossRef]
- Piechowiak, T.; Antos, P.; Kosowski, P.; Skrobacz, K.; Józefczyk, R.; Balawejder, M. Impact of ozonation process on the microbiological and antioxidant status of raspberry (Rubus ideaeus L.) fruit during storage at room temperature. Agric. Food Sci. 2019, 28, 35–44. [Google Scholar] [CrossRef]
- Contigiani, E.V.; Jaramillo-Sánchez, G.; Castro, M.A.; Gómez, P.L.; Alzamora, S.M. Postharvest Quality of Strawberry Fruit (Fragaria x Ananassa Duch cv. Albion) as Affected by Ozone Washing: Fungal Spoilage, Mechanical Properties, and Structure. Food Bioprocess. Technol. 2018, 11, 1639–1650. [Google Scholar] [CrossRef]
- Jaramillo-Sánchez, G.; Contigiani, E.V.; Castro, M.; Hodara, K.; Alzamora, S.; Loredo, A.; Nieto, A. Freshness maintenance of blueberries (Vaccinium corymbosum L.) during postharvest using ozone in aqueous phase: Microbiological, structure, and mechanical issues. Food Bioprocess. Technol. 2019, 12, 2136–2147. [Google Scholar] [CrossRef]
- Lv, Y.; Tahir, I.I.; Olsson, M.E. Effect of ozone application on bioactive compounds of apple fruit during short-term cold storage. Sci. Hortic. 2019, 253, 49–60. [Google Scholar] [CrossRef]
- Guidi, L.; Degl’Innocenti, E.; Soldatini, G.F. Assimilation of CO2, enzyme activation and photosynthetic electron transport in bean leaves, as affected by high light and ozone. New Phytol. 2002, 156, 377–388. [Google Scholar] [CrossRef]
- Zardzewiały, M.; Matlok, N.; Piechowiak, T.; Gorzelany, J.; Balawejder, M. Ozone Treatment as a Process of Quality Improvement Method of Rhubarb (Rheum. rhaponticum L.) Petioles during Storage. Appl. Sci. 2020, 10, 8282. [Google Scholar] [CrossRef]
- Xu, Y.; Charles, M.T.; Luo, Z.; Mimee, B.; Veronneau, P.Y.; Rolland, D.; Roussel, D. Preharvest Ultraviolet C Irradiation Increased the Level of Polyphenol Accumulation and Flavonoid Pathway Gene Expression in Strawberry Fruit. J. Agric. Food Chem. 2017, 65, 9970–9979. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Charles, M.T.; Luo, Z.; Mimee, B.; Tong, Z.; Véronneau, P.Y.; Roussel, D.; Rolland, D. Ultraviolet-C priming of strawberry leaves against subsequent Mycosphaerella fragariae infection involves the action of reactive oxygen species, plant hormones, and terpenes. Plant Cell Environ. 2019, 42, 815–831. [Google Scholar] [CrossRef] [PubMed]
- Sànchez-Ballesta, M.T.; Zacarias, L.; Granell, A.; Lafuente, M.T. Accumulation of PAL transcript and PAL activity as affected by heat-conditioning and low temperature storage and its relation to chilling sensitivity inmandarin fruits. J. Agric. Food Chem. 2000, 48, 2726–2731. [Google Scholar] [CrossRef]
- Restuccia, C.; Giusino, F.; Licciardello, F.; Randazzo, C.; Caggia, C.; Muratore, G. Biological control of peach fungal pathogens by commercial products and indigenous yeasts. J. Food Prot. 2006, 69, 2465–2470. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lu, Z.; Yu, Z.; Gao, X. Preservation of fresh-cut celery by treatment of ozonated water. Food Control 2005, 16, 279–283. [Google Scholar] [CrossRef]
- Grignani, E.; Mansi, A.; Cabella, R.; Castellano, P.; Tirabasso, A.; Sisto, R.; Spagnoli, M.; Fabrizi, G.; Frigerio, F.; Tranfo, G. Safe and Effective Use of Ozone as Air and Surface Disinfectant in the Conjuncture of COVID-19. Gases 2021, 1, 19–32. [Google Scholar] [CrossRef]
- Ozkan, R.; Smilanick, J.L.; Karabulut, O.A. Toxicity of ozone gas to conidia of Penicillium digitatum, Penicillium italicum and Botyris cinerea and control of gray mold on table grapes. Postharvest Biol. Technol. 2011, 60, 47–51. [Google Scholar]
- Sharma, R.R.; Demirci, A.; Beuchat, L.R.; Fett, W.F. Inactivation of Escherichia coli O157:H7 on inoculated alfalfa seeds with ozonated water and heat treatment. J. Food Prot. 2002, 65, 447–451. [Google Scholar] [CrossRef]
- Palou, L.; Crisoto, C.H.; Smilanick, J.L.; Adaskaveg, J.E.; Zoffoli, J.P. Effects of continuous 0.3 ppm ozone exposure on decay development and physical responses of peaches and table grapes in cold storage. Postharvest Biol. Technol. 2002, 24, 39–48. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, B.; Jin, W. Experimental investigation on decomposition of ethylene by ozone: Harmful product, food safety, and control strategy. J. Food Process. Preserv. 2021, 45, 15861. [Google Scholar] [CrossRef]
- Hwang, E.S.; Cash, J.N.; Zabik, M.J. Postharvest treatments for the reduction of mancozeb in fresh apples. J. Agric. Food Chem. 2001, 44, 3127–3132. [Google Scholar] [CrossRef]
- Djordjević, B.; Šavikin, K.; Zdunic, G.; Janković, T.; Vulić, T.; Oparnica, Č.; Radivojević, D. Biochemical properties of red currant varieties in relation to storage. Plant Foods Hum. Nutr. 2010, 65, 326–332. [Google Scholar] [CrossRef] [PubMed]
- McClurkin, J.D.; Maier, D.E.; Ileleji, K.E. Half-life time of ozone as a function of air movement and conditions in a sealed container. J. Stored Prod. Res. 2013, 55, 41–47. [Google Scholar] [CrossRef]
- Zapałowska, A.; Matłok, N.; Zardzewiały, M.; Piechowiak, T.; Balawejder, M. Effect of Ozone Treatment on the Quality of Sea Buckthorn (Hippophae rhamnoides L.). Plants 2021, 10, 847. [Google Scholar] [CrossRef] [PubMed]
- Matlok, N.; Piechowiak, T.; Gorzelany, J.; Zardzewiały, M.; Balawejder, M. Effect of Ozone Fumigation on Physiological Processes and Bioactive Compounds of Red-Veined Sorrel (Rumex sanguineus ssp. sanguineus). Agronomy 2020, 10, 1726. [Google Scholar] [CrossRef]
- Matłok, N.; Gorzelany, J.; Piechowiak, T.; Antos, P.; Zardzewiały, M.; Balawejder, M. Impact of Ozonation Process on the Content of Bioactive Compounds with Antioxidant Properties in Scots Pine (Pinus sylvestris L.) Shoots as Well as Yield and Composition of Essential Oils. Acta Univ. Cibiniensis Ser. E Food Technol. 2020, 2, 146–155. [Google Scholar] [CrossRef]
- Matłok, N.; Piechowiak, T.; Zardzewiały, M.; Gorzelany, J.; Balawejder, M. Effects of Ozone Treatment on Microbial Status and the Contents of Selected Bioactive Compounds in Origanum majorana L. Plants. Plants 2020, 9, 1637. [Google Scholar] [CrossRef]
- Gorzelany, J.; Migut, D.; Matłok, N.; Antos, P.; Kuźniar, P.; Balawejder, M. Impact of Pre-Ozonation on Mechanical Properties of Selected Genotypes of Cucumber Fruits During the Souring Process. Ozone Sci. Eng. 2017, 39, 188–195. [Google Scholar] [CrossRef]
- Lombardo, S.; Restuccia, C.; Pandino, G.; Licciardello, F.; Muratore, G.; Mauromicale, G. Influence of an O3-atmosphere storage on microbial growth and antioxidant contents of globe artichoke as affected by genotype and harvest time. Innov. Food Sci. Emerg. Technol. 2015, 27, 121–128. [Google Scholar] [CrossRef]
- Lin, F.; Lv, K.; Shufeng, M.A.; Wang, F.; Li, J.; Wang, L. Effects of ozone treatment on storage quality and antioxidant capacity of fresh-cut water fennel [Oenanthe javanica]. Food Sci. Technol. 2023, 43, 1–11. [Google Scholar] [CrossRef]
- Pretell-Vásquez, C.; Márquez-Villacorta, L.; Siche, R.; Hayayumi-Valdivia, M. Optimization of ozone concentration and storage time in green asparagus (Asparagus officinalis L.) using response surface methodology. Vitae 2022, 28, 3. [Google Scholar] [CrossRef]
- Horvitz, S.; Cantalejo, M.J. Effects of ozone and chlorine postharvest treatments on quality of fresh-cut red bell peppers. Int. J. Food Sci. Technol. 2012, 47, 1935–1943. [Google Scholar] [CrossRef]
- Liu, H.; Xu, L.; Yu, F.; Tan, J.; Cao, L.; Xing, Y.; Xu, Q.; Yang, S.; Liu, X.; Yang, P.; et al. Effects of different ozone treatments on the storage quality and stability of fresh peeled garlic. RSC Adv. 2021, 11, 22530–22543. [Google Scholar] [CrossRef] [PubMed]
- Karaca, H.; Velioglu, Y.S. Effects of Ozone Treatments on Microbial Quality and Some Chemical Properties of Lettuce, Spinach, and Parsley. Postharvest Biol. Technol. 2014, 88, 46–53. [Google Scholar] [CrossRef]
- El-Moniem, A.; Eman, A.A.; Yousef, A.R.; Abdel-Razek, A.G.; Badr, A.N.; Mahmoud, T.S.M. Effects of Postharvest Gaseous Ozone Treatment on Quality Attributes and Extending Storage Life of Fresh Cut ‘Hass’ Avocado Fruits. Egypt. J. Chemistry. Egypt. J. Chem. 2022, 65, 27–37. [Google Scholar] [CrossRef]
- Li, C.; Wang, S.; Wang, J.; Wu, Z.; Xu, Y.; Wu, Z. Ozone treatment promotes physicochemical properties and antioxidant capacity of fresh-cut red pitaya based on phenolic metabolism. Front. Nutr. 2022, 9, 1016607. [Google Scholar] [CrossRef] [PubMed]
- Alothman, M.; Kaur, B.; Fazilah, A.; Bhat, R.; Karim, A. Ozone-induced changes of antioxidant capacity of fresh-cut tropical fruits. Innov. Food Sci. Emerg. Technol. 2010, 11, 666–671. [Google Scholar] [CrossRef]
- Rodoni, L.; Casadei, N.; Concellón, A.; Chaves, A.; Vicente, A.R. Effect of short-term ozone treatments on tomato (Solanum lycopersicum L.) fruit quality and cell wall degradation. J. Agric. Food Chem. 2010, 58, 594–599. [Google Scholar] [CrossRef]
- Barth, M.M.; Zhou, C.; Mercier, J.; Payne, F.A. Ozone storage effects on anthocyanin content and fungal growth in blackberries. J. Food Sci. 1995, 60, 1286–1288. [Google Scholar] [CrossRef]
- Minas, I.S.; Karaoglanidis, G.S.; Manganaris, G.A.; Vasilakakis, M. Effect of ozone application during cold storage of kiwifruit on the development of stem-end rot caused by Botrytis cinerea. Postharvest Biol. Technol. 2010, 58, 203–210. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Restuccia, C.; Muratore, G.; Licciardello, F.; Mauro, R.P.; Pesce, R.; Mauromicale, G. Effect of cultivar x ozone treatment interaction on the total polyphenols content and antioxidant activity of globe artichoke. Ital. J. Agron. 2015, 10, 105–107. [Google Scholar] [CrossRef]
- Balawejder, M.; Matłok, N.; Sowa, W.; Kończyk, N.; Piechowiak, T.; Zapałowska, A. Effect of two types of ozone treatments on the quality of apple fruits. Acta Univ. Cibiniensis. Ser. E Food Technol. 2021, 25, 285–292. [Google Scholar] [CrossRef]
- Vettraino, A.M.; Bianchini, L.; Caradonna, V.; Forniti, R.; Goffi, V.; Zambelli, M.; Testa, A.; Vinciguerra, V.; Botondi, R. Ozone gas as a storage treatment to control Gnomoniopsis castanea, preserving chestnut quality. J. Sci. Food Agric. 2019, 99, 6060–6065. [Google Scholar] [CrossRef]
- Glowacz, M.; Colgan, R.; Rees, D. Influence of continuous exposure to gaseous ozone on the quality of red bell peppers, cucumbers and zucchini. Postharvest Biol. Technol. 2015, 99, 1–8. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef] [PubMed]
- Anam, C.; Perwitasari, A.; Nur, J.; Hanifah, Y.; Nurul, E.; Sasmita, E.; Restiwijaya, M.; Kinandana, A.; Arianto, F.; Nur, M. Effect of ozone treatment on microbiological and physicochemical properties of soymilk beverage. IOP Conf. Ser. Earth Environ. Sci. 2020, 443, 012100. [Google Scholar] [CrossRef]
- Panebianco, F.; Rubiola, S.; Di Ciccio, P.A. The Use of Ozone as an Eco-Friendly Strategy against Microbial Biofilm in Dairy Manufacturing Plants: A Review. Microorganisms 2022, 10, 162. [Google Scholar] [CrossRef]
- Ong, M.K.; Ali, A. Antifungal action of ozone against Colletotrichum gloeosporioides and control of papaya anthracnose. Postharvest Biol. Technol. 2015, 100, 113–119. [Google Scholar] [CrossRef]
- Zeynep, B.; Guzel-Seydim, A.; Greene, A.C. Use of ozone in the food industry. LWT Food Sci. Technol. 2004, 37, 453–460. [Google Scholar] [CrossRef]
- Rangel, K.; Cabral, F.O.; Lechuga, G.C.; Carvalho, J.P.R.S.; Villas-Bôas, M.H.S.; Midlej, V.; De-Simone, S.G. Detrimental Effect of Ozone on Pathogenic Bacteria. Microorganisms 2022, 10, 40. [Google Scholar] [CrossRef]
- Xue, W.; Macleod, J.; Blaxland, J. The Use of Ozone Technology to Control Microorganism Growth, Enhance Food Safety and Extend Shelf Life: A Promising Food Decontamination Technology. Foods 2023, 12, 814. [Google Scholar] [CrossRef] [PubMed]
- Porto, Y.D.; Trombete, F.M.; Freitas-Silva, O.; de Castro, I.M.; Direito, G.M.; Ascheri, J.L.R. Gaseous Ozonation to Reduce Aflatoxins Levels and Microbial Contamination in Corn Grits. Microorganisms 2019, 7, 220. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H. Effect of ozone application on postharvest quality and microbiological state of “Zaghloul” date palm fruits. J. Plant Prod. 2019, 7, 43–51. [Google Scholar] [CrossRef]
- Pérez, A.G.; Sanz, C.; Ríos, J.J.; Olías, R.; Olías, J.M. Effects of ozone treatment on postharvest strawberry quality. J. Agric. Food Chem. 1999, 47, 1652–1656. [Google Scholar] [CrossRef] [PubMed]
- Yaseen, T.; D’Onghia, A.; Ricelli, A. The use of ozone in strawberry post harvest conservation. IOBC-WPRS Bull. 2013, 86, 143–148. [Google Scholar]
- Palou, L.; Smilanick, J.L.; Crisosto, C.H.; Mansour, M. Effect of gaseous ozone exposure on the development of green and blue molds on cold stored citrus fruit. Plant Dis. 2001, 85, 632–638. [Google Scholar] [CrossRef]
- Zorlugenç, B.; Kiroğlu Zorlugenç, F.; Oztekin, S.; Evliya, I.B. The influence of gaseous ozone and ozonated water on microbial flora and degradation of aflatoxin B (1) in dried figs. Food Chem. Toxicol. 2008, 46, 3593–3597. [Google Scholar] [CrossRef]
- Pinto, L.; Palma, A.; Cefola, M.; Pace, B.; D’Aquino, S.; Carboni, C.; Baruzzi, F. Effect of modified atmosphere packaging (MAP) and gaseous ozone pre-packaging treatment on the physico-chemical, microbiological and sensory quality of small berry fruit. Food Packag. Shelf Life 2020, 26, 100573. [Google Scholar] [CrossRef]
- Ölmez, H.; Akbas, M.Y. Optimization of ozone treatment of fresh-cut green leaf lettuce. J. Food Eng. 2009, 90, 487–494. [Google Scholar] [CrossRef]
- Restuccia, C.; Lombardo, S.; Pandino, G.; Licciardello, F.; Muratore, G.; Mauromicale, G. An innovative combined water ozonisation/O3-atmosphere storage for preserving the overall quality of two globe artichoke cultivars. Innov. Food Sci. Emerg. Technol. 2014, 21, 82–89. [Google Scholar] [CrossRef]
- Liew, C.L.; Prange, R.K. Effect of ozone and storage temperature on postharvest diseases and physiology of carrots (Daucus carota L.). J. Am. Soc. Hortic. Sci. 1994, 119, 563–567. [Google Scholar] [CrossRef]
- Cárdenas, F.C.; Andrés, S.; Giannuzzi, L.; Zaritzky, N. Antimicrobial action and effects on beef quality attributes of a gaseous ozone treatment at refrigeration temperatures. Food Control 2011, 22, 1442–1447. [Google Scholar] [CrossRef]
- Giménez, B.; Graiver, N.; Giannuzzi, L.; Zaritzky, N. Treatment of beef with gaseous ozone: Physicochemical aspects and antimicrobial effects on heterotrophic microflora and listeria monocytogenes. Food Control 2021, 121, 107602. [Google Scholar] [CrossRef]
- Ayranci, U.G.; Ozunlu, O.; Ergezer, H.; Karaca, H. Effects of Ozone Treatment on Microbiological Quality and Physicochemical Properties of Turkey Breast Meat. Ozone Sci. Eng. 2020, 42, 95–103. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zardzewiały, M.; Matłok, N.; Piechowiak, T.; Balawejder, M. The Influence of Ozonation Carried Out during Vegetation on the Content of Selected Bioactive Phytochemicals and the Microbiological Load of Tubers of Raphanus sativus var. sativus. Agriculture 2023, 13, 2153. https://doi.org/10.3390/agriculture13112153
Zardzewiały M, Matłok N, Piechowiak T, Balawejder M. The Influence of Ozonation Carried Out during Vegetation on the Content of Selected Bioactive Phytochemicals and the Microbiological Load of Tubers of Raphanus sativus var. sativus. Agriculture. 2023; 13(11):2153. https://doi.org/10.3390/agriculture13112153
Chicago/Turabian StyleZardzewiały, Miłosz, Natalia Matłok, Tomasz Piechowiak, and Maciej Balawejder. 2023. "The Influence of Ozonation Carried Out during Vegetation on the Content of Selected Bioactive Phytochemicals and the Microbiological Load of Tubers of Raphanus sativus var. sativus" Agriculture 13, no. 11: 2153. https://doi.org/10.3390/agriculture13112153
APA StyleZardzewiały, M., Matłok, N., Piechowiak, T., & Balawejder, M. (2023). The Influence of Ozonation Carried Out during Vegetation on the Content of Selected Bioactive Phytochemicals and the Microbiological Load of Tubers of Raphanus sativus var. sativus. Agriculture, 13(11), 2153. https://doi.org/10.3390/agriculture13112153







