Mixed Silage of Banana Pseudostem and Maize Stover on Ethiopian Smallholder Farms: Effect of Fermentation Package and Location on Microbiological and Nutritional Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw material Collection and Experimental Plan
2.2. Filling and Compaction of Crums and Bags
2.3. Sampling
2.4. pH Determination
2.5. Microbial Counts
2.6. Nutritional Analyses
2.7. Statistical Analysis
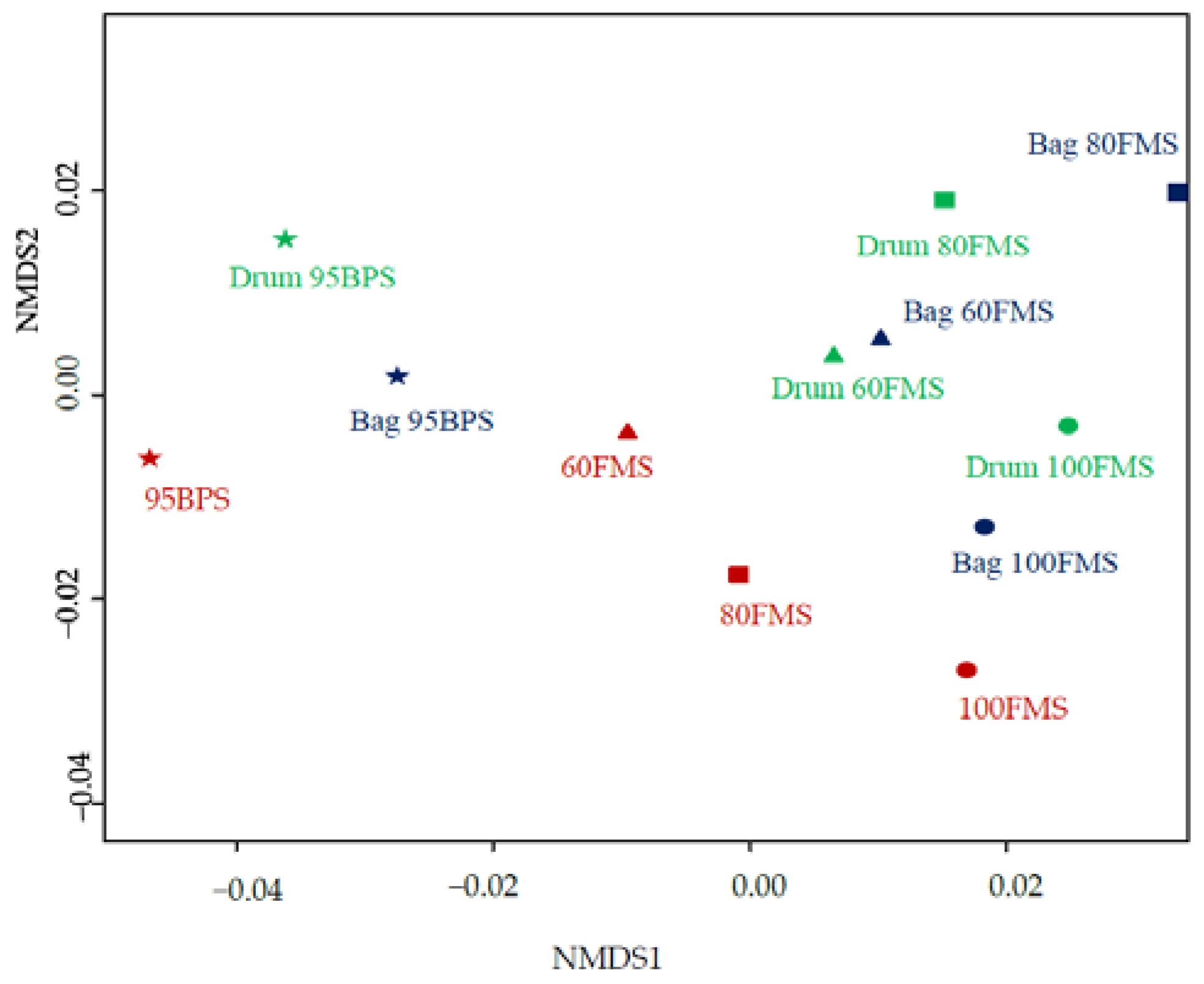
3. Results
3.1. pH and Microbial Counts
3.1.1. Fresh Mixtures
3.1.2. Mixed Silages
3.2. Nutritional Value Evaluation
3.2.1. Fresh Mixtures
3.2.2. Mixed Silages
4. Discussion
4.1. pH and Microbial Counts
4.1.1. Fresh Mixtures
4.1.2. Mixed Silages
4.2. Nutritional Value Evaluation
4.2.1. Fresh Mixtures
4.2.2. Mixed Silages
4.3. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Yisehak, K.; Biruk, K.; Abegaze, B.; Janssens, G.P.J. Growth of Sheep Fed Tannin-Rich Albizia Gummifera with or without Polyethylene Glycol. Trop. Anim. Health Prod. 2014, 46, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Wallie, M.; Mekasha, Y.; Urge, M.; Abebe, G.; Goetsch, A.L. Effects of Form of Leftover Khat (Catha edulis) on Feed Intake, Digestion, and Growth Performance of Hararghe Highland Goats. Small Rumin. Res. 2012, 102, 1–6. [Google Scholar] [CrossRef]
- Saylor, B.A.; Min, D.H.; Bradford, B.J. Productivity of Lactating Dairy Cows Fed Diets with Teff Hay as the Sole Forage. J. Dairy Sci. 2018, 101, 5984–5990. [Google Scholar] [CrossRef] [PubMed]
- Geberemariyam, T.; Getu, K.; Mulugeta, W.; Dereje, F.; Aeimro, K.; Mesfin, D.; Betlehem, M.; Endale, Y. Feed Intake and Growth Performance of Jersey Calves in Maize Stover Silage Based Total Mixed Ration. J. Biol. Agric. Healthc. 2020, 10, 9–13. [Google Scholar] [CrossRef]
- Mekuriaw, S.; Tsunekawa, A.; Ichinohe, T.; Tegegne, F.; Haregeweyn, N.; Kobayashi, N.; Tassew, A.; Mekuriaw, Y.; Walie, M.; Tsubo, M.; et al. Effect of Feeding Improved Grass Hays and Eragrostis Tef Straw Silage on Milk Yield, Nitrogen Utilization, and Methane Emission of Lactating Fogera Dairy Cows in Ethiopia. Animals 2020, 10, 1021. [Google Scholar] [CrossRef]
- Kassa, A. Review of Performance, Marketing and Milk Processing of Dairy Cattle Production System in Ethiopia. J. Dairy Vet. Anim. Res. 2019, 8, 1–9. [Google Scholar]
- Abate, T.; Shiferaw, B.; Menkir, A.; Wegary, D.; Kebede, Y.; Tesfaye, K.; Kassie, M.; Bogale, G.; Tadesse, B.; Keno, T. Factors That Transformed Maize Productivity in Ethiopia. Food Secur. 2015, 7, 965–981. [Google Scholar] [CrossRef]
- Mellisse, B.T.; Descheemaeker, K.; Giller, K.E.; Abebe, T.; van de Ven, G.W.J. Are Traditional Home Gardens in Southern Ethiopia Heading for Extinction? Implications for Productivity, Plant Species Richness and Food Security. Agric. Ecosyst. Environ. 2018, 252, 1–13. [Google Scholar] [CrossRef]
- Moselhy, M.A.; Borba, J.P.; Borba, A.E.S. Improving the Nutritive Value, in Vitro Digestibility and Aerobic Stability of Hedychium Gardnerianum Silage through Application of Additives at Ensiling Time. Anim. Feed Sci. Technol. 2015, 206, 8–18. [Google Scholar] [CrossRef]
- Tadesse, M.; Fentahun, M.; Tadesse, G. Dairy Farming and Its Economic Importance in Ethiopia: A Review. World J. Dairy Food Sci. 2017, 12, 42–51. [Google Scholar]
- Gallo, A.; Bernardes, T.F.; Copani, G.; Fortunati, P.; Giuberti, G.; Bruschi, S.; Bryan, K.A.; Nielsen, N.G.; Witt, K.L.; Masoero, F. Effect of Inoculation with Lactobacillus Buchneri LB1819 and Lactococcus Lactis O224 on Fermentation and Mycotoxin Production in Maize Silage Compacted at Different Densities. Anim. Feed Sci. Technol. 2018, 246, 36–45. [Google Scholar] [CrossRef]
- Wilkinson, J.M.; Davies, D.R. The Aerobic Stability of Silage: Key Findings and Recent Developments. Grass Forage Sci. 2013, 68, 1–19. [Google Scholar] [CrossRef]
- Mitiku, A.A.; Vandeweyer, D.; Lievens, B.; Bossaert, S.; Crauwels, S.; Aernouts, B.; Kechero, Y.; Van Campenhout, L. Microbial Profile during Fermentation and Aerobic Stability of Ensiled Mixtures of Maize Stover and Banana Pseudostem in South Ethiopia. J. Appl. Microbiol. 2022, 132, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Mitiku, A.A.; Andeta, A.F.; Borremans, A.; Lievens, B.; Bossaert, S.; Crauwels, S.; Aernouts, B.; Kechero, Y.; Van Campenhout, L. Silage Making of Maize Stover and Banana Pseudostem under South Ethiopian Conditions: Evolution of PH, Dry Matter and Microbiological Profile. Microb. Biotechnol. 2020, 13, 1477–1488. [Google Scholar] [CrossRef]
- Andeta, A.F.; Vandeweyer, D.; Teffera, E.F.; Woldesenbet, F.; Verreth, C.; Crauwels, S.; Lievens, B.; Vancampenhout, K.; Van Campenhout, L. Effect of Fermentation System on the Physicochemical and Microbial Community Dynamics during Enset (Ensete ventricosum) Fermentation. J. Appl. Microbiol. 2019, 126, 842–853. [Google Scholar] [CrossRef]
- Andeta, A.F.; Vandeweyer, D.; Woldesenbet, F.; Eshetu, F.; Hailemicael, A.; Woldeyes, F.; Crauwels, S.; Lievens, B.; Ceusters, J.; Vancampenhout, K.; et al. Fermentation of Enset (Ensete ventricosum) in the Gamo Highlands of Ethiopia: Physicochemical and Microbial Community Dynamics. Food Microbiol. 2018, 73, 342–350. [Google Scholar] [CrossRef]
- Santos, A.O.; Ávila, C.L.S.; Schwan, R.F. Selection of Tropical Lactic Acid Bacteria for Enhancing the Quality of Maize Silage. J. Dairy Sci. 2013, 96, 7777–7789. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists: Washington DC, USA, 1995. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Tilley, J.M.A.; Terry, R.A. A Two-Stage Technique for the Invitro Digestions of Forage Crops. Grass Forage Sci. 1963, 18, 104–111. [Google Scholar] [CrossRef]
- Fievez, V.; Dohme, F.; Danneels, M.; Raes, K.; Demeyer, D. Fish Oils as Potent Rumen Methane Inhibitors and Associated Effects on Rumen Fermentation in Vitro and in Vivo. Anim. Feed Sci. Technol. 2003, 104, 41–58. [Google Scholar] [CrossRef]
- Ørskov, E.R.; McDonald, I. The Estimation of Protein Degradability in the Rumen from Incubation Measurements Weighted According to Rate of Passage. J. Agric. Sci. 1979, 92, 499–503. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D. R Package, version 2; Vegan: Community Ecology Package; 2019; Available online: https://cran.r-project.org/package=vegan (accessed on 20 September 2023).
- McEniry, J.; O’Kiely, P.; Clipson, N.J.; Forristal, P.D.; Doyle, E.M. The Microbiological and Chemical Composition of Baled and Precision-Chop Silages on a Sample of Farms in County Meath. Ir. J. Agric. Food Res. 2006, 45, 73–83. [Google Scholar]
- Wambacq, E.; Latré, J.P.; Haesaert, G. The Effect of Lactobacillus Buchneri Inoculation on the Aerobic Stability and Fermentation Characteristics of Alfalfa-Ryegrass, Red Clover and Maize Silage. Agric. Food Sci. 2013, 22, 127–136. [Google Scholar] [CrossRef]
- Ni, K.; Wang, F.; Zhu, B.; Yang, J.; Zhou, G.; Pan, Y.; Tao, Y.; Zhong, J. Effects of Lactic Acid Bacteria and Molasses Additives on the Microbial Community and Fermentation Quality of Soybean Silage. Bioresour. Technol. 2017, 238, 706–715. [Google Scholar] [CrossRef]
- Dolci, P.; Tabacco, E.; Cocolin, L.; Borreani, G. Microbial Dynamics during Aerobic Exposure of Corn Silage Stored under Oxygen Barrier or Polyethylene Films. Appl. Environ. Microbiol. 2011, 77, 7499–7507. [Google Scholar] [CrossRef]
- Ni, K.; Zhao, J.; Zhu, B.; Su, R.; Pan, Y.; Ma, J.; Zhou, G.; Tao, Y.; Liu, X.; Zhong, J. Assessing the Fermentation Quality and Microbial Community of the Mixed Silage of Forage Soybean with Crop Corn or Sorghum. Bioresour. Technol. 2018, 265, 563–567. [Google Scholar] [CrossRef]
- Rochana, A.; Dhalika, T.; Budiman, A.; Kamil, K.A. Nutritional Value of a Banana Stem (Musa Paradisiaca Val) of Anaerobic Fermentation Product Supplemented with Nitrogen, Sulphur and Phosphorus Sources. Pak. J. Nutr. 2017, 16, 738–742. [Google Scholar] [CrossRef]
- Wang, C.F.; Muhammad, A.U.R.; Liu, Z.Y.; Huang, B.Z.; Cao, B.H.C.B.H. Effects of Ensiling Time on Banana Pseudo-Stem Silage Chemical Composition, Fermentation and in Sacco Rumen DegradationEffects of Ensiling Time on Banana Pseudo-Stem Silage Chemical Composition, Fermentation and in Sacco Rumen Degradation. J. Anim. Plant Sci. 2016, 26, 339–346. [Google Scholar]
- Lima, E.M.d.; Gonçalves, L.C.; Keller, K.M.; Rodrigues, J.A.d.S.; Santos, F.P.C.; Michel, P.H.F.; Raposo, V.S.; Jayme, D.G. Re-Ensiling and Its Effects on Chemical Composition, in Vitro Digestibility, and Quality of Corn Silage after Different Lengths of Exposure to Air. Can. J. Anim. Sci. 2016, 97, 250–257. [Google Scholar] [CrossRef]
- Filya, I.; Sucu, E. The Effects of Lactic Acid Bacteria on the Fermentation, Aerobic Stability and Nutritive Value of Maize Silage. Grass Forage Sci. 2010, 65, 446–455. [Google Scholar] [CrossRef]

| pH and Microbial Counts (log10 cfu/g) | Before Fermentation (Day 0) | Chano Dorga | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drums (Day 60) | Bags (Day 60) | |||||||||||
| 100FMS | 80FMS | 60FMS | 95BPS | 100FMS | 80FMS | 60FMS | 95BPS | 100FMS | 80FMS | 60FMS | 95BPS | |
| pH | 5.385 | 4.844,5 | 5.315 | 5.956 | 3.561,2 | 3.771,2 | 3.611,2 | 3.501 | 4.333,4 | 3.971,2,3 | 4.072,3 | 3.831,2,3 |
| Total viable count | 8.5410 | 8.147,8,9,10 | 8.379,10 | 7.766,7,8,9 | 7.605,6,7,8 | 7.403,4,5,6,7 | 7.676,7,8,9 | 6.451,2 | 8.188,9,10 | 7.786,7,8,9,10 | 8.087,8,9,10 | 6.701,2,3 |
| Enterobacteria-ceae count | 6.843 | 7.143 | 6.633 | 6.623 | <11 | <11 | <11 | <11 | 5.062,3 | 2.681,2 | 2.151,2 | <1.01 |
| Lactic acid bacteria count | 7.032,3,4 | 8.156 | 7.092,3,4,5 | 7.192,3,4,5,6 | 7.533,4,5,6 | 7.453,4,5,6 | 7.6745,6 | 6.291,2 | 8.066 | 7.343,4,5,6 | 8.076 | 6.651,2,3 |
| Yeasts and molds count | 6.856 | 6.734,5 | 6.846 | 5.481,2,3,4 | 5.021,2,3 | 4.941,2,3 | 5.191,2,3 | 4.621,2,3 | 5.672,3,4,5 | 5.853,4,5 | 5.521,2,3,4 | 4.341 |
| Clostridium endospore count | 4.632 | 4.452 | 4.592 | 4.091,2 | 4.682 | 4.562 | 4.592 | 4.121,2 | 4.842 | 4.622 | 4.682 | 4.412 |
| pH and microbial counts (log10 cfu/g) | Kolla Shelle | p-value M × P × L interaction | ||||||||||
| Drums (day 60) | Bags (day 60) | |||||||||||
| 100FMS | 80FMS | 60FMS | 95BPS | 100FMS | 80FMS | 60FMS | 95BPS | |||||
| pH | 3.731,2 | 3.861,2,3 | 3.741,2 | 3.691,2 | 3.711,2 | 3.811,2,3 | 3.731,2 | 3.761,2 | 0.001 | |||
| Total viable count | 7.886,7,8,9,10 | 8.107,8,9,10 | 7.494,5,6,7,8 | 6.871,2,3,4,5 | 7.172,3,4,5,6 | 7.484,5,6,7,8 | 6.771,2,3,4 | 6.221 | 0.001 | |||
| Enterobacteria-ceae count | <11 | <11 | <11 | <11 | 2.291,2 | 2.341,2 | 2.231,2 | 1.701,2 | 0.001 | |||
| Lactic acid bacteria count | 7.884,5,6 | 8.055,6 | 7.513,4,5,6 | 6.641,2,3 | 6.992,3,4 | 7.433,4,5,6 | 6.681,2,3 | 6.011 | 0.001 | |||
| Yeasts and molds count | 5.221,2,3 | 4.771,2,3 | 4.481,2 | 5.071,2,3 | 4.761,2,3 | 5.571,2,3,4,5 | 5.471,2,3,4 | 4.601,2,3 | 0.001 | |||
| Clostridium endospore count | 4.412 | 4.512 | 4.171,2 | 3.401 | 4.232 | 4.672 | 4.151,2 | 4.532 | 0.001 | |||
| Nutritional Values | Mixture (M) | Package (P) | M × P |
|---|---|---|---|
| DM (g/kg FM) | 0.023 | ||
| Ash (g/kg DM) | <0.001 | <0.001 | 0.087 |
| OM (g/kg DM) | <0.001 | <0.001 | 0.188 |
| CP (g/kg DM) | 0.001 | ||
| NDF (g/kg DM) | 0.024 | ||
| ADF (g/kg DM) | 0.047 | ||
| ADL (g/kg DM) | 0.027 | ||
| IVDMD (%) | 0.001 | ||
| IVOMD (%) | 0.001 | ||
| ME (MJ/kg DM) | 0.001 | ||
| ED (%) | <0.001 | <0.001 | 0.052 |
| Nutritional Values | Before Fermentation (day 0) | Drums (Day 60) | Bags (Day 60) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100FMS | 80FMS | 60FMS | 95BPS | 100FMS | 80FMS | 60FMS | 95BPS | 100FMS | 80FMS | 60FMS | 95BPS | |
| DM (g/kg FM) | 623.763,4 | 594.791,2 | 580.891 | 603.241,2,3 | 652.104,5 | 610.702,3 | 608.301,2,3 | 605.301,2,3 | 662.505 | 605.401,2,3 | 601.101,2,3 | 599.791,2,3 |
| Ash (g/kg DM) | 89.40aA | 104.27bA | 104.23bA | 91.03aA | 95.57aB | 119.63cB | 109.03bB | 95.73aB | 107.20aC | 123.80cC | 113.40bC | 103.93aC |
| OM (g/kg DM) | 910.03bC | 894.53aC | 893.83aC | 909.53bC | 904.43cB | 880.37aB | 890.97bB | 904.27cB | 892.80cA | 876.20aA | 886.60bA | 896.07dA |
| CP (g/kg DM) | 55.133,4 | 44.302 | 43.631,2 | 33.431 | 71.035 | 71.275 | 63.104,5 | 46.372,3 | 69.335 | 82.206 | 65.835 | 46.972,3 |
| NDF (g/kg DM) | 588.035 | 590.275 | 540.103,4 | 413.271 | 629.275,6 | 595.135 | 584.834,5 | 473.932 | 672.806 | 617.305 | 609.105 | 503.072,3 |
| ADF (g/kg DM) | 344.503,4 | 355.173,4 | 320.502,3 | 270.971 | 359.134 | 364.934 | 349.573,4 | 286.931,2 | 409.035 | 377.774,5 | 356.803,4 | 300.401,2 |
| ADL (g/kg DM) | 32.301 | 38.531,2,3 | 38.701,2,3 | 45.473 | 35.071,2 | 40.772,3 | 42.233 | 54.074 | 43.173 | 43.873 | 42.132,3 | 54.204 |
| IVDMD (%) | 52.081 | 53.522,3 | 55.063,4 | 50.521 | 60.586 | 64.087 | 60.076 | 58.755,6 | 58.805,6 | 57.525 | 60.536 | 55.223,4 |
| IVOMD (%) | 42.761,2 | 44.122,3 | 46.073,4 | 41.141 | 51.876 | 55.587 | 51.326 | 49.925,6 | 49.975,6 | 48.614,5 | 51.826 | 46.173,4 |
| ME (MJ/kg DM) | 6.901,2 | 7.122,3 | 7.502,3,4 | 6.581 | 8.307 | 8.898 | 8.216,7 | 8.005,6,7 | 7.995,6,7 | 7.784,5,6 | 8.297 | 7.402,3,4 |
| ED (%) | 3.64cA | 3.55cA | 3.28bA | 3.18aA | 4.07cB | 4.06cB | 3.84bB | 3.53aB | 3.90bB | 4.39cB | 3.91bB | 3.45aB |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitiku, A.A.; Vandeweyer, D.; Adriaens, I.; Kechero, Y.; Van Campenhout, L.; Aernouts, B. Mixed Silage of Banana Pseudostem and Maize Stover on Ethiopian Smallholder Farms: Effect of Fermentation Package and Location on Microbiological and Nutritional Evaluation. Agriculture 2023, 13, 2152. https://doi.org/10.3390/agriculture13112152
Mitiku AA, Vandeweyer D, Adriaens I, Kechero Y, Van Campenhout L, Aernouts B. Mixed Silage of Banana Pseudostem and Maize Stover on Ethiopian Smallholder Farms: Effect of Fermentation Package and Location on Microbiological and Nutritional Evaluation. Agriculture. 2023; 13(11):2152. https://doi.org/10.3390/agriculture13112152
Chicago/Turabian StyleMitiku, Ashenafi Azage, Dries Vandeweyer, Ines Adriaens, Yisehak Kechero, Leen Van Campenhout, and Ben Aernouts. 2023. "Mixed Silage of Banana Pseudostem and Maize Stover on Ethiopian Smallholder Farms: Effect of Fermentation Package and Location on Microbiological and Nutritional Evaluation" Agriculture 13, no. 11: 2152. https://doi.org/10.3390/agriculture13112152
APA StyleMitiku, A. A., Vandeweyer, D., Adriaens, I., Kechero, Y., Van Campenhout, L., & Aernouts, B. (2023). Mixed Silage of Banana Pseudostem and Maize Stover on Ethiopian Smallholder Farms: Effect of Fermentation Package and Location on Microbiological and Nutritional Evaluation. Agriculture, 13(11), 2152. https://doi.org/10.3390/agriculture13112152





