Analyzing a Saturation Effect of Nitrogen Fertilization on Baking Volume and Grain Protein Concentration in Wheat
Abstract
1. Introduction
2. Material and Methods
2.1. Plant Cultivation
2.2. Determination of Yield Variables, and Grain N and S Concentration
2.3. Quantification of Grain Protein Sub Fractions
2.3.1. Osborne Fractionation
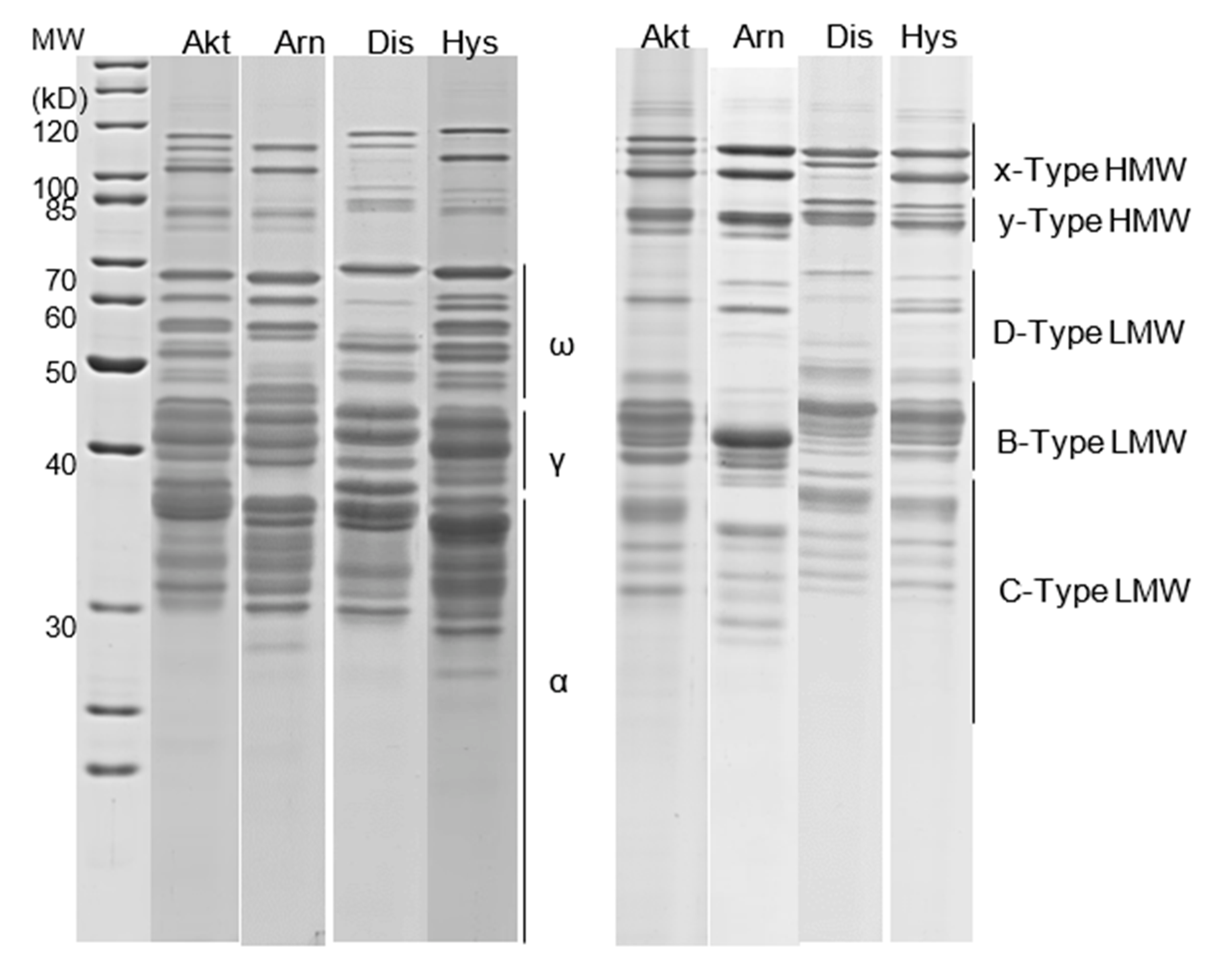
2.3.2. SDS-PAGE
2.3.3. Quantification of GLIA and GLUT Fractions
2.4. Quantification of GLUT Macropolymer
2.5. Micro-Scale Baking Tests
2.6. Statistical Analysis
3. Results
3.1. Grain Yield, Grain Protein Concentration and Mean N and S Content per Grain
3.2. Concentration of Grain Protein Fractions and GMP
3.3. Ratios between Different Gluten Fractions
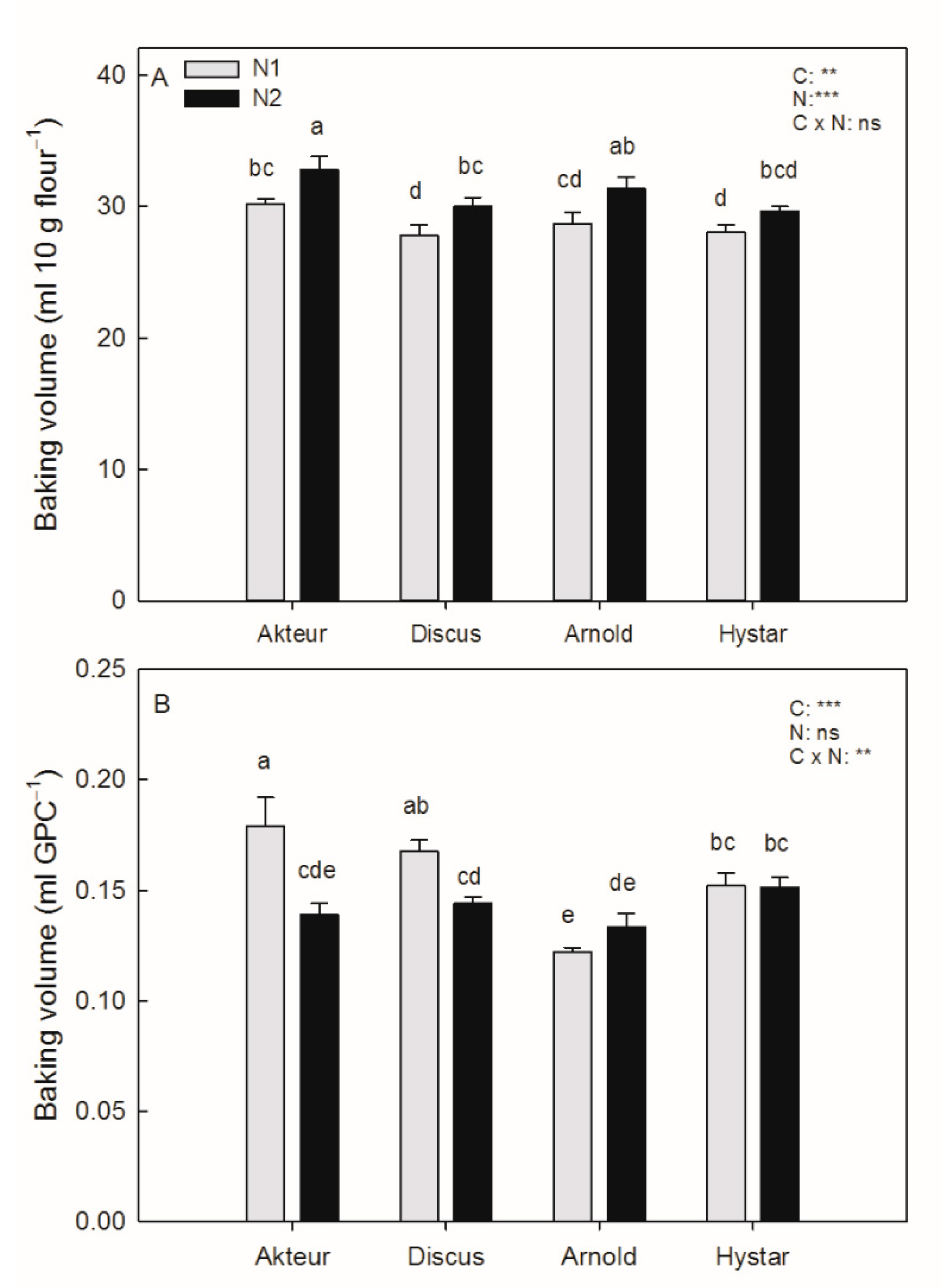
3.4. Baking Volume
3.5. Effect of Grain Protein Fractions on Baking Volume
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef] [PubMed]
- Dier, M.; Hüther, L.; Schulze, W.X.; Erbs, M.; Köhler, P.; Weigel, H.J.; Zörb, C. Elevated atmospheric CO2 concentration has limited effect on wheat grain quality regardless of nitrogen supply. J. Agric. Food Chem. 2020, 68, 3711–3721. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, D.; Pfitzner, C.; Haase, N.U.; Hüsken, A.; Prüfer, H.; Greef, J.M.; Rühl, G. New strategies for a reliable assessment of baking quality of wheat–Rethinking the current indicator protein content. J. Cereal Sci. 2017, 77, 126–134. [Google Scholar] [CrossRef]
- Rossmann, A.; Buchner, P.; Savill, G.P.; Hawkesford, M.J.; Scherf, K.A.; Mühling, K.H. Foliar N application at anthesis alters grain protein composition and enhances baking quality in winter wheat only under a low N fertiliser regimen. Eur. J. Agron. 2019, 109, 125909. [Google Scholar] [CrossRef]
- Zörb, C.; Ludewig, U.; Hawkesford, M.J. Perspective on Wheat Yield and Quality with Reduced Nitrogen Supply. Trends Plant Sci. 2018, 23, 1029–1037. [Google Scholar] [CrossRef]
- Lafiandra, D.; Shewry, P.R. Wheat Glutenin polymers 2, the role of wheat glutenin subunits in polymer formation and dough quality. J. Cereal Sci. 2022, 106, 103487. [Google Scholar] [CrossRef]
- Wieser, H.; Kieffer, R. Correlations of the amount of gluten protein types to the technological properties of wheat flours determined on a micro-scale. J. Cereal Sci. 2001, 34, 19–27. [Google Scholar] [CrossRef]
- Bonilla, J.C.; Erturk, M.Y.; Kokini, J.L. Understanding the role of gluten subunits (LMW, HMW glutenins and gliadin) in the networking behavior of a weak soft wheat dough and a strong semolina wheat flour dough and the relationship with linear and non-linear rheology. Food Hydrocoll. 2020, 108, 106002. [Google Scholar] [CrossRef]
- Gianibelli, M.C.; Larroque, O.R.; MacRitchie, F.; Wrigley, C.W. Biochemical, genetic, and molecular characterization of wheat endosperm proteins. Cereal Chem. 2001, 78, 635–646. [Google Scholar] [CrossRef]
- Don, C.; Lookhart, G.; Naeem, H.; MacRitchie, F.; Hamer, R.J. Heat stress and genotype affect the glutenin particles of the glutenin macropolymer-gel fraction. J. Cereal Sci. 2005, 42, 69–80. [Google Scholar] [CrossRef]
- Shewry, P.R.; Popineau, Y.; Lafiandra, D.; Belton, P. Wheat glutenin subunits and dough elasticity: Findings of the EUROWHEAT project. Trends Food Sci. Technol. 2000, 11, 433–441. [Google Scholar] [CrossRef]
- Rekowski, A.; Wimmer, M.A.; Henkelmann, G.; Zörb, C. Is a change of protein composition after late application of nitrogen sufficient to improve the baking quality of winter wheat? Agriculture 2019, 9, 101. [Google Scholar] [CrossRef]
- Rossmann, A.; Scherf, K.A.; Rühl, G.; Greef, J.M.; Mühling, K.H. Effects of a late N fertiliser dose on storage protein composition and bread volume of two wheat varieties differing in quality. J. Cereal Sci. 2020, 93, 102944. [Google Scholar] [CrossRef]
- Yue, H.; Jiang, D.; Dai, T.; Qin, X.; Jing, Q.; Cao, W. Effect of nitrogen application rate on content of glutenin macropolymer and high molecular weight glutenin subunits in grains of two winter wheat cultivars. J. Cereal Sci. 2007, 45, 248–256. [Google Scholar] [CrossRef]
- Békés, F. New aspects in quality related wheat research: II. New methodologies for better quality wheat. Cereal Res. Commun. 2012, 40, 307–333. [Google Scholar] [CrossRef][Green Version]
- Henkelmann, G.; Volkheimer, B.; Zörb, C.; von Tucher, S.; Haase, N.U. Backversuche im Vergleich: Rapid-Mix-Test, Mikro- und Kleinbackversuch. Getreide Mehl Brot. 2020, 2, 50–57. [Google Scholar]
- Dier, M.; Sickora, J.; Erbs, M.; Weigel, H.J.; Zörb, C.; Manderscheid, R. Positive effects of free air CO2 enrichment on N remobilization and post-anthesis N uptake in winter wheat. Field Crops Res. 2019, 234, 107–118. [Google Scholar] [CrossRef]
- Gooding, M.J.; Gregory, P.J.; Ford, K.E.; Ruske, R.E. Recovery of nitrogen from different sources following applications to winter wheat at and after anthesis. Field Crops Res. 2007, 100, 143–154. [Google Scholar] [CrossRef]
- Terman, G.L. Yields and Protein Content of Wheat Grain as Affected by Cultivar, N, and Environmental Growth Factors 1. Agron. J. 1979, 71, 437–440. [Google Scholar] [CrossRef]
- Meier, U.; Bleiholder, H.; Buhr, L.; Feller, C.; Hack, H.; Hess, M.; Klose, R.; Lancashire, P.D.; Edmunds, B.S.; Stauss, R.; et al. Growth Stages of Mono-and Dicotyledonous Plants. BBCH Monograph; Federal Biological Research Centre for Agriculture and Forestry-Julius Kühn-Institut: Quedlinburg, Germany, 2001; Available online: https://www.julius-kuehn.de/media/Veroeffentlichungen/bbch%20epaper%20en/page.pdf (accessed on 20 January 2021).
- ICC 167-2000; Determination of Crude Protein in Grain and Grain Products for Food and Feed by the Dumas Combustion Principle. International Association for Cereal Science and Technology: Vienna, Austria, 2000.
- Wieser, H.; Seilmeier, W. The influence of nitrogen fertilisation on quantities and proportions of different protein types in wheat flour. J. Sci. Food Agric. 1998, 76, 49–55. [Google Scholar] [CrossRef]
- Schalk, K.; Lexhaller, B.; Koehler, P.; Scherf, K.A. Isolation and characterization of gluten protein types from wheat, rye, barley and oats for use as reference materials. PLoS ONE 2017, 12, e0172819. [Google Scholar] [CrossRef] [PubMed]
- Available online: http://www.gelanalyzer.com/index.html (accessed on 14 December 2020).
- Shewry, P.R.; Tatham, A.S.; Halford, N.G. Nutritional control of storage protein synthesis in developing grain of wheat and barley. Plant. Growth Regul. 2001, 34, 105–111. [Google Scholar] [CrossRef]
- Thanhaeuser, S.M.; Wieser, H.; Koehler, P. Correlation of quality parameters with baking performance of wheat flours. Cereal Chem. 2014, 91, 333–341. [Google Scholar] [CrossRef]
- Van Eckert, R.; Berghofer, E.; Ciclitira, P.J.; Chirdo, F.; Denery-Papini, S.; Ellis, H.J.; Ferranti, P.; Goodwin, P.; Immer, U.; Wieser, H.; et al. Towards a new gliadin reference material–isolation and characterisation. J. Cereal Sci. 2006, 43, 331–341. [Google Scholar] [CrossRef]
- Martre, P.; Porter, J.R.; Jamieson, P.D.; Triboï, E. Modeling grain nitrogen accumulation and protein composition to understand the sink/source regulations of nitrogen remobilization for wheat. Plant. Physiol. 2003, 133, 1959–1967. [Google Scholar] [CrossRef]
- Martre, P.; Jamieson, P.D.; Semenov, M.A.; Zyskowski, R.F.; Porter, J.R.; Triboi, E. Modelling protein content and composition in relation to crop nitrogen dynamics for wheat. Eur. J. Agron. 2006, 25, 138–154. [Google Scholar] [CrossRef]
- Wan, Y.; Gritsch, C.S.; Hawkesford, M.J.; Shewry, P.R. Effects of nitrogen nutrition on the synthesis and deposition of the ω-gliadins of wheat. Ann. Bot. 2014, 113, 607–615. [Google Scholar] [CrossRef]
- Triboï, E.; Martre, P.; Triboï-Blondel, A.M. Environmentally-induced changes in protein composition in developing grains of wheat are related to changes in total protein content. J. Exp. Bot. 2003, 54, 1731–1742. [Google Scholar] [CrossRef]
- Call, L.; Kapeller, M.; Grausgruber, H.; Reiter, E.; Schoenlechner, R.; D’Amico, S. Effects of species and breeding on wheat protein composition. J. Cereal Sci. 2020, 93, 102974. [Google Scholar] [CrossRef]
- Geisslitz, S.; Wieser, H.; Scherf, K.A.; Koehler, P. Gluten protein composition and aggregation properties as predictors for bread volume of common wheat, spelt, durum wheat, emmer and einkorn. J. Cereal Sci. 2018, 83, 204–212. [Google Scholar] [CrossRef]
- Pronin, D.; Börner, A.; Weber, H.; Scherf, K.A. Wheat (Triticum aestivum L.) breeding from 1891 to 2010 contributed to increasing yield and glutenin contents but decreasing protein and gliadin contents. J. Agric. Food Chem. 2020, 68, 13247–13256. [Google Scholar] [CrossRef]
- Pechanek, U.; Karger, A.; Gröger, S.; Charvat, B.; Schöggl, G.; Lelley, T. Effect of nitrogen fertilization on quantity of flour protein components, dough properties, and breadmaking quality of wheat. Cereal Chem. 1997, 74, 800–805. [Google Scholar] [CrossRef]
- Triboi, E.; Abad, A.; Michelena, A.; Lloveras, J.; Ollier, J.L.; Daniel, C. Environmental effects on the quality of two wheat genotypes: 1. Quantitative and qualitative variation of storage proteins. Eur. J. Agron. 2000, 13, 47–64. [Google Scholar] [CrossRef]
- Godfrey, D.; Hawkesford, M.J.; Powers, S.J.; Millar, S.; Shewry, P.R. Effects of crop nutrition on wheat grain composition and end use quality. J. Agric. Food Chem. 2010, 58, 3012–3021. [Google Scholar] [CrossRef]

| Quantity (g N pot−1) | |||||||
|---|---|---|---|---|---|---|---|
| N Level | EC0 * | EC30 | EC39 | EC51 | EC65 | EC75 | Σ |
| N1 | 0.30 | 0.50 | 1.20 | 0.20 | 0.00 | 0.00 | 2.20 |
| N2 | 0.30 | 0.50 | 1.20 | 0.75 | 0.75 | 0.75 | 4.25 |
| Cultivar (C) | Nitrogen (N) | C × N | |
|---|---|---|---|
| Grain yield (g pot−1) | *** | ns | ns |
| TGW (g) | ns | * | ns |
| GPC (%) | *** | *** | *** |
| N content per grain (mg) | *** | *** | *** |
| S content per grain (mg) | *** | ns | ns |
| N:S | ns | * | ns |
| ALGL (mg g−1) | *** | ** | ns |
| GLIA (mg g−1) | *** | *** | ns |
| ω GLIA (mg g−1) | *** | *** | ** |
| γ GLIA (mg g−1) | *** | *** | ns |
| α GLIA (mg g−1) | *** | *** | ns |
| GLUT (mg g−1) | *** | ns | *** |
| x HMW (mg g−1) | *** | ns | *** |
| y HMW (mg g−1) | *** | * | *** |
| D LMW (mg g−1) | *** | ns | *** |
| B LMW (mg g−1) | *** | ns | *** |
| C LMW (mg g−1) | ** | ns | ** |
| GMP | *** | *** | ** |
| ALGL (GMP) (mg g−1) | *** | *** | ns |
| HMW (GMP) (mg g−1) | *** | *** | ** |
| LMW (GMP) (mg g−1) | *** | *** | ** |
| GLIA:GLUT | *** | ** | * |
| GLIA:HMW | *** | * | ns |
| HMW:LMW | *** | ns | ns |
| x:y | *** | ** | ** |
| HMW:LMW (GMP) | *** | ns | * |
| GMP:GPC | *** | *** | ns |
| Akteur | Discus | Arnold | Hystar | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N Level | N1 | N2 | % | N1 | N2 | % | N1 | N2 | % | N1 | N2 | % |
| Grain yield (g pot−1) | 55.1 bc | 56.2 bc | 2 | 66.5 a | 68.4 a | 3 | 53.5 c | 64.0 ab | 20 | 67.7 a | 68.6 a | 1 |
| TGW (g) | 44.9 a | 42.8 abc | −5 | 41.4 abc | 39.0 c | −6 | 42.3 abc | 41.2 abc | −3 | 43.0 ab | 39.9 bc | −7 |
| GPC (%) | 16.6 c | 23.7 a | 43 | 17.1 c | 20.7 b | 21 | 23.2 a | 23.9 a | 3 | 18.7 bc | 19.6 b | 5 |
| N content per grain (mg) | 1.30 bc | 1.78 a | 37 | 1.21 c | 1.41 b | 17 | 1.70 a | 1.72 a | 1 | 1.40 b | 1.37 b | −2 |
| S content per grain (mg) | 0.084 bc | 0.099 ab | 17 | 0.066 d | 0.081 cd | 23 | 0.101 a | 0.100 ab | −1 | 0.089 abc | 0.086 abc | −4 |
| N:S ratio | 15.4 d | 18.0 a | 17 | 15.7 d | 17.5 ab | 11 | 16.9 bc | 17.2 ab | 2 | 15.7 d | 16.0 cd | 2 |
| Cultivar | Akteur | Discus | Arnold | Hystar | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N Level | N1 | N2 | % | N1 | N2 | % | N1 | N2 | % | N1 | N2 | % |
| GLIA:GLUT | 0.86 cd | 0.84 d | −2 | 0.94 bcd | 0.96 bcd | 2 | 0.86 cd | 1.05 b | 22 | 0.98 bc | 1.21 a | 23 |
| GLIA:HMW | 2.45 cd | 2.30 cd | −6 | 2.68 bc | 2.66 bc | −1 | 2.19 d | 2.70 bc | 23 | 3.02 b | 3.56 a | 18 |
| HMW:LMW | 0.54 cd | 0.58 b | 7 | 0.54 cd | 0.56 bc | 4 | 0.65 a | 0.64 a | −2 | 0.48 e | 0.52 de | 6 |
| x:y | 1.59 a | 1.42 b | −10 | 0.91 e | 0.90 e | −2 | 1.30 c | 1.30 c | 0 | 1.21 d | 1.15 d | −5 |
| GMP:GPC | 0.0623 b | 0.0755 a | 21 | 0.0449 e | 0.0531 cd | 18 | 0.0573 bc | 0.0719 a | 25 | 0.0444 e | 0.0476 de | 7 |
| HMW:LMW GMP | 0.34 ab | 0.34 a | 2 | 0.27 c | 0.27 c | −2 | 0.31 b | 0.37 a | 18 | 0.25 cd | 0.24 d | −6 |
| Akteur | Discus | Arnold | Hystar | |
|---|---|---|---|---|
| BV (mL 10 g−1) | BV (mL 10 g−1) | BV (mL 10 g−1) | BV (mL 10 g−1) | |
| GPC (%) | 0.64 | 0.81 ** | 0.29 | 0.25 |
| N:S | 0.68 | 0.82 ** | 0.34 | 0.43 |
| ALGL (mg g−1) | 0.63 | 0.42 | 0.14 | 0.36 |
| GLIA (mg g−1) | 0.34 | 0.68 * | 0.55 | 0.10 |
| ω (mg g−1) | 0.48 | 0.75 * | 0.48 | 0.30 |
| γ (mg g−1) | −0.41 | 0.57 | 0.56 | −0.03 |
| α (mg g−1) | 0.17 | 0.55 | 0.48 | −0.15 |
| GLUT (mg g−1) | 0.73 * | 0.92 *** | −0.27 | −0.45 |
| x HMW (mg g−1) | 0.72 * | 0.90 *** | −0.31 | −0.28 |
| y HMW (mg g−1) | 0.69 | 0.94 *** | −0.35 | −0.26 |
| D LMW (mg g−1) | 0.78 * | 0.72 * | 0.08 | −0.22 |
| B LMW (mg g−1) | 0.69 | 0.95 *** | −0.39 | −0.27 |
| C LMW (mg g−1) | 0.71 * | 0.83 ** | −0.24 | −0.62 |
| GMP (mg g−1) | 0.60 | 0.81 ** | 0.86 ** | 0.61 * |
| GLIA:GLUT | −0.75 * | −0.60 | 0.57 | 0.33 |
| GLIA:HMW | −0.83 ** | −0.72 * | 0.60 | 0.16 |
| HMW:LMW | 0.34 | 0.58 | −0.51 | 0.66 * |
| x:y | −0.56 | −0.52 | 0.38 | −0.31 |
| GMP:GPC | 0.52 | 0.73 * | 0.89 ** | 0.46 |
| HMW:LMW (GMP) | −0.42 | −0.03 | 0.51 | −0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dier, M.; Hüsken, A.; Mikolajewski, S.; Langenkämper, G.; Zörb, C. Analyzing a Saturation Effect of Nitrogen Fertilization on Baking Volume and Grain Protein Concentration in Wheat. Agriculture 2023, 13, 20. https://doi.org/10.3390/agriculture13010020
Dier M, Hüsken A, Mikolajewski S, Langenkämper G, Zörb C. Analyzing a Saturation Effect of Nitrogen Fertilization on Baking Volume and Grain Protein Concentration in Wheat. Agriculture. 2023; 13(1):20. https://doi.org/10.3390/agriculture13010020
Chicago/Turabian StyleDier, Markus, Alexandra Hüsken, Sabine Mikolajewski, Georg Langenkämper, and Christian Zörb. 2023. "Analyzing a Saturation Effect of Nitrogen Fertilization on Baking Volume and Grain Protein Concentration in Wheat" Agriculture 13, no. 1: 20. https://doi.org/10.3390/agriculture13010020
APA StyleDier, M., Hüsken, A., Mikolajewski, S., Langenkämper, G., & Zörb, C. (2023). Analyzing a Saturation Effect of Nitrogen Fertilization on Baking Volume and Grain Protein Concentration in Wheat. Agriculture, 13(1), 20. https://doi.org/10.3390/agriculture13010020







