Changes over the Years in Soil Chemical Properties Associated with the Cultivation of Ginseng (Panax ginseng Meyer) on Andosol Soil
Abstract
:1. Introduction
2. Materials and Methods
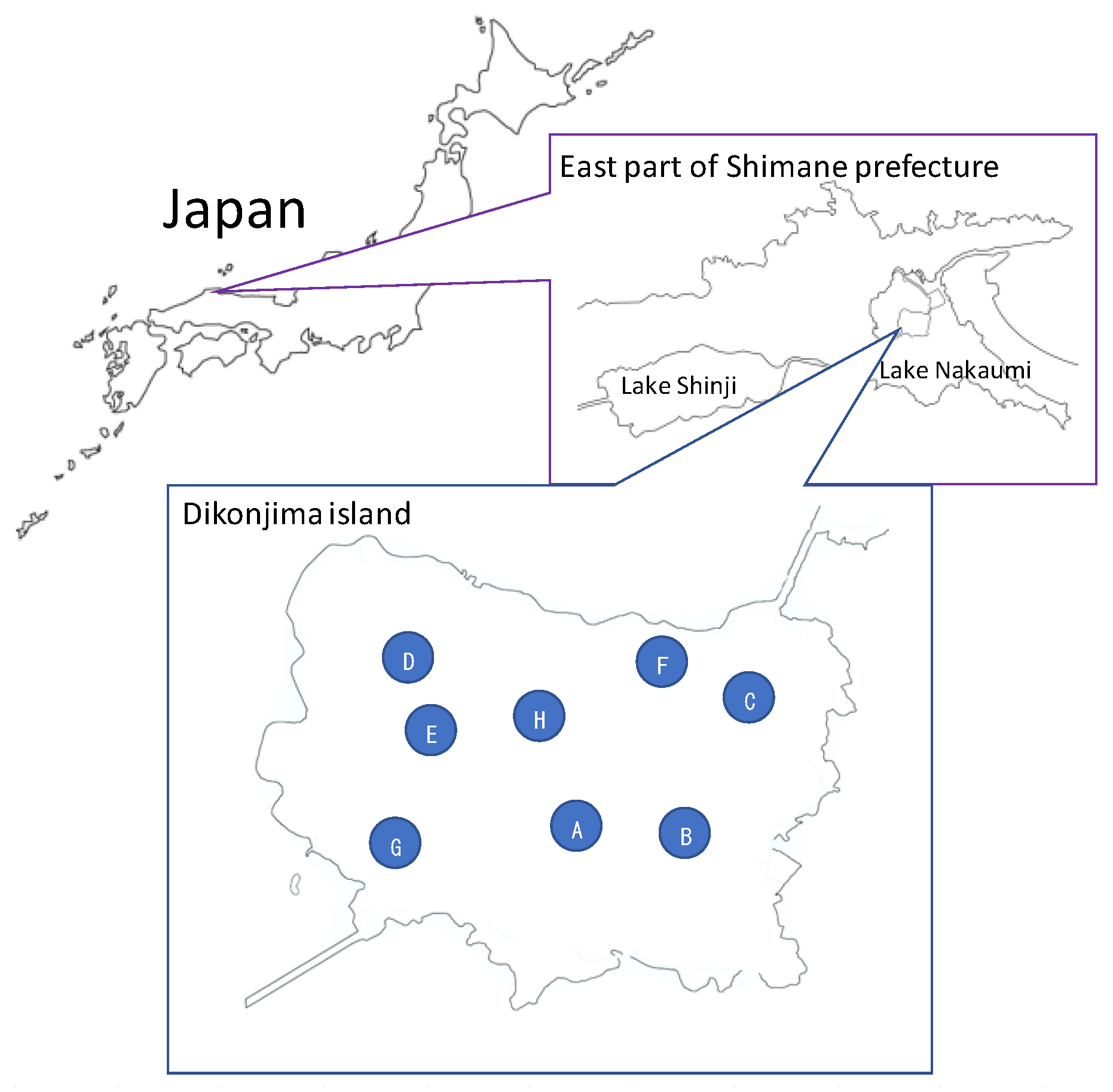
2.1. Study Sites and Sampling
2.2. Soil Analyses
2.3. Plant Analyses
2.4. Statistical Analyses
3. Results
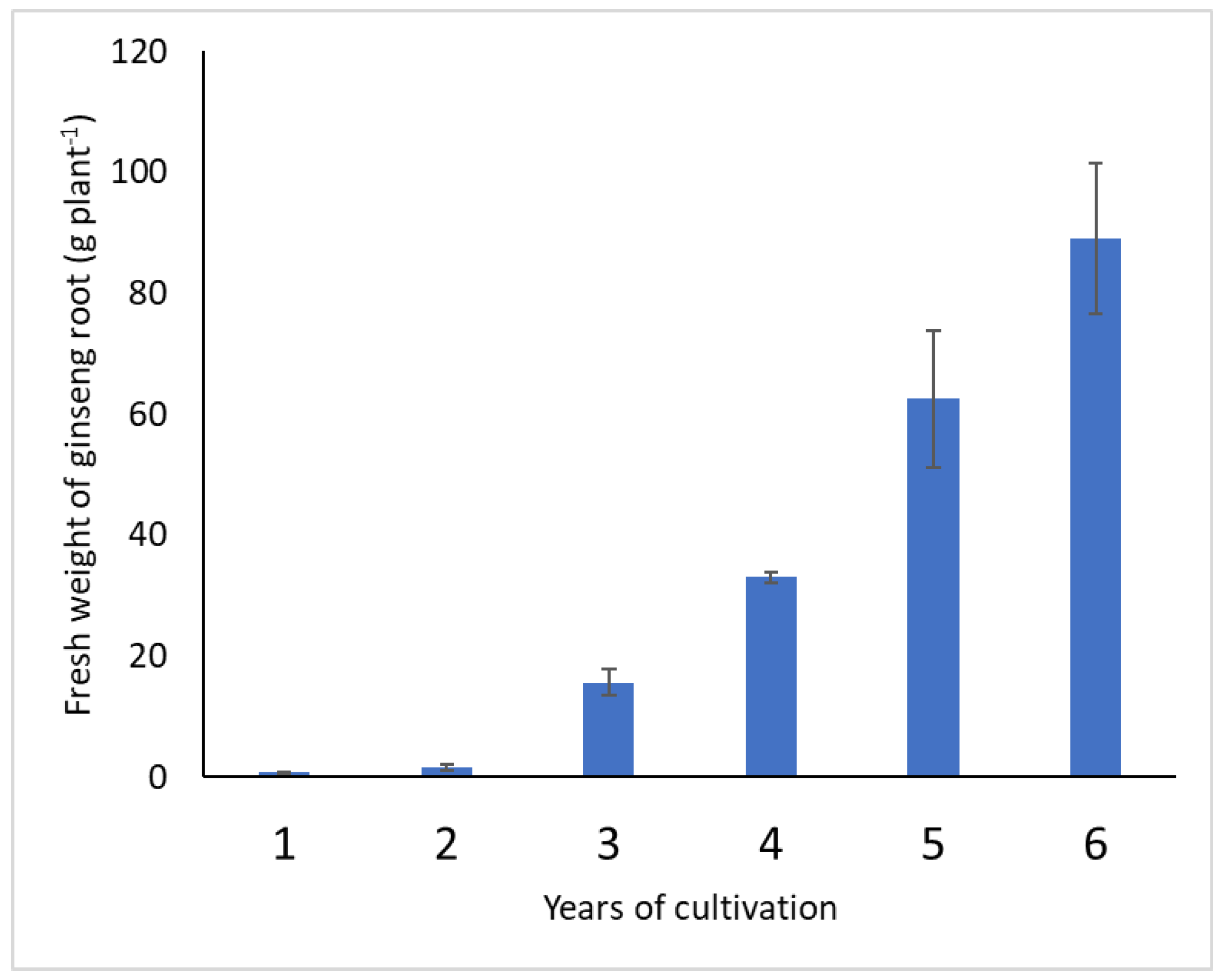
3.1. Changes over Cultivation Years in Fresh Root Weights and Mineral Concentrations in the Ginseng
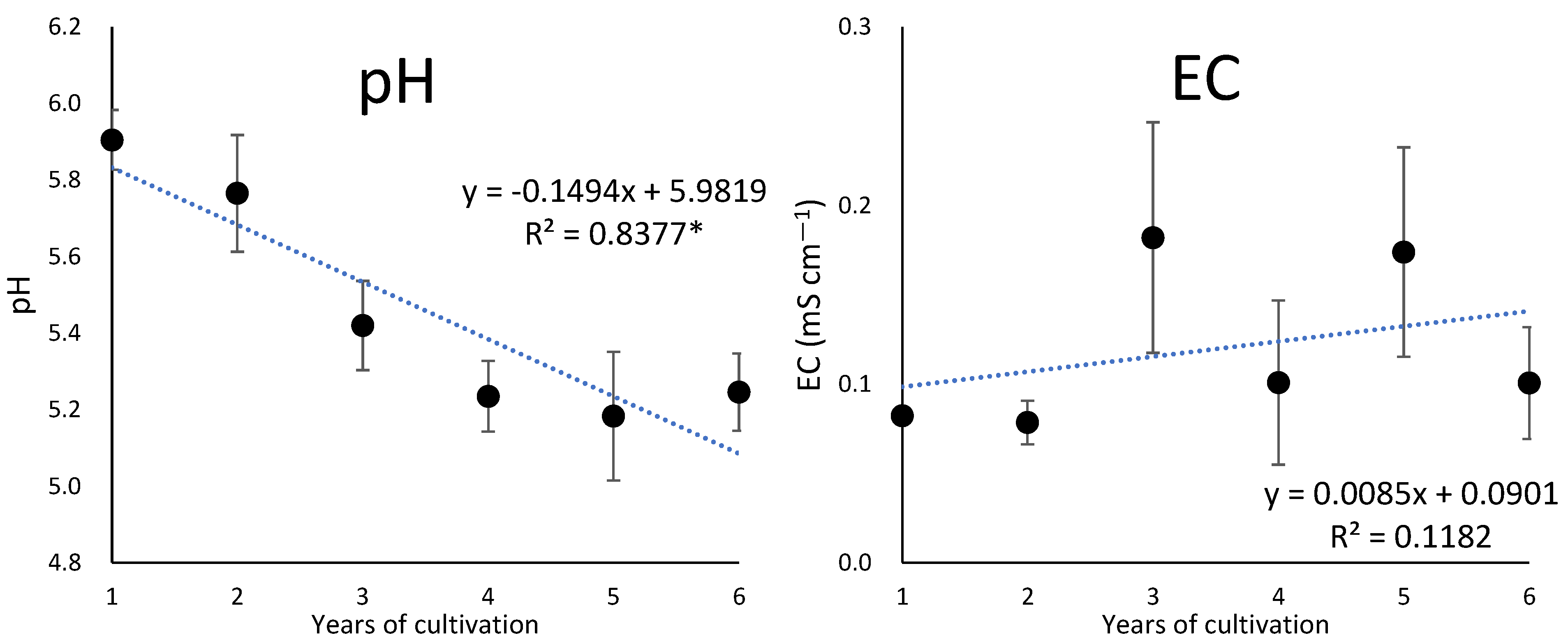
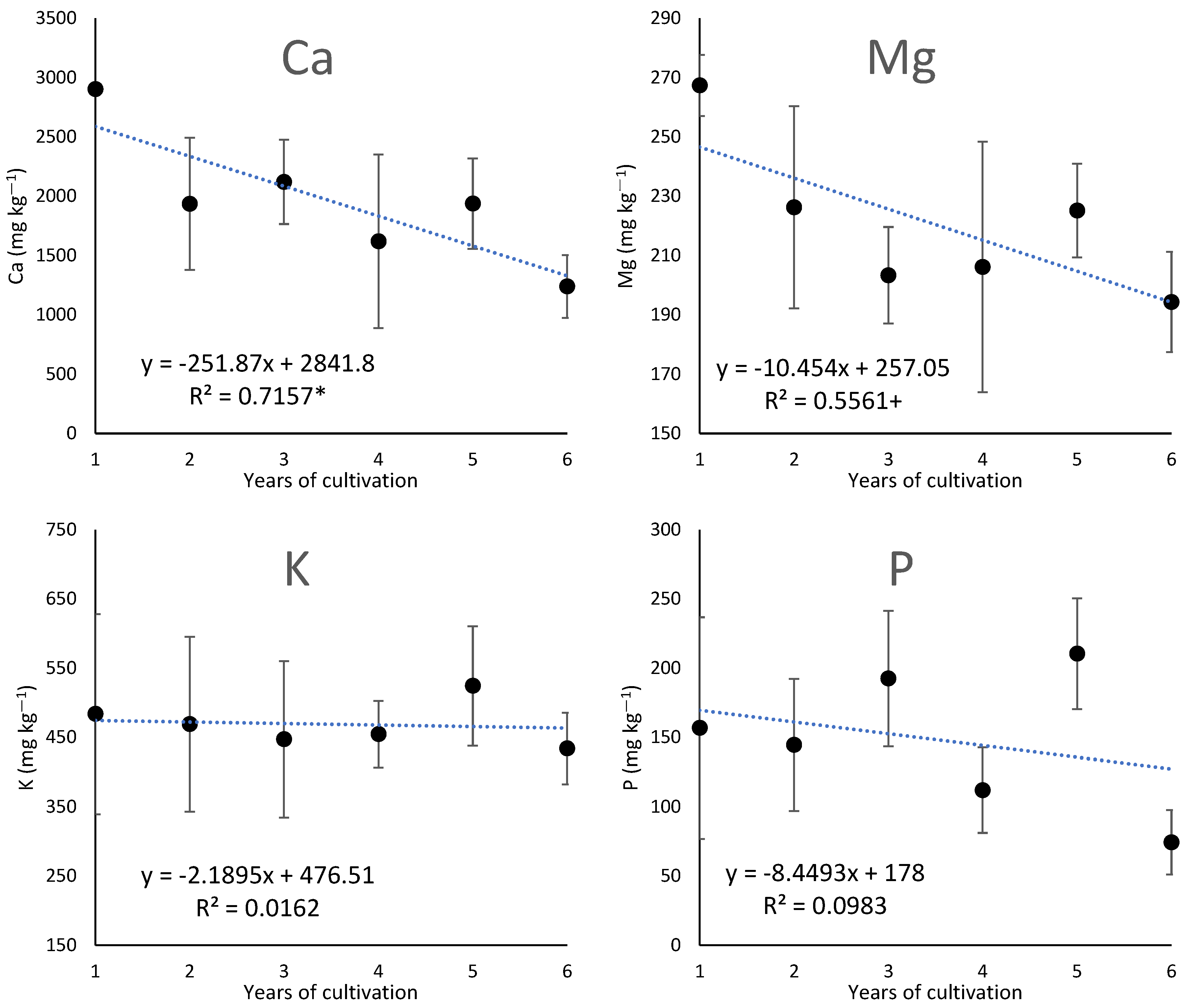
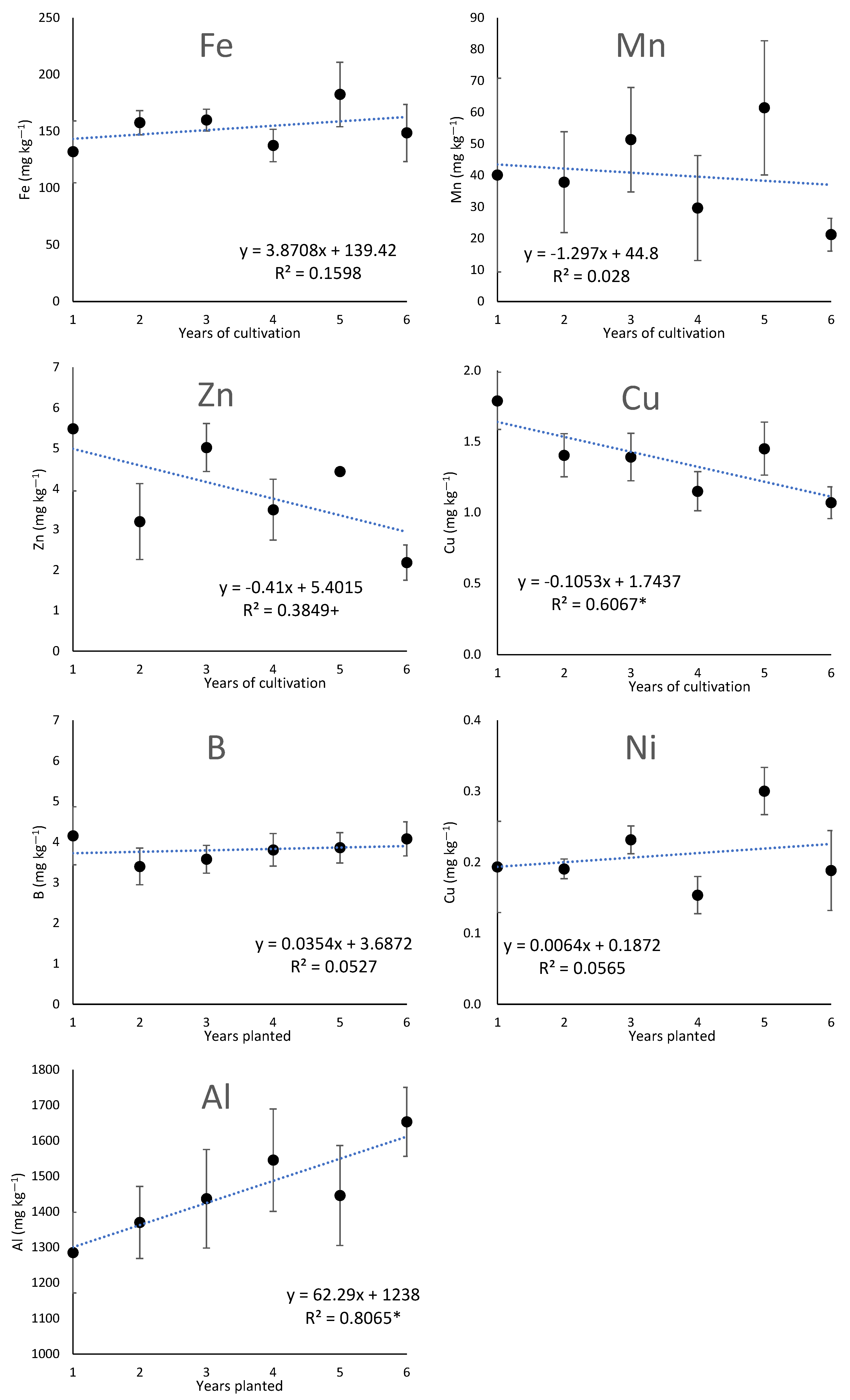
3.2. Changes over Years in the Chemical Properties of the Soil under Cultivation of Ginseng
4. Discussion
4.1. Ginseng Yield and Background
4.2. Validity of the Mineral Concentrations in the Ginseng Roots in This Study
4.3. Factors Contributing to Acidification with Increasing Years of Ginseng Cultivation and Their Effect on the Dynamics of Inorganic Elements
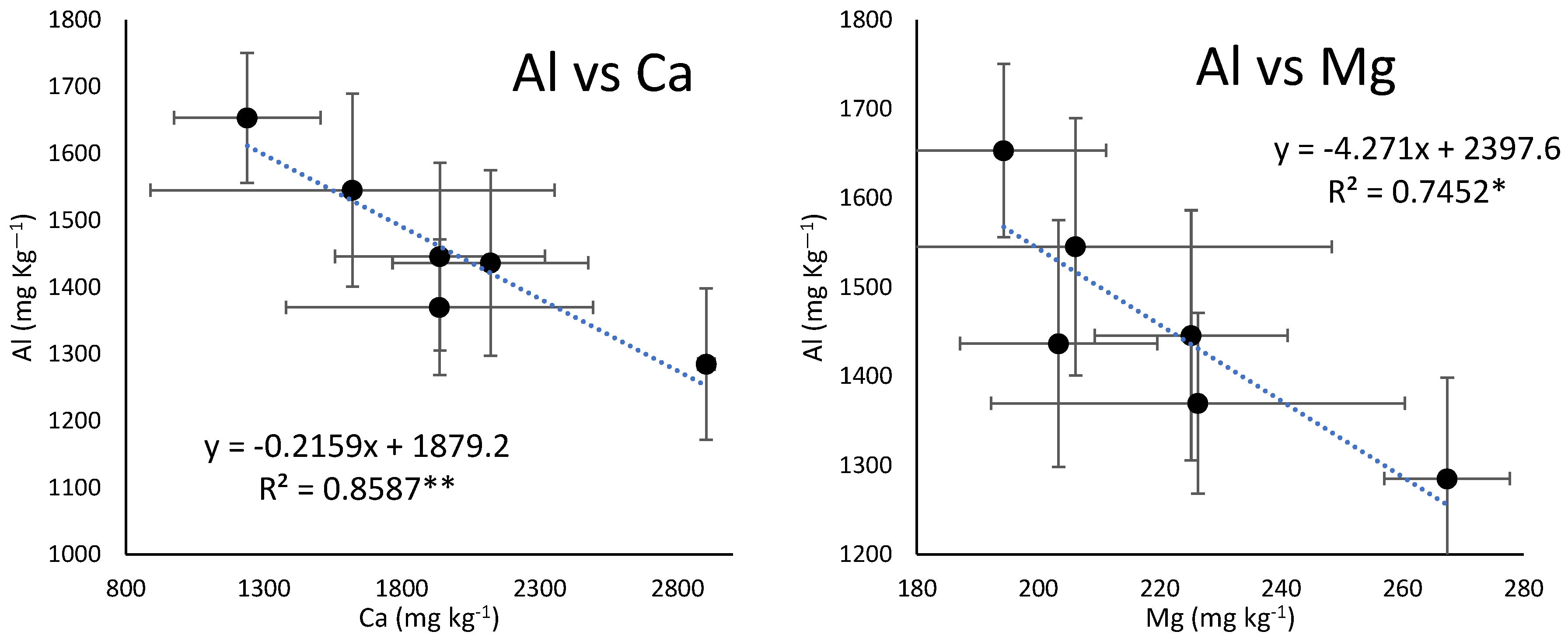
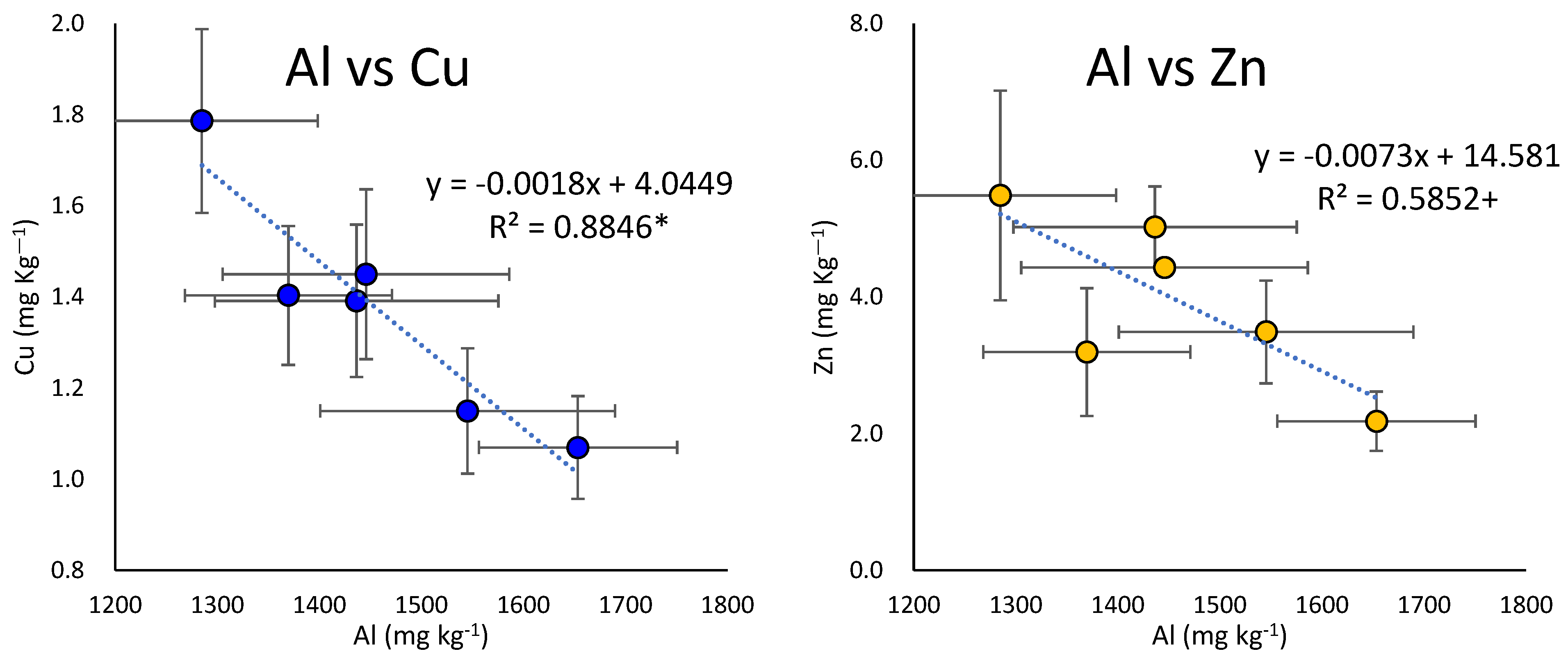
4.4. Increase in Aluminum Leaching with the Increase in the Number of Years of Ginseng Cultivation
4.5. The Effects of Acidification with Increasing Years of Cultivation on Trace Elements’ Availability
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, L.; Jin, Y.; Yin, C.; Bai, L. Co-transformation of Panax major ginsenosides Rb1 and Rg1 to minor ginsenosides C-K and F1 by Cladosporium cladosporioides. J. Ind. Microbiol. Biotechnol. 2012, 39, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-P.; Huo, Y.; Yang, D.-U.; Yang, D.-C. Influence of the plant growth promoting Rhizobium panacihumi on aluminum resistance in Panax ginseng. J. Ginseng Res. 2021, 45, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Baeg, I.-H.; So, S.-H. The world ginseng market and the ginseng (Korea). J. Ginseng Res. 2013, 37, 1–7. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Liu, X.; Zhang, B.; Xie, Z.; Hou, Z.; Yang, Z. Seasonal changes in soil acidity and related properties in ginseng artificial bed soils under a plastic shade. J. Ginseng Res. 2015, 39, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.-Y. A Contribution to Our Knowledge of Ginseng. Am. J. Chin. Med. 1977, 5, 1–23. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, Z.; Gao, L.; Liu, W.; Liu, R.; Zhao, J.; You, J. Changes in element accumulation, phenolic metabolism, and antioxidative enzyme activities in the red-skin roots of Panax ginseng. J. Ginseng Res. 2017, 41, 307–315. [Google Scholar] [CrossRef]
- Duke, J.A. Ginseng: A Concise Handbook; Reference Pubns: Algonac, MI, USA, 1989. [Google Scholar]
- Douglas, M.H.; Smallfield, B.M.; Parmenter, G.A.; Burton, L.C.; Heaney, A.J.; Johnstone, P.D. Effect of growing media on the production of ginseng (Panax ginseng) in Central Otago, New Zealand. N. Z. J. Crop Hortic. Sci. 2000, 28, 195–207. [Google Scholar] [CrossRef]
- Nadeau, I.; Simard, R.R.; Olivier, A. The impact of lime and organic fertilization on the growth of wild-simulated American ginseng. Can. J. Plant Sci. 2003, 83, 603–609. [Google Scholar] [CrossRef]
- Matsumoto, S. Soils and Agriculture in Shimane. Pedologist 2014, 58, 88–92. [Google Scholar] [CrossRef]
- Sruamsiri, P.; Ogaki, K.; Sugino, M. Production of Ginseng (Panax ginseng) in Nagano Prefecture, Japan. Mem. Fac. Agri. Kinki Univ. 1991, 24, 71–87. [Google Scholar]
- Yamaoka, D.; Ito, T.; Asama, H.; Sahashi, Y.; Mitani, K.; Kang, D.; Yasui, H.; Watanabe, H. The Current Situation and Problems of Domestic Crude Drug Production. Kampo Med. Nihon Toyo Igaku Zasshi 2017, 68, 270–280. [Google Scholar] [CrossRef]
- Li, T.S.C.; Mazza, G. Correlations between Leaf and Soil Mineral Concentrations and Ginsenoside Contents in American Ginseng. HortScience 1999, 34, 85–87. [Google Scholar] [CrossRef]
- Keum, Y.S.; Park, K.K.; Lee, J.M.; Chun, K.S.; Park, J.H.; Lee, S.K.; Kwon, H.; Surh, Y.J. Antioxidant and anti-tumor promoting activities of the methanol extract of heat-processed ginseng. Cancer Lett. 2000, 150, 41–48. [Google Scholar] [CrossRef]
- Wang, N.; Wang, X.; He, M.; Zheng, W.; Qi, D.; Zhang, Y.; Han, C.C. Ginseng polysaccharides: A potential neuroprotective agent. J. Ginseng Res. 2021, 45, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Xu, J.; Li, Y.; Fang, H.; Niu, W.; Li, X.; Zhang, Y.; Ding, W.; Chen, S. Manipulation of microbial community in the rhizosphere alleviates the replanting issues in Panax ginseng. Soil Biol. Biochem. 2018, 125, 64–74. [Google Scholar] [CrossRef]
- Yuan, Y.; Zuo, I.; Zhang, H.; Zu, M.; Liu, S. The Chinese medicinal plants rhizosphere: Metabolites, microorganisms, and interaction. Rhizosphere 2022, 22, 100540. [Google Scholar] [CrossRef]
- Konsler, T.R.; Shelton, J.E. Lime and Phosphorus Effects on American Ginseng: I. Growth, Soil Fertility, and Root Tissue Nutrient Status Response. J. Amer. Soc. Hort. Sci. 1990, 115, 570–574. [Google Scholar] [CrossRef]
- He, C.; Wang, R.; Ding, W.; Li, Y. Effects of cultivation soils and ages on microbiome in the rhizosphere soil of Panax ginseng. Appl. Soil Ecol. 2022, 174, 104397. [Google Scholar] [CrossRef]
- Hirasawa, F. On the physical and chemical features of ginseng cultivated soil. Bull. Nagano Veg. Ornam. Crops Exp. Stn. 1986, 4, 93–98. [Google Scholar]
- Tsukui, M. Temporal variation in chemical composition of phenocrysts and magmatic temperature at Daisen volcano, southwest Japan. J. Volcanol. Geotherm. Res. 1985, 26, 317–336. [Google Scholar] [CrossRef]
- Mehlich, A. Mehlich 3 soil test extractant: A modification of Mehlich 2 extractant. Commun. Soil Sci. Plant Anal. 1984, 15, 1409–1416. [Google Scholar] [CrossRef]
- Malmir, M.; Tahmasbian, I.; Xu, Z.; Farrar, M.B.; Bai, S.H. Prediction of soil macro- and micro-elements in sieved and ground air-dried soils using laboratory-based hyperspectral imaging technique. Geoderma 2019, 340, 70–80. [Google Scholar] [CrossRef]
- Yanai, M.; Uwasawa, M.; Shimizu, Y. Development of a New Multinutrient Extraction Method for Macro- and Micro-Nutrients in Arable Land Soil. Soil Sci. Plant Nutr. 2019, 46, 299–313. [Google Scholar] [CrossRef]
- Dhar, P.; Kobayashi, K.; Ujiie, K.; Adachi, F.; Kasuga, J.; Akahane, I.; Arao, T.; Matsumoto, S. The increase in the arsenic concentration in brown rice due to high temperature during the ripening period and its reduction by silicate material treatment. Agriculture 2020, 10, 289. [Google Scholar] [CrossRef]
- Penn, C.; Camberato, J. A Critical Review on Soil Chemical Processes that Control How Soil pH Affects Phosphorus Availability to Plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, H.; Wang, Y.; Gao, Y.; Zhang, L. The response of ginseng grown on farmland to foliar-applied iron, zinc, manganese and copper. Ind. Crops Prod. 2013, 45, 388–394. [Google Scholar] [CrossRef]
- Proctor, J.T.A.; Shelp, B.J. Effect of boron nutrition on American ginseng in field and in nutrient cultures. J. Ginseng Res. 2014, 38, 73–77. [Google Scholar] [CrossRef]
- Kim, C.; Choo, G.C.; Cho, H.S.; Lim, J.T. Soil properties of cultivation sites for mountain-cultivated ginseng at local level. J. Ginseng Res. 2015, 39, 76–80. [Google Scholar] [CrossRef]
- Xia, P.; Guo, H.; Zhao, H.; Jiao, J.; Deyholos, M.K.; Yan, X.; Liu, Y.; Liang, Z. Optimal fertilizer application for Panax notoginseng and effect of soil water on root rot disease and saponin contents. J. Ginseng Res. 2016, 40, 38–46. [Google Scholar] [CrossRef]
- Kochian, L.V. Cellular mechanisms of aluminum toxicity and resistance in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995, 46, 237–260. [Google Scholar] [CrossRef]
- Blamey, F.P.C.; Edmeades, D.C.; Wheeler, D.M. Empirical models to approximate calcium and magnesium ameliorative effects and genetic differences in aluminium tolerance in wheat. Plant Soil 1992, 144, 281–287. [Google Scholar] [CrossRef]
- Brunet, J. Interacting effects of pH, aluminium and base cations on growth and mineral composition of the woodland grasses Bromus benekenii and Hordelymus europaeus. Plant Soil 1994, 161, 157–166. [Google Scholar] [CrossRef]
- Farh, M.E.-A.; Kim, Y.-J.; Sukweenadhi, J.; Singh, P.; Yang, D.-C. Aluminium resistant, plant growth promoting bacteria induce overexpression of Aluminium stress related genes in Arabidopsis thaliana and increase the ginseng tolerance against Aluminium stress. Microbiol. Res. 2017, 200, 45–52. [Google Scholar] [CrossRef]
- Shetty, R.; Vidya, C.S.-N.; Prakash, N.B.; Lux, A.; Vaculík, M. Aluminum toxicity in plants and its possible mitigation in acid soils by biochar: A review. Sci. Total Environ. 2021, 765, 142744. [Google Scholar] [CrossRef]
- Ayala-Silva, T.; Al-Hamdani, S. Interactive Effects of Polylactic Acid with Different Aluminum Concentrations on Growth, Pigment Concentrations, and Carbohydrate Accumulation of Azolla. Am. Fern J. 1997, 87, 120–126. [Google Scholar] [CrossRef]
- Takahashi, T.; Ikeda, Y.; Fujita, K.; Nanzyo, M. Effect of liming on organically complexed aluminum of nonallophanic Andosols from northeastern Japan. Geoderma 2006, 130, 26–34. [Google Scholar] [CrossRef]
- Oshima, H.; Goto, D.; Goto, I.; Maeda, Y. Effect of organic matter removal treatments and addition of aluminum-containing substances on incidence of Fusarium wilt of lettuce. Soil Sci. Plant Nutr. 2015, 61, 613–619. [Google Scholar] [CrossRef]
- Sadamoto, H.; Iimura, K.; Honna, T.; Yamamoto, S. Examination of fractionation of heavy metals in soils. Jpn. J. Soil Sci. Plant Nutr. 1994, 65, 645–653. [Google Scholar]
- Liang, S.; Wang, X.; Li, Z.; Gao, N.; Sun, H. Fractionation of heavy metals in contaminated soils surrounding non-ferrous metals smelting area in the North China Plain. Chem. Speciat. Bioavailab. 2014, 26, 59–64. [Google Scholar] [CrossRef]
- Kotoky, P.; Bora, B.J.; Baruah, N.K.; Baruah, J.; Baruah, P.; Borah, G.C. Chemical fractionation of heavy metals in soils around oil installations, Assam. Chem. Speciat. Bioavailab. 2003, 15, 115–126. [Google Scholar] [CrossRef]
- Meite, F.; Granet, M.; Imfeld, G. Ageing of Copper, Zinc and Synthetic Pesticides in Particle-Size and Chemical Fractions of Agricultural Soils. Sci. Total Environ. 2022, 824, 153860. [Google Scholar] [CrossRef] [PubMed]


| Years of Cultivation | K | P | Ca | Mg | Fe | Mn | Zn | Cu | B |
|---|---|---|---|---|---|---|---|---|---|
| (g kg−1) | (g kg−1) | (g kg−1) | (g kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | (mg kg−1) | |
| 2 | 20.9±1.3 | 1.0 ± 0.1 | 2.9 ± 0.2 | 1.9 ± 0.3 | 190.9 ± 57.1 | 47.2 ± 9.1 | 31.6 ± 8.4 | 5.6 ± 0.7 | 12.6 ± 1.0 |
| 3 | 16.1±2.7 | 1.0 ± 0.1 | 2.3 ± 0.3 | 1.3 ± 0.3 | 95.6 ± 69.0 | 37.1 ± 8.3 | 23.6 ± 8.7 | 5.5 ± 0.7 | 9.2 ± 0.1 |
| 4 | 15.3 ± 2.6 | 0.7 ± 0.1 | 2.1 ± 0.3 | 1.4 ± 0.1 | 109.3 ± 27.8 | 39.4 ± 7.4 | 19.7 ± 9.9 | 5.6 ± 0.5 | 9.8 ± 0.5 |
| 5 | 15.7 ± 2.0 | 1.0 ± 0.1 | 2.3 ± 0.2 | 1.5 ± 0.1 | 112.9 ± 17.1 | 41.0 ± 4.7 | 15.7 ± 5.6 | 4.4 ± 0.3 | 11.0 ± 0.2 |
| 6 | 18.9 ± 2.5 | 0.9 ± 0.2 | 3.0 ± 0.2 | 1.4 ± 0.1 | 141.6 ± 16.5 | 45.9 ± 4.9 | 18.3 ± 6.5 | 6.3 ± 0.2 | 11.6 ± 0.5 |
| pH | EC | P | K | Ca | Mg | Al | Fe | Mn | B | Zn | Cu | Ni | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p value | 0.010 | 0.505 | 0.545 | 0.810 | 0.034 | 0.089 | 0.015 | 0.432 | 0.751 | 0.662 | 0.189 | 0.068 | 0.650 |
| Significance | * | ns | ns | ns | * | + | * | ns | ns | ns | ns | + | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, S.; Doi, H.; Kasuga, J. Changes over the Years in Soil Chemical Properties Associated with the Cultivation of Ginseng (Panax ginseng Meyer) on Andosol Soil. Agriculture 2022, 12, 1223. https://doi.org/10.3390/agriculture12081223
Matsumoto S, Doi H, Kasuga J. Changes over the Years in Soil Chemical Properties Associated with the Cultivation of Ginseng (Panax ginseng Meyer) on Andosol Soil. Agriculture. 2022; 12(8):1223. https://doi.org/10.3390/agriculture12081223
Chicago/Turabian StyleMatsumoto, Shingo, Haruno Doi, and Junko Kasuga. 2022. "Changes over the Years in Soil Chemical Properties Associated with the Cultivation of Ginseng (Panax ginseng Meyer) on Andosol Soil" Agriculture 12, no. 8: 1223. https://doi.org/10.3390/agriculture12081223
APA StyleMatsumoto, S., Doi, H., & Kasuga, J. (2022). Changes over the Years in Soil Chemical Properties Associated with the Cultivation of Ginseng (Panax ginseng Meyer) on Andosol Soil. Agriculture, 12(8), 1223. https://doi.org/10.3390/agriculture12081223






