Gene Expression, Activity and Localization of Beta-Galactosidases during Late Ripening and Postharvest Storage of Tomato Fruit
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Growth Conditions
2.2. Fruit Firmness and Total Soluble Solids Assay
2.3. qPCR Expression Analysis
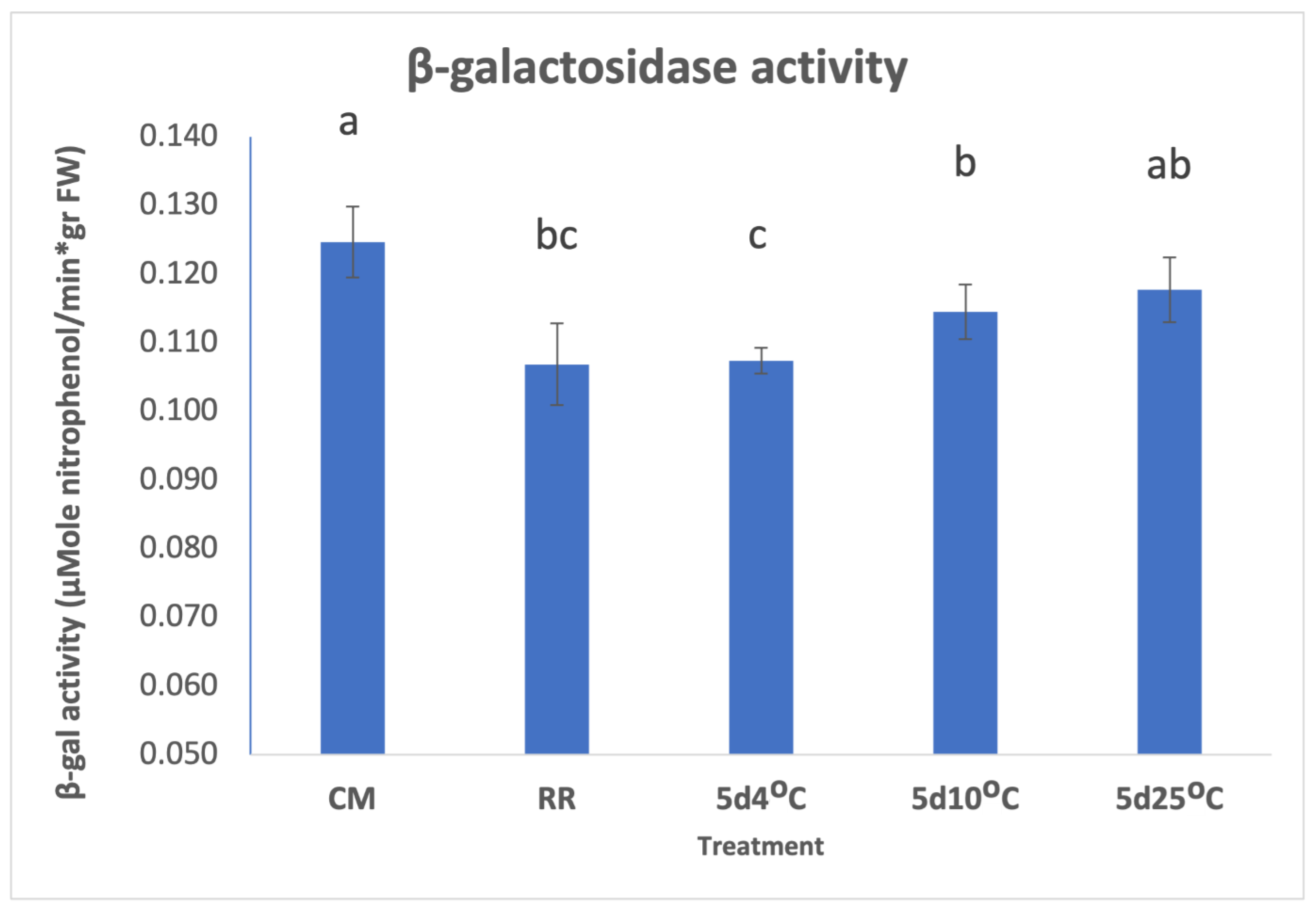
2.4. Enzyme Extraction and Estimation of Total β-GAL Enzyme Activity
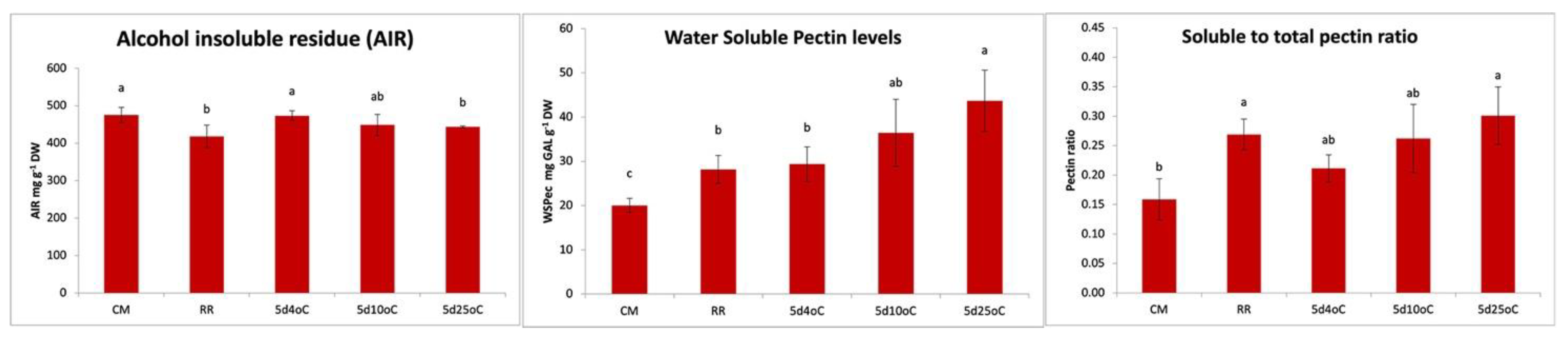
2.5. Extraction Procedure of Alcohol Insoluble Residue (AIR) for Total and Soluble Pectins
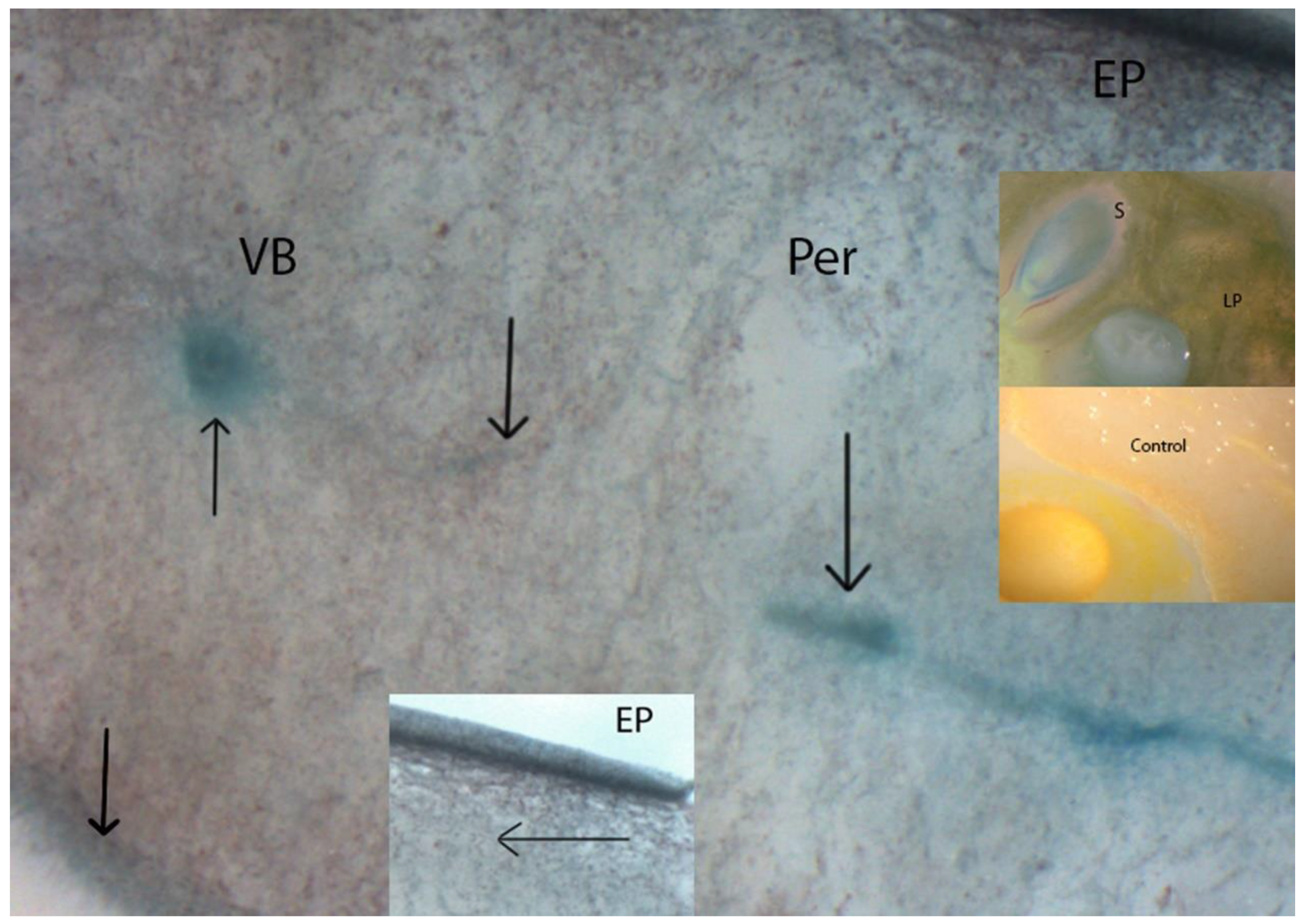
2.6. In situ Localization of β-GAL Activity
2.7. Statistical Analysis
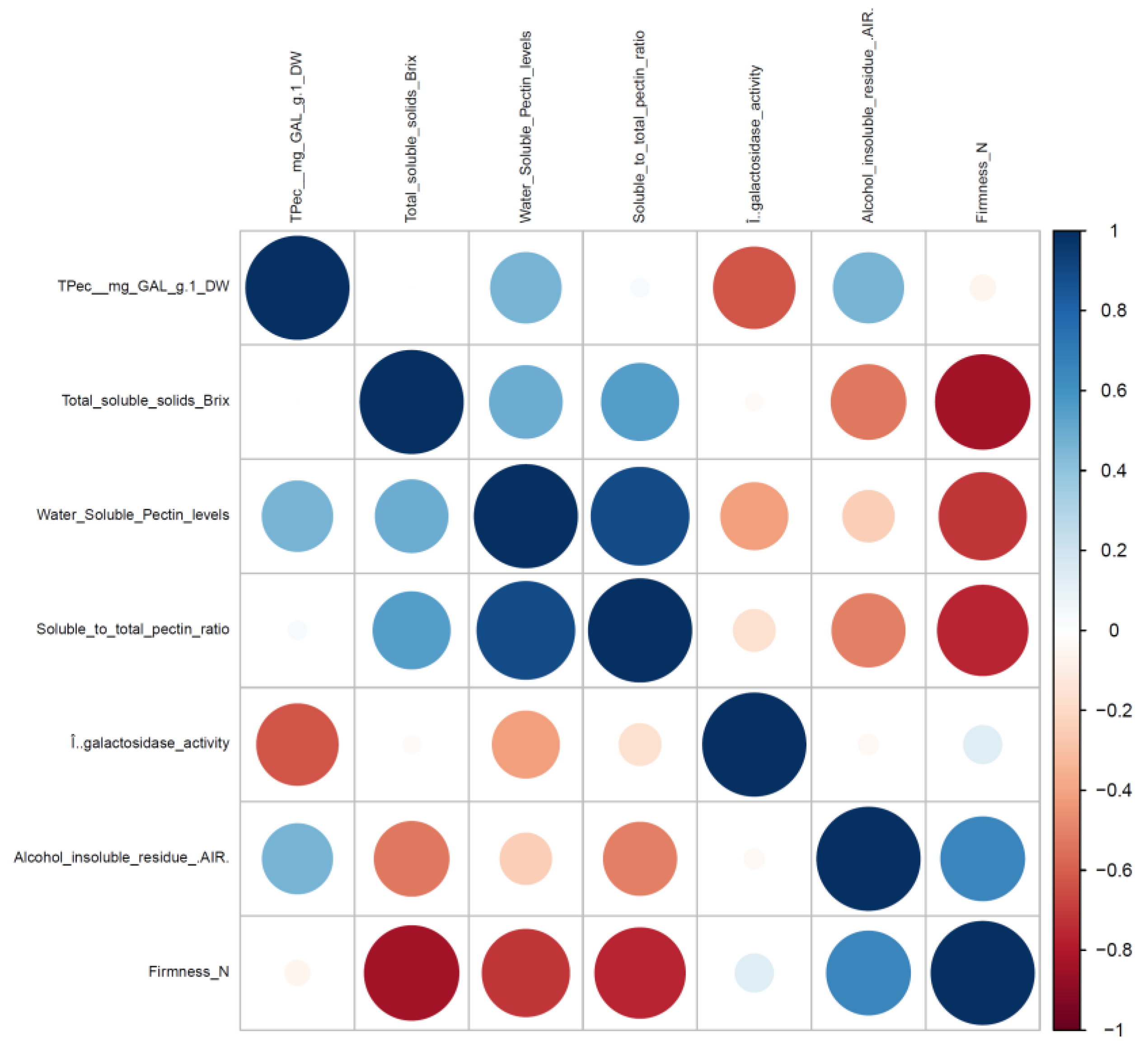
3. Results and Discussion
3.1. β-GAL during Late On-Plant Tomato Maturation
3.2. β-GAL during Postharvest Tomato Storage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Mwaniki, M.W.; Mathooko, F.M.; Matsuzaki, M.; Hiwasa, K.; Tateishi, A.; Ushijima, K.; Nakano, R.; Inaba, A.; Kubo, Y. Expression characteristics of seven members of the β-GALactosidase gene family in ‘La France’ pear (Pyrus communis L.) fruit during growth and their regulation by 1-methylcyclopropene during postharvest ripening. Postharvest Biol. Technol. 2005, 36, 253–263. [Google Scholar] [CrossRef]
- Brummell, D.A. Primary cell wall during fruit ripening. N. Z. J. For. Sci. 2006, 36, 99–111. [Google Scholar]
- Jarvis, M.C.; Briggs, S.P.H.; Knox, J.P. Intercellular adhesion and cell separation in plants. Plant Cell Environ. 2003, 26, 977–989. [Google Scholar] [CrossRef]
- Yang, H.; Liu, J.; Dang, M.; Zhang, B.; Li, H.; Meng, R.; Qu, D.; Yang, Y.; Zhao, Z. Analysis of β-GALactosidase during fruit development and ripening in two different texture types of apple cultivars. Front. Plant Sci. 2018, 9, 539. [Google Scholar] [CrossRef]
- Tsaniklidis, G.; Benovias, A.; Delis, C.; Aivalakis, G. Acidic alpha galactosidase during the maturation and cold storage of cherry tomatoes. Acta Physiol. Plant. 2016, 38, 57. [Google Scholar] [CrossRef]
- Smith, D.L.; Gross, K.C. A family of at least seven β-GALactosidase genes is expressed during tomato fruit development. Plant Physiol. 2000, 123, 1173–1183. [Google Scholar] [CrossRef] [Green Version]
- Ross, G.S.; Redgwell, R.J.; MacRae, E.A. Kiwifruit betagalactosidase-isolation and activity against specific fruit cell wall polysaccharides. Planta 1993, 109, 499–506. [Google Scholar]
- Buckeridge, M.S. Seed cell wall storage polysaccharides: Models to understand cell wall biosynthesis and degradation. Plant Physiol. 2010, 154, 1017–1023. [Google Scholar] [CrossRef] [Green Version]
- Goulao, L.F.; Oliveira, C.M. Cell wall modifications during fruit ripening: When a fruit is not the fruit. Trends Food Sci. Technol. 2008, 19, 4–25. [Google Scholar] [CrossRef] [Green Version]
- Gwanpua, S.G.; Mellidou, I.; Boeckx, J.; Kyomugasho, C.; Bessemans, N.; Verlinden, B.E.; Hertog, M.L.A.T.M.; Hendrickx, M.; Nicolai, B.; Geeraerd, A.H. Expression analysis of candidate cell wall-related genes associated with changes in pectin biochemistry during postharvest apple softening. Postharvest Biol. Technol. 2016, 112, 176–185. [Google Scholar] [CrossRef]
- Guo, S.; Song, J.; Zhang, B.; Jiang, H.; Ma, R.; Yu, M. Genome-wide identification and expression analysis of beta-galactosidase family members during fruit softening of peach [Prunus persica (L.) Batsch]. Postharvest Biol. Technol. 2018, 136, 111–123. [Google Scholar] [CrossRef]
- Brummell, D.A.; Harpster, M.H. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol. Biol. 2001, 47, 311–339. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, B.; van der Hoorn, R.A. Beta galactosidases in Arabidopsis and tomato—A mini review. Biochem. Soc. Trans. 2016, 44, 150–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carey, A.T.; Smith, D.L.; Harrison, E.; Bird, C.R.; Gross, K.C.; Seymour, G.B.; Tucker, G.A. Down-regulation of a ripening-related beta-galactosidase gene (TBG1) in transgenic tomato fruits. J. Exp. Bot. 2001, 52, 663–668. [Google Scholar] [CrossRef] [Green Version]
- Pressey, R. β -Galactosidases in ripening tomatoes. Plant Physiol. 1983, 71, 132–135. [Google Scholar] [CrossRef] [Green Version]
- Belge, B.; Goulao, L.F.; Comabella, E.; Graell, J.; Lara, I. Refrigerated storage and calcium dips of ripe ‘Celeste’ sweet cherryfruit: Combined effects on cell wall metabolism. Sientia Hort. 2017, 219, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Tsaniklidis, G.; Charova, S.N.; Fanourakis, D.; Tsafouros, A.; Nikoloudakis, N.; Goumenaki, E.; Tsantili, E.; Roussos, P.A.; Spiliopoulos, I.K.; Paschalidis, K.; et al. The role of temperature in mediating postharvest polyamine homeostasis in tomato fruit. Postharvest Biol. Technol. 2021, 179, 111586. [Google Scholar] [CrossRef]
- Zhang, Y.; Ntagkas, N.; Fanourakis, D.; Tsaniklidis, G.; Zhang, Y.; Zou, J.; Cheng, R.; Yang, Q.; Li, T. The role of light intensity in mediating ascorbate content during postharvest tomato ripening: A transcriptomic analysis. Postharvest Biol. Technol. 2021, 180, 111622. [Google Scholar] [CrossRef]
- Huang, S. Global Trade Patterns in Fruits and Vegetables; USDA-ERS Agriculture and Trade Report No. WRS-04-06; USDA: Washington, DC, USA, 2005. [Google Scholar]
- Yao, B.N.; Tano, K.; Konan, H.K.; Bédié, G.K.; Oulé, M.K.; Koffi-Nevry, R.; Arul, J. The role of hydrolases in the loss of firmness and of the changes in sugar content during the post-harvest maturation of Carica papaya L. var solo 8. J. Food Sci. Technol. 2014, 51, 3309–3316. [Google Scholar] [CrossRef] [Green Version]
- Rose, J.K.; Bennett, A.B. Cooperative disassembly of the cellulose–xyloglucan network of plant cell walls: Parallels between cell expansion and fruit ripening. Trends Plant Sci. 1999, 4, 176–183. [Google Scholar] [CrossRef]
- Smith, D.L.; Starrett, D.A.; Gross, K.C. A gene coding for tomato fruit b-galactosidase II is expressed during fruit ripening. Plant Physiol. 1998, 117, 417–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posé, S.; Marcus, S.E.; Knox, J.P. Differential metabolism of pectic galactan in tomato and strawberry fruit: Detection of the LM26 branched galactan epitope in ripe strawberry fruit. Physiol. Plant 2018, 164, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Seymour, G.B. Molecular and biochemical basis of softening in tomato. Mol. Hort. 2022, 2, 5. [Google Scholar] [CrossRef]
- Dangcham, S.; Bowen, J.; Ferguson, I.; Ketsa, S. Effect of temperature and low oxygen on pericarp hardening of mangosteen fruit stored at low temperature. Postharvest Biol. Technol. 2008, 50, 37–44. [Google Scholar] [CrossRef]
- Renard, C.M.G.C. Variability in cell wall preparations, quantification and comparison of common methods. Carbohydr. Polym. 2005, 60, 512–522. [Google Scholar] [CrossRef]
- Kafkaletou, M.; Fasseas, C.; Tsantili, E. Increased firmness and modified cell wall composition by ethylene were reversed by the ethylene inhibitor 1-methylcyclopropene (1-MCP) in the non-climacteric olives harvested at dark green stage—Possible implementation of ethylene for olive quality. J. Plant Physiol. 2019, 238, 63–71. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Durst, R.W.; Lee, J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci.Technol. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Blumenkrantz, N.; Asboe-Hanssen, G. New method for quantitative determination of uronic acids. Anal. Biochem. 1974, 54, 484–489. [Google Scholar] [CrossRef]
- Peet, M.M. Fruit cracking in tomato. HortTechnology 1992, 2, 216–223. [Google Scholar] [CrossRef] [Green Version]
- Moctezuma, E.; Smith, D.L.; Gross, K.C. Effect of ethylene on mRNA abundance of three β-GALactosidase genes in wild type and mutant tomato fruit. Postharvest Biol. Technol. 2003, 28, 207–217. [Google Scholar] [CrossRef]
- Ireland, H.S.; Gunaseelan, K.; Muddumage, R.; Tacken, E.J.; Putterill, J.; Johnston, J.W.; Schaffer, R.J. Ethylene regulates apple (Malus × domestica) fruit softening through a dose x time-dependent mechanism and through differential sensitivities and dependencies of cell wall-modifying genes. Plant Cell Physiol. 2014, 55, 1005–1016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tateishi, A.; Nagashima, K.; Mathooko, F.W.; Mwaniki, M.W.; Kubo, Y.; Inaba, A.; Yamaki, S.; Inoue, H. Differential expression of members of the β-GALactosidase gene family during Japanese pear (Pyrus pyrifolia) fruit growth and on-tree ripening. J. Am. Soc. Hort. Sci. 2005, 130, 819–829. [Google Scholar] [CrossRef] [Green Version]
- Saladié, M.; Matas, A.J.; Isaacson, T.; Jenks, M.A.; Goodwin, S.M.; Niklas, K.J.; Xiaolin, R.; Labavitch, J.M.; Shackel, K.A.; Fernie, A.R.; et al. A reevaluation of the key factors that influence tomato fruit softening and integrity. Plant Physiol. 2007, 144, 1012–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsilikochrisos, G.; Tsaniklidis, G.; Delis, C.; Nikoloudakis, N.; Aivalakis, G. Glutamate dehydrogenase is differentially regulated in seeded and parthenocarpic tomato fruits during crop development and postharvest storage. Sci. Hort. 2015, 181, 34–42. [Google Scholar] [CrossRef]
- Van de Poel, B.; Vandenzavel, N.; Smet, C.; Nicolay, T.; Bulens, I.; Mellidou, I.; Vandoninck, S.; Hertog, M.L.; Derua, R.; Spaepen, S.; et al. Tissue specific analysis reveals a differential organization and regulation of both ethylene biosynthesis and E8 during climacteric ripening of tomato. BMC Plant Biol. 2014, 14, 11. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Zhang, Y.; Jiang, L.; Shao, H. Roles of plant soluble sugars and their responses to plant cold stress. Afr. J. Biotechnol. 2009, 8, 2004–2010. [Google Scholar]
- Cantu, D.; Vicente, A.R.; Greve, L.C.; Dewey, F.M.; Bennett, A.B.; Labavitch, J.M.; Powell, A.L.T. The intersection between cell wall disassembly, ripening, and fruit susceptibility to Botrytis cinerea. Proc. Natl. Acad. Sci. USA 2008, 105, 859–864. [Google Scholar] [CrossRef] [Green Version]
- Tenhaken, R. Cell wall remodeling under abiotic stress. Front. Plant Sci. 2014, 5, 771. [Google Scholar] [CrossRef] [Green Version]
- Ishimaru, M.; Smith, D.L.; Mort, A.J.; Gross, K.C. Enzymatic activity and substrate specificity of recombinant tomato β-galactosidases 4 and 5. Planta. 2009, 229, 447–456. [Google Scholar] [CrossRef]
- Lee, D.H.; Kang, S.G.; Suh, S.G.; Byun, J.K. Purification, and characterization of a beta-galactosidase from peach (Prunus persica). Mol. Cells 2003, 15, 68–74. [Google Scholar]
- Bai, C.; Wu, C.; Ma, L.; Fu, A.; Zheng, Y.; Han, J.; Li, C.; Yuan, S.; Zheng, S.; Gao, L.; et al. Transcriptomics and Metabolomics Analyses Provide Insights into Postharvest Ripening and Senescence of Tomato Fruit Under Low Temperature. Hort. Plant J. 2022, in press. [CrossRef]
- Saltveit, M.E., Jr. Internal carbon dioxide and ethylene levels in ripening tomato fruit attached to or detached from the plant. Physiol. Plant. 1993, 89, 204–210. [Google Scholar] [CrossRef]
- Balasubramaniam, S.; Lee, H.C.; Lazan, H.; Othman, R.; Ali, Z.M. Purification and properties of a b-galactosidase from carambola fruit with significant activity towards cell wall polysaccharides. Phytochem. 2005, 66, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Ma, F.; Shi, S.; Qi, X.; Zhu, X.; Yuan, J. Changes and postharvest regulation of activity and gene expression of enzymes related to cell wall degradation in ripening apple fruit. Postharv. Biol. Technol. 2010, 56, 147–154. [Google Scholar] [CrossRef]


| Stage | Duration/Storage Temperature | ||||
|---|---|---|---|---|---|
| Commercial Maturity (CM) | Red Ripe (RR) | 5 d 4 °C | 5 d 10 °C | 5 d 25 °C | |
| Total soluble solids (° Brix) | 8.20 | 9.70 | 8.90 | 9.30 | 10.40 |
| SD | 0.42 | 0.57 | 0.40 | 0.54 | 0.48 |
| Homogenous groups | c | ab | bc | b | a |
| Firmness (N) | 1.15 | 0.94 | 1.03 | 0.96 | 0.73 |
| SD | 0.03 | 0.06 | 0.08 | 0.05 | 0.06 |
| Homogenous groups | a | b | a | b | c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fanourakis, D.; Nikoloudakis, N.; Paschalidis, K.; Christopoulos, M.V.; Goumenaki, E.; Tsantili, E.; Delis, C.; Tsaniklidis, G. Gene Expression, Activity and Localization of Beta-Galactosidases during Late Ripening and Postharvest Storage of Tomato Fruit. Agriculture 2022, 12, 778. https://doi.org/10.3390/agriculture12060778
Fanourakis D, Nikoloudakis N, Paschalidis K, Christopoulos MV, Goumenaki E, Tsantili E, Delis C, Tsaniklidis G. Gene Expression, Activity and Localization of Beta-Galactosidases during Late Ripening and Postharvest Storage of Tomato Fruit. Agriculture. 2022; 12(6):778. https://doi.org/10.3390/agriculture12060778
Chicago/Turabian StyleFanourakis, Dimitrios, Nikolaos Nikoloudakis, Konstantinos Paschalidis, Miltiadis V. Christopoulos, Eleni Goumenaki, Eleni Tsantili, Costas Delis, and Georgios Tsaniklidis. 2022. "Gene Expression, Activity and Localization of Beta-Galactosidases during Late Ripening and Postharvest Storage of Tomato Fruit" Agriculture 12, no. 6: 778. https://doi.org/10.3390/agriculture12060778
APA StyleFanourakis, D., Nikoloudakis, N., Paschalidis, K., Christopoulos, M. V., Goumenaki, E., Tsantili, E., Delis, C., & Tsaniklidis, G. (2022). Gene Expression, Activity and Localization of Beta-Galactosidases during Late Ripening and Postharvest Storage of Tomato Fruit. Agriculture, 12(6), 778. https://doi.org/10.3390/agriculture12060778











