Impact of Freshwater Macroalga (Cladophora glomerata) Extract on the Yield and Morphological Responses of Glycine max (L.) Merr.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soybean Varieties
2.2. Production of the Cladophora glomerata Extract
2.3. Field Experiment
- Algae extract (factor A)—main plot:
- I—Control—H2O (distillated water)
- II—20% Cladophora glomerata extract used as a foliar spray
- Cultivars (factor B)—subplot:
- Enrei
- Erica
2.4. Statistical Analysis
3. Results and Discussion
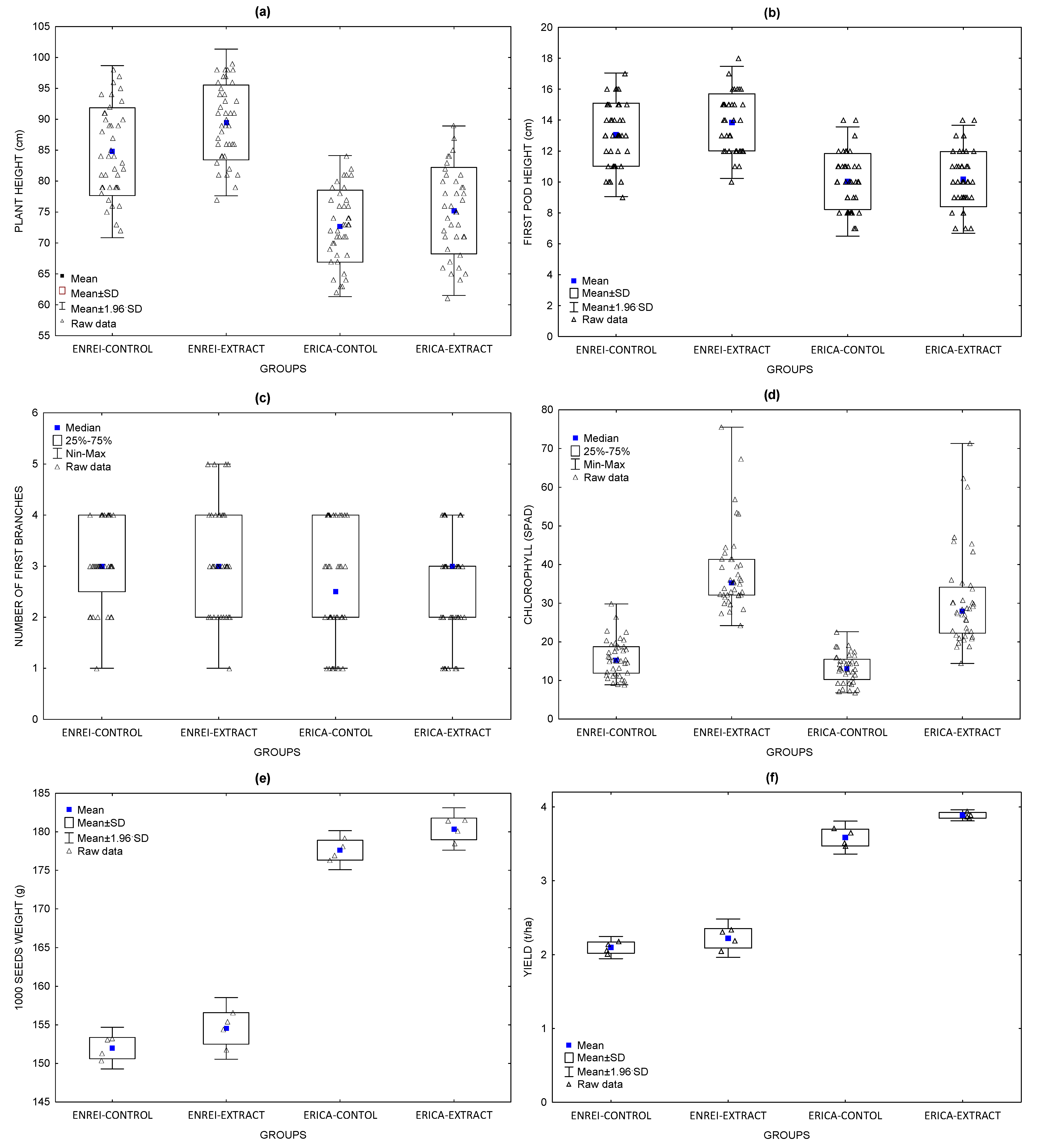
3.1. Effect of the Macroalgal Extracts on the Soybean Height
3.2. Effect of Macroalgal Extracts on the Height of the First Soybean Pod
3.3. Effect of the Macroalgal Extracts on the Number of First Branches in Soybean
3.4. Effect of the Macroalgal Extracts on the Chlorophyll Content in the Soybean Seedlings
3.5. Effect of Macroalgal Extracts on the Soybean 1000-Seed Weight
3.6. Effect of the Macroalgal Extracts on the Soybean Yield
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Faostat. 2018. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 1 January 2022).
- Holmberg, S.A. Soybeans for cool temperature climates. Agric. Hort. Genet. 1973, 31, 1–20. [Google Scholar]
- Kamp, J.A.L.M.; van Berkum, S.; Timmer, R.D.; van Reeuwijk, P. Verkenning Naar de Mogelijkheden van Eiwithoudende Teelten in Europa; Wageningen University & Research: Wageningen, The Netherlands, 2010. [Google Scholar]
- Kusano, M.; Baxter, I.; Fukushima, A.; Oikawa, A.; Okazaki, Y.; Nakabayashi, R.; Bouvrette, D.J.; Achard, F.; Jakubowski, A.R.; Ballam, J.M.; et al. Assessing metabolomic and chemical diversity of a soybean lineage representing 35 years of breeding. Metabolomics 2015, 11, 261–270. [Google Scholar] [CrossRef]
- Carrera, C.S.; Dardanelli, J.L.; Soldini, D.O. Chemical compounds related to nutraceutical and industrial qualities of non–transgenic soybean genotypes. J. Sci. Food Agric. 2014, 94, 1463–1469. [Google Scholar] [CrossRef]
- Häusling, M. The EU Protein Deficit: What Solution for a Long–Standing Problem? (2010/2111(INI)). (European Parliament, 2011). Available online: https://www.europarl.europa.eu/sides/getDoc.do?type=REPORT&reference=A7%E2%80%932011%E2%80%930026&language=%20EN (accessed on 12 September 2014).
- Podleśny, J. Rośliny strączkowe w Polsce—Perspektywy uprawy i wykorzystania nasion. Acta Agrophys. 2005, 6, 213–224. [Google Scholar]
- Lee, H.; Garlich, A. Effect of overcooked soybean meal on chicken performance and amino acid availability. Poult. Sci. 1992, 71, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Engram, D.; Parsons, S.; Parsons, C.M. In Methods for determining quality of soybean meal important. Feedstuffs 1999, 71, 10–11. [Google Scholar]
- Xu, S.; Liu, X.; Wie, J.; Ma, L.; Sun, Z. Effects of different fermented soybean meals on growth performance and intestinal morphology in piglets. Feed Ind. 2013, 21, 1–9. [Google Scholar]
- De Visser, C.L.M.; Schreuder, R.; Stoddard, F.L. The EU’s dependency on soya bean import for the animal feed industry and potential for EU produced alternative. Oilseed Fat Crops Lipids 2014, 21, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Brown, P.B. Soybean Meal in Aquaculture. Soybean INFO. March 2002. Available online: www.soymeal.org (accessed on 1 January 2022).
- Willis, S. The use of soybean meal and full fat soybean meal by the animal feed industry. In Proceedings of the 12th Australian Soybean Conference, Bundaberg, Australia, 2–6 February 2003; pp. 1–8. [Google Scholar]
- Biermann, U.; Friedt, W.; Lang, S.; Lühs, W.; Machmüller, G.; Metzger, J.O.; Klaas, M.R.; Schäfer, H.J.; Schneider, M.P. New syntheses with oils and fats as renewable raw materials for the chemical industry. Angew. Chem. Int. Ed. 2000, 39, 2206–2224. [Google Scholar] [CrossRef]
- Karczmarczuk, I. Soja—roślina ze wszech miar użyteczna. Wiadomości Zielar. 1999, 41, 6–7. [Google Scholar]
- Ohyama, T.; Tewari, K.; Ishikawa, S.; Tanaka, K.; Kamiyama, S.; Ono, Y.; Hatano, S.; Ohtake, N.; Sueyoshi, K.; Hasegawa, H.; et al. Role of nitrogen on growth and seed yield of soybean and a new fertilization technique to promote nitrogen fixation and seed yield. Open access peer-reviewed chapter in a book. In Soybean—The Basis of Yield, Biomass and Productivity; BoD–Books on Demand: Norderstedt, Germany, 2017. [Google Scholar] [CrossRef] [Green Version]
- Haegele, J.W.; Below, F.E. The Six Secrets of Soybean Success: Improving Management Practices for High Yield Soybean Production. 2013. Available online: http://cropphysiology.cropsci.illinois.edu/documents/2012%20Six%20Secrets%20of%20Soybean%20Success%20report.pdf (accessed on 8 November 2015).
- Summerfield, R.J.; Lawn, R.J.; Qi, A.; Ellis, R.H.; Roberts, E.H.; Chay, P.M.; Brouwer, J.B.; Rose, J.L.; Shanmugasundaram, S.; Yeates, S.J.; et al. Towards the reliable prediction of time to flowering in six annual crops. II. Soyabean (Glycine Max). Exp. Agric. 2008, 29, 253–289. [Google Scholar] [CrossRef]
- Szyrmer, J.; Federowska, B. Kierunki badań i hodowli roślin soi. Biul. IHAR 1975, 3–4, 3–7. [Google Scholar]
- Wilcox, J.R.; Boerma, H.R.; Specht, J.E. Soybeans: Improvement, production, and uses. In American Society of Agronomy, Crop Science Society of America, Soil Science Society of America; Boerma, H.R., Specht, J.E., Eds.; Agronomy: Madison, WI, USA, 2004; Volume 16, pp. 303–416. [Google Scholar]
- Hyten, D.L.; Choi, I.Y.; Costa, J.M.; Cregan, P.B.; Nelson, R.L.; Song, Q.; Specht, J.E.; Shoemaker, R.C.; Zhu, Y. Impacts of genetic bottlenecks on soybean genome diversity. Proc. Natl. Acad. Sci. USA 2006, 103, 16666–16671. [Google Scholar] [CrossRef] [Green Version]
- Jaranowski, J.; Muszyński, A.; Skorupska, H.; Torz, L. Charakterystyka zmienności i ważniejszych pod względem gospodarczym cech użytkowych soi (Glycine max L.). In Poznańskie Towarzystwo Przyjaciół Nauk, Wydział Nauk Rolniczych i Leśnych; Prace Komisji Nauk Rolniczych i Komisji Nauk Leśnych: Poznań, Poland, 1984; Volume LVII, pp. 149–159. [Google Scholar]
- Nawracała, J.; Konieczny, G. Możliwości wykorzystania zmienności introdukowanej, mutacyjnej i rekombinacyjnej w hodowli soi. Hod. Roślin–I Kraj. Konf. 1997, XXII, 91–94. [Google Scholar]
- Jaranowski, J.; Konieczny, G.; Muszyński, A.; Skorupska, H.; Torz, L. Charakterystyka zmienności ważniejszych pod względem gospodarczych cech użytkowych soi. Zesz. Probl. Post. Nauk. Rol. 1983, 253, 7–24. [Google Scholar]
- Criswell, J.G.; Hume, D.J. Variation in sensitivity to photoperiod among early maturing soybean strains. Crop Sci. 1972, 12, 657–660. [Google Scholar] [CrossRef]
- Polson, D.E. Day-Neutrality in Soybeans. Crop Sci. 1972, 12, 6. [Google Scholar] [CrossRef]
- Runge, E.C.A.; Odell, R.T. The relation between precipitation, temperature and the yield of soybeans on the Agronomy South Farm, Urbana, Illinois. Agron. J. 1960, 52, 245–247. [Google Scholar] [CrossRef]
- Shanmu-Gasundaram, S.; Tsou, S.C.S. Photoperiod and critical duration for flower induction in soybean. Crop Sci. 1978, 18, 598–601. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ashour, M.; Sakai, N.; Zhang, L.; Hassanien, H.A.; Ammar, G.A.G.; Ammar, G. Impact of seaweed liquid extract biostimulant on growth, yield, and chemical composition of cucumber (Cucumis sativus). Agriculture 2021, 11, 320. [Google Scholar] [CrossRef]
- Mohan, V.R.; Venkataraman Kumar, V.; Murugeswari, R.; Muthuswami, S. Effect of crude and commercial seaweed extract on seed germination and seedling growth in Cajanus cajan L. Phykos 1994, 33, 47–51. [Google Scholar]
- Sivasankari, S.; Venkatesalu, V.; Anantharaj, M.; Chandrasekaran, M. Effect of seaweed extracts on the growth and biochemical constituents of Vigna sinensis. Bioresour. Technol. 2006, 97, 1745–1751. [Google Scholar] [CrossRef]
- Cober, E.R.; Voldeng, H.D. A new soybean maturity and photoperiod-sensitive locus linked to E1 and T. Crop Sci. 2001, 41, 698–701. [Google Scholar] [CrossRef]
- Heatherly, L.G.; Elmore, R.W. Managing inputs for peak production. In Soybeans: Improvement, Production, and Uses, 3rd ed.; Specht, J.E., Boerma, H.R., Eds.; Agronomy Monographs; American Society of Agronomy: Madison, WI, USA, 2004; pp. 451–536. [Google Scholar]
- Major, D.L.; Anderson, I.C.; Johnson, D.J.; Tanner, J.W. Effects of day length and temperature on soybean development. Crop Sci. 1975, 15, 174–179. [Google Scholar] [CrossRef]
- Lewandowska, S.; Michalak, I. Effect of algal extracts on germination ability of different varieties of soybean. In Seed and Seedlings; XIV Scientific and Technical Seminar; 978-80-213-2922-5; Czech University of Life Sciences Prague: Prague, Czech Republic, 2019; pp. 84–90. [Google Scholar]
- Dziergowska, K.; Lewandowska, S.; Mech, R.; Pol, M.; Detyna, J.; Michalak, I. Soybean germination response to algae extract and a static magnetic field treatment. Appl. Sci. 2021, 11, 8597. [Google Scholar] [CrossRef]
- Mooney, P.A.; Van Staden, J. Algae and cytokinins. J. Plant. Physiol. 1986, 123, 1–21. [Google Scholar] [CrossRef]
- Ashour, M.; Hassan, S.M.; Elshobary, M.E.; Ammar, G.A.G.; Gaber, A.; Alsanie, W.F.; Mansour, A.T.; El-Shenody, R. Impact of commercial seaweed liquid extract (TAM®) biostimulant and its bioactive molecules on growth and antioxidant activities of hot pepper (Capsicum annuum). Plants 2021, 10, 1045. [Google Scholar] [CrossRef]
- Dziergowska, K.; Wełna, M.; Szymczycha-Madeja, A.; Chęcmanowski, J.; Michalak, I. Valorization of Cladophora glomerata biomass and obtained bioproducts into biostimulants of plant growth and as sorbents (biosorbents) of metal ions. Molecules 2021, 26, 6917. [Google Scholar] [CrossRef]
- Blunden, G. Agricultural uses of seaweeds and seaweed products. In Seaweed Resources in Europe: Uses and Potential; Guiry, M.D., Blunden, G., Eds.; John Wiley and Sons: Chichester, UK, 1991; pp. 65–81. [Google Scholar]
- Michalak, I.; Messyasz, B. Concise review of Cladophora spp.: Macroalgae of commercial interest. J. Appl. Phycol. 2021, 33, 133–166. [Google Scholar] [CrossRef]
- Fraser, J.; Eaton, G.W. Applications of yield component analysis to crop research. Field Crop. Abstr. 1983, 36, 787–796. [Google Scholar]
- Mohammadi, S.A.; Prasanna, S.A.; Singh, N.N. Sequential path model for determining interrelationships among grain yield and related characters in maize. Crop Sci. 2003, 43, 1690–1697. [Google Scholar] [CrossRef]
- Rasmusson, D.C.; Cannell, R.Q. Selection for grain yield and components of yield in barley. Crop Sci. 1970, 10, 51–54. [Google Scholar] [CrossRef]
- Egli, D.B.; Guffy, R.D.; Heitholt, J.J. Factors associated with reduced yields of delayed plantings of soybean. J. Agron. Crop Sci. 1987, 159, 176–185. [Google Scholar] [CrossRef]
| Year | 2019 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Month | I | II | III | IV | V | VI | VII | VIII | IX | X | XI | XII | |
| Decade | |||||||||||||
| Temperature (°C) | |||||||||||||
| I | 0.7 | 1.8 | 6.7 | 10.5 | 9.5 | 20.9 | 17.7 | 20.2 | 16.3 | 10.1 | 7.8 | 2.4 | |
| II | 1.0 | 4.1 | 6.1 | 8.3 | 11.6 | 23.0 | 17.6 | 19.0 | 13.0 | 13.9 | 7.2 | 3.6 | |
| III | −2.7 | 3.8 | 7.2 | 13.5 | 14.8 | 22.3 | 22.4 | 21.4 | 13.9 | 9.9 | 6.2 | 3.6 | |
| Monthly mean | −0.9 | 3.2 | 6.7 | 10.8 | 12.1 | 22.1 | 19.3 | 20.3 | 14.4 | 11.2 | 7.1 | 3.2 | |
| Multi-year means for 1981–2010 | −0.8 | 0.3 | 3.8 | 8.9 | 14.4 | 17.1 | 19.3 | 18.3 | 13.6 | 9.1 | 3.9 | 0.2 | |
| Rainfall (mm) | |||||||||||||
| I | 26.0 | 16.6 | 10.3 | 0.0 | 9.4 | 1.3 | 3.2 | 13.6 | 34.4 | 7.6 | 4.7 | 3.1 | |
| II | 26.3 | 2.7 | 8.4 | 5.2 | 20.6 | 4.2 | 1.5 | 38.6 | 3.9 | 3.2 | 28.2 | 1.6 | |
| III | 3.9 | 8.4 | 3.8 | 19.0 | 46.8 | 21.5 | 39.8 | 7.6 | 3.7 | 7.3 | 1.6 | 10.4 | |
| Total sums | 56.2 | 27.7 | 22.5 | 24.2 | 76.8 | 27.0 | 44.5 | 59.8 | 42.0 | 18.1 | 34.5 | 15.1 | |
| Multi-year means for 1981–2010 | 31.9 | 26.7 | 31.7 | 30.5 | 51.3 | 59.5 | 78.9 | 61.7 | 45.3 | 32.3 | 36.6 | 37.4 | |
| Parameter | Enrei | Erica | ||
|---|---|---|---|---|
| Control | Extract | Control | Extract | |
| Plant height (cm); Mean ± SD ** | 84.8 ± 7.1 a | 89.5 ± 6.1 a | 72.7 ± 5.8 | 75.2 ± 7.0 |
| First pod height (cm); Mean ± SD ** | 13.1 ± 2.0 | 13.9 ± 1.8 | 10.0 ± 1.8 | 10.2 ± 1.8 |
| Number of first branches; Median * | 3 | 3 | 3 | 3 |
| Chlorophyll (SPAD); Median * | 15.3 a | 35.2 a | 13.1 a | 28.2 a |
| 1000 seed weight (g); Mean ± SD ** | 152 ± 1 | 155 ± 2 | 178 ± 1 a | 180 ± 1 a |
| Yield (t/ha); Mean ± SD ** | 2.10 ± 0.08 | 2.22 ± 0.13 | 3.58 ± 0.11 a | 3.89 ± 0.04 a |
| Parameter | Enrei | Erica | ||
|---|---|---|---|---|
| Control | Extract | Control | Extract | |
| Number of pods per plant * | 21.1 ± 1.2 | 23.8 ± 1.7 | 28.4 ± 1.0 | 31.3 ± 0.6 |
| Number of seeds per plant ** | 41.3 ± 0.8 | 41.7 ± 0.9 | 56.8 ± 1.1 | 64.3 ± 1.5 |
| Seed weight per plant *** (g) | 6.37 ± 0.14 | 6.50 ± 0.14 | 10.1 ± 0.2 | 11.7 ± 0.2 |
| Seed weight per pod **** (g) | 0.303 ± 0.024 | 0.274 ± 0.021 | 0.358 ± 0.006 | 0.375 ± 0.003 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewandowska, S.; Marczewski, K.; Kozak, M.; Ohkama-Ohtsu, N.; Łabowska, M.; Detyna, J.; Michalak, I. Impact of Freshwater Macroalga (Cladophora glomerata) Extract on the Yield and Morphological Responses of Glycine max (L.) Merr. Agriculture 2022, 12, 685. https://doi.org/10.3390/agriculture12050685
Lewandowska S, Marczewski K, Kozak M, Ohkama-Ohtsu N, Łabowska M, Detyna J, Michalak I. Impact of Freshwater Macroalga (Cladophora glomerata) Extract on the Yield and Morphological Responses of Glycine max (L.) Merr. Agriculture. 2022; 12(5):685. https://doi.org/10.3390/agriculture12050685
Chicago/Turabian StyleLewandowska, Sylwia, Krzysztof Marczewski, Marcin Kozak, Naoko Ohkama-Ohtsu, Magdalena Łabowska, Jerzy Detyna, and Izabela Michalak. 2022. "Impact of Freshwater Macroalga (Cladophora glomerata) Extract on the Yield and Morphological Responses of Glycine max (L.) Merr." Agriculture 12, no. 5: 685. https://doi.org/10.3390/agriculture12050685
APA StyleLewandowska, S., Marczewski, K., Kozak, M., Ohkama-Ohtsu, N., Łabowska, M., Detyna, J., & Michalak, I. (2022). Impact of Freshwater Macroalga (Cladophora glomerata) Extract on the Yield and Morphological Responses of Glycine max (L.) Merr. Agriculture, 12(5), 685. https://doi.org/10.3390/agriculture12050685










