Abstract
The world population is increasing, and our current agricultural practices are not sustainable enough to address the concerns. Alternative proteins including plant-based proteins would provide a more sustainable source of food and feed ingredients. Among food systems, the aquaculture industry is rapidly growing, while still depending on marine sources as a main source of protein. Thus, using alternative plant-based proteins as a source for developing aquafeed would make this industry more viable. Sorghum is a valuable grain with high protein contents, proper mineral and fatty acids balance, and is available all around the world. However, sorghum has not been used widely for aquafeed development. In this review article, we cover sorghum production, composition, sorghum as a protein source for aquafeed development, and bioprocessing methods for enhancing the quality of sorghum.
1. Introduction
As the world population increases to 10 billion by 2050, total food and meat production must rise by 70 to 100% to satisfy global demand [1]. The USA food production system faces several issues in meeting this demand due to the limited available agricultural water and land and increased greenhouse gas emissions. Increasing water scarcity in major production regions and increasing vulnerability to disruptions from natural disasters due to climate change are just some of the growing issues that prompt the need for new technologies in meat production. Therefore, the new sustainable protein sources would help alleviate these concerns and supply sufficient protein for the world population [2,3].
Sorghum is a drought-resistant cereal grain typically cultivated in semi-arid conditions. This grain has been ranked fifth worldwide after wheat, corn, rice, and barley in terms of both production and area planted [4,5,6]. Global sorghum production accounted for 2.2% of total worldwide grain production in 2013 [7] and reached 62.3 MT in 2020 [8]. The United States is the main producer of sorghum, with 15.21% of the total production.
Sorghum’s special characteristics, such as mostly being grown by subsistence farmers, resistance to wild plants, and adaptability to poor soils and climate, increase its utilization above the other cereal grains [9]. Sorghum has long been a principal food crop and a major source of protein, energy, and minerals for millions of people in Asia and Africa [10,11]. This grain has gained a global reputation in the production of fermented foods due to its wide adaptability and low production cost [11]. Sorghum demand is growing by consumer choice because the grain is a non-GMO (non-genetically modified organism), gluten-free, and high in antioxidants. In the USA sorghum is being used mainly for animal feed [12] and biofuel production [13,14]. In 2018, researchers indicated that by converting 10% of the pastureland and cropland to sorghum, the total ethanol production could be increased to 17 billion gallons [15]. Sorghum is one of the few crops that can fit into all of the current bioenergy frameworks, including grain-to-ethanol, sweet sorghum sugar into bio-fuels, and as lignocellulosic and cellulosic biomass feedstock for bio-fuels [16]. Sorghum has also been used as a novel ingredient for developing sustainable aquafeeds [5,17,18,19].
Fish and fishery products are an important source of essential nutrients in the human food chain, and demand is growing along with the increasing population [20]. Aquaculture is a rapidly-growing segment of the food industry, and production growth is dependent on the utilization of resources other than the fish meal for aquafeeds. With such a huge demand for fish feed, agricultural ingredients such as cereal grains and oilseeds have been introduced in progressively increasing amounts, replacing fish meal and marine protein sources [21]. Among these ingredients, sorghum grain has potential due to its extensive global production and suitability of nutritional properties [22]; although like any other ingredient, sorghum has its own cons and pros as a feed ingredient for aquafeed development (Table 1 and Table 2).

Table 1.
A summary of sorghum pros and cons for aquafeed development [2,4,5,6,8,9,11,18,23].

Table 2.
Different grains’ price in 2020.
In addition, compared to other plant-based protein sources, the sorghum price is much lower, making it a cost-effective feed ingredient (Table 2).
Despite the potential use of sorghum grain in aquafeeds, there are no review papers related to the effect of sorghum on the nutritional status and health of fish and crustaceans. The present review includes an overview of studies on sorghum grain characteristics, composition, and application in aquafeed, emphasizing improving fish productivity when fed high sorghum-based diets.
2. Sorghum Biology and Agriculture
Sorghum is a genus of grasses native to Australia and certain regions in Africa, Asia, the Indo-Pacific, and Middle America, but widely cultivated and naturalized throughout the world [24]. Although the genus name Sorghum is part of the formal taxonomy of these plants, “sorghum” or “sorghums” is also used in common parlance and throughout the present text. Sorghums are more heat- and drought-resistant than other cereal or forage crops, less prone to fungal infections and mycotoxin contamination, and better-suited to marginal cropland. Sorghums are typically categorized according to their use:
- -
- Grain sorghums (Caffrotum group) are cultivated for their grain, similar to corn/maize, and used to make human foods and beverages and in animal feeding.
- -
- Sweet sorghums (Saccharatum group) are cultivated for their stalks and foliage, which are used to produce syrups, silage, and, increasingly, biofuels.
- -
- Grass sorghums (Sudan grass) are cultivated as animal fodder consumed directly as a pasture or hay crop or as silage.
- -
- Broom corn (Technicum group) are woody varieties cultivated for their fibrous structures used to make brooms and brushes [24].
Although sweet or grass sorghums could be developed for use in aquaculture, grain sorghums (hereafter, simply “sorghums”), specifically varieties of S. bicolor, are more similar to other commonly used feed ingredients and likely have greater potential for use in aquafeeds.
Grain sorghums are also classified into three types according to their tannin contents, including type Ⅰ, type Ⅱ, and type Ⅲ, in which type Ⅰ is tannin-free and types Ⅱ and Ⅲ contain low and high levels of tannin, respectively [25,26,27]. Sorghums are raised in warm, semi-arid regions throughout the world and, as shown in Table 3, the USA, Nigeria, Ethiopia, Sudan, Mexico, India, China, Argentina, Brazil, and Niger are the countries with the highest production of sorghum grains. However, sorghum production in these countries has changed from 2019 to 2018. Argentina and Sudan have shown the highest production growth of 28% and 25% during 2020–2021, respectively, while sorghum production by India and Brazil has dropped by 18.7% and 6.8%, respectively. Selective breeding has produced a wide range of sorghum varietals and hybrids varying in their tolerance of poor climatic conditions, resistance to pathogens, higher yield, and suitable time of maturity, as well as grain shape, size, color, hardness, and nutritional value [28].

Table 3.
Production of sorghum and changers percentage during 2019 to 2021 in different countries.
Varietals are commonly grouped according to grain color, e.g., black, brown, red, yellow, and white. Grain color is indicative of other attributes, including nutrient levels and the presence and concentrations of phenolic compounds, including tannins produced by the plant to discourage grazing by herbivores. Whereas some of these phytochemicals are thought to have beneficial effects, perhaps nutraceutical and biological activities [29], tannins impart a bitter or astringent flavor and are generally considered anti-nutritional factors (ANF) in animal feeding. White tan sorghums have low levels of phenolic compounds in general and contain little or no tannins. Yellow and red varieties have modest and moderately high levels of phenolic compounds, respectively, but do not contain tannins. Black varieties are genetically red and compositionally similar to red varieties, but the grain darkens in response to sunlight as it matures. Brown varieties contain high levels of tannins and are sometimes referred to as “tannin sorghums” [30]. Consequently, while tan, cream, and white varieties are used to make flours for human consumption and the food industry, black and burgundy varieties are raised for their antioxidant properties and are used in specialty foods. Additionally, red, orange, and bronze are the most commonly raised varieties and have various applications in human foods and animal feeding. Notably, sorghum varieties’ diversity and characteristic are the results of conventional selective breeding only; genetic modification has not been implemented in sorghum agriculture and all commercially produced crops are non-genetically modified.
3. Nutrient Composition of Sorghum
The sorghum kernel has three distinct parts, including a pericarp or bran on the outside, the germ or embryo, and the endosperm or storage tissue. The pericarp represents a small proportion of the sorghum kernel and available nutrients, with a content of 4% total protein, 11% fat, and 4% starch; the remaining tissue is cellulose and hemicellulose [31,32]. Endosperm represents 85% of the whole grain on average and is filled with starch granules [31]. The germ, a rich source of lipid (28% of the germ), also has high levels of protein (19%) and ash (10%) [31] (Figure 1).
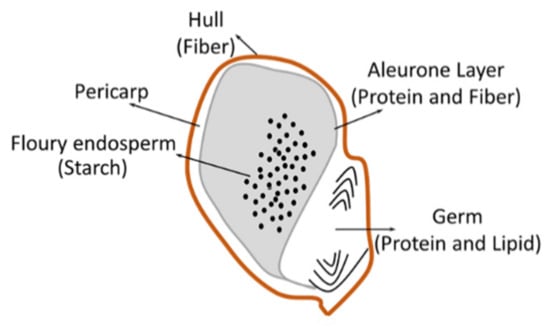
Figure 1.
Sorghum grain structure.
The proximate composition and nutrient content of various sorghum grains is different according to their varieties. As shown in Table 1, in a study performed by Gassem and Osman [33] on the varieties of Hamra, Shahla and Baidah, the protein content was 14.80%, 14.51%, and 14.75%, respectively, while three varieties of sorghum including white, red, and black analyzed by Pontieri and Troisi [34] showed 6.14%, 6.85%, and 7.28%, respectively. Another significant difference between the varieties was in crude fiber, for which an analysis of the sorghum varieties in the Pontieri and Troisi [34] study revealed 6.5% on average, while the Gassem and Osman [33] study showed 1.87% crude fiber for the three varieties on average. Despite the crude fiber, the crude fat content showed a reverse pattern in their studies [33,34]. Furthermore, in another study, Udachan and Sahoo [35] determined the proximate composition of the four varieties dadar, parbhani, CSH-5, and CSH-9. As shown, similar to the other analyses, the total carbohydrate was not significantly different; however, protein content, crude fiber and fat as well as the ash content were different from the other two analyses. Moreover, according to the analyses that the authors carried out on the four varieties of Texas, red, white/tan, and super sack, their protein contents were 14.9%, 15%, 10.9%, and 13.4%, respectively. In addition, as indicated in Table 4, the crude fat is comparable among the Hamra, Shahla, and Baidha varieties. However, it was higher than the varieties analyzed by Udachan and Sahoo [35] and Pontieri and Troisi [34], whereas the crude fiber content was almost in agreement with the results reported by Udachan and Sahoo [35] and Gassem and Osman [33].

Table 4.
Proximate composition of some sorghum varieties.
Overall, these components yield a macronutrient profile with abundant carbohydrate and energy levels and modest amounts of lipids and protein (Table 4).
3.1. Protein Content and Amino Acids Compositions
The protein content of sorghum varies with environmental conditions (particularly rainfall), growing region, and soil type, and among varieties [36], with average levels ranging from 8 to 15% [4,36,37] on a dry matter basis. The sorghum proteins are divided based on their solubility in different solvents as: water-soluble proteins (albumins), salt-soluble (globulins), prolamins, aqueous alcohol-soluble (kafirins), aqueous alcohol + reducing agent-soluble (cross-linked kafirins), detergent + reducing agent + alkaline pH-soluble (cross-linked glutelins), and unextracted protein residue [38]. Another classification method is based on the homogeneous nature and different origin of the kafirin storage prolamins relative to the heterogeneous nature of the non-kafirin proteins (albumins, globulins, and glutelins) which will divide the proteins into kafririn and non-kafirin proteins.
As discussed before, the amino acid composition of sorghum varies along with the protein content. Sorghum cultivars have been proven to contain smaller amounts of lysine, threonine, and total sulfur amino acids compared to soybean [9,39]. In comparison with yellow corn protein, sorghum grain protein typically contains higher levels of Ala, Asp, Glu, Leu, Ile, Phe, Tyr, and Val, and lower levels of Arg, Gly, His, and sulfur amino acids (methionine and cystine) [36,40]. Despite the variation in amino acid profiles of sorghum varieties and types, sorghum proteins (similar to other plant proteins) are generally lower in the essential amino acids such as lysine, tryptophan, and threonine than are animal-based ingredients [40]. In sorghum grains with higher levels of protein, there is usually a lower ratio of lysine [41] (Figure 2).
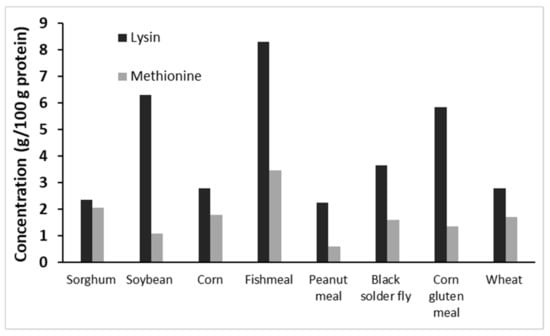
Figure 2.
Lysine and methionine contents of common feed ingredients of aquafeed [36,42,43].
Sorghum protein is less digestible as compared with that of corn for humans [44,45], and cooked sorghum also has lower protein digestibility than that of other grains [9,46]. Reduced digestibility is primarily associated with the formation of disulfide bonds [47]. This condition also affects the starch digestion, since starch granules in sorghum grains are usually entrapped in the protein matrix [48]. In addition, an association has been observed between protein and the pericarp or endosperm cell walls in sorghum [49,50] and barley [51]. Starch granules and protein of the endosperm are surrounded by cell walls [52] and bound to dietary fiber [50]. This bonding between protein and non-starch polysaccharides can reduce protein digestibility either by reducing accessibility to enzymes or forming indigestible complexes [23].
Sorghum proteins also could be divided into two groups, including prolamins and non-prolamins. Prolamins are a group of storage proteins in plants with high proline and glutamine content. They solubilize best in strong alcohol (70–80%), light acid, and alkaline solutions. Kafirins, the predominant protein in sorghum, are classified as prolamin storage proteins and are soluble in alcohol–water mixtures [53,54]. Sorghum varieties are further classified into α-, β-, γ-, and δ-kafirin. α-Kafirin is the main protein store in sorghum (80–84%) [55]. Increasing the α-kafirin ratio can improve the protein digestibility [56]. This protein is rich in nonpolar amino acids and is found primarily as monomers and oligomers. It is thought that inter- and intra-molecular disulfide bonds between β- and γ-kafirin result in an increased degree of protease resistance in protein bodies, thereby lowering the digestibility [57,58,59]. It has been suggested by researchers that genetical modification of sorghum may lower the outer layer of the protein body (β, γ) to improve digestibility (20%) by allowing greater access of proteinase to α-kafirin [38].
3.2. Fat and Fatty Acids
Sorghum, rice and wheat fat contents are 3.4, 5.5, and 7.5%, respectively [7]. The germ and aleurone layers are the major sources of fat; the germ contributes approximately 80% of the total fat [60]. Sorghum lipids are valuable nutrients that influence the taste and storage time of sorghum meals; they consist primarily of unsaturated fatty acids, with polyunsaturated fatty acids being the most abundant [30]. Usually, sorghum oil is extracted using wet-milled germ fraction through a water-intensive technology, which applies water for extracting and separating different fractions including starch, oil, and protein from seeds [31].
The major lipid class in sorghum seeds is triacylglycerols (accounting for approximately 90% of total lipids), with linoleic acid being the predominant fatty acid. Oleic acid is the second-most abundant fatty acid in sorghum grain followed by palmitic acid and stearic acid [7]. The fatty acid composition of sorghum oil is as follows: linoleic, 52%; oleic, 32%; palmitic, 10%; stearic, 4%; and linolenic, 1% [61]. Generally, sorghum oil is similar to corn oil, and due to its higher content of essential fatty acids, sorghum has a high potential to be used as another grain in human and animal nutrition [62].
3.3. Micronutrients
Sorghum contains high levels of minerals but with variable bioavailability, ranging from less than 1% for some forms of iron to greater than 90% for sodium and potassium. The reasons for this are varied and complicated since many factors interact to determine the ultimate bioavailability of a nutrient [37].
Sorghum is a rich source of B-complex vitamins, contains fat-soluble vitamins, namely D, E, and K, and is not a rich source of vitamin C. The concentrations of thiamin, riboflavin, and niacin in sorghum are high. Sorghum does not contain vitamin A, although certain yellow endosperm varieties contain small amounts of β-carotene, a precursor of vitamin A.
3.4. Fiber
Sorghum bran is low in ash and protein and rich in fiber. Cellulose, the major insoluble fiber component of sorghum, varies from 1.19 to 5.23% among sorghum varieties [63]. Processing removes the outer pericarp, and thus proportionally increases the protein content and reduces the cellulose, lipid, and mineral contents of the grain [64].
3.5. Starch
Starch is the major component of sorghum meal and constitutes 83% of the endosperm and 69.5% of the whole grain [65]. Starch granules of sorghum are similar to those of corn in general size, range, and shape [31]; however, the starch and sugar in sorghum are released more slowly than those in other cereals [66]. Sorghum starch has a higher gelatinization temperature range (68–76 °C) than that of both corn starch (62–68 °C) and wheat starch (58–64 °C) [31].
Sorghum starch digestibility is relatively lower than that of corn due to being bound in a protein matrix, limiting accessibility to enzymes [56]. The release of starch granules from the protein matrix using processing methods renders them more susceptible to enzymatic digestion [67]. The low starch digestibility has also been attributed to the high dietary fiber content [50].
The chemical nature of the starch, particularly the amylose and amylopectin content, is yet another factor that affects its digestibility. Starch digestibility has been reported to be higher in low-amylose, i.e., waxy sorghum, than that in normal sorghum [68]. The presence of tannins in the grain contributes to the poor digestibility of starch in some varieties of sorghum [69]. Tannins isolated from sorghum grain have been shown to inhibit the enzyme X-amylose and also bind to grain starches to varying degrees [70].
3.6. Anti-Nutritional Factors (ANFs)
Like many other plant ingredients, sorghum contains various ANFs, including trypsin and amylase inhibitors, phytic acid, and tannins. These compounds are known to have a negative impact on protein, carbohydrate, and mineral metabolism [11].
Tannins are the primary ANFs, limiting the utilization of sorghums in animal feeding. Seeds from a large number of sorghum varieties contain tannins, primarily of the condensed type. Atteh [71] observed that sorghum, especially the brown variety, contains high levels of tannins; only the white variety of sorghum is reported to be tannin-free [72] or contain negligible levels. Purseglove [73] highlighted that sorghum grain contains tannins in varying proportions depending on the variety, with certain strains containing up to 5%.
Tannins are responsible for the bulk of protein binding activity and are able to precipitate gelatin and other proteins from aqueous solutions [74]. The presence of sorghum’s tannin has a huge negative economic impact on the livestock industry and has been associated with a lower nutritive value, a lower biological availability of macromolecules such as proteins, carbohydrates, amino acids, and vitamins, and a lower protein efficiency ratio and weight [72]. Tannins also have a negative effect on feed intake [4], leading to poor feed efficiency [75], reduced nutrient digestibility [76], and weight gain in poultry and swine. Tannins bind to proteins, carbohydrates, and minerals, reducing the digestibility of these nutrients [77,78] and decreasing the utilization of energy, protein, and specific amino acids [79,80]. Tannins also diminish the permeability of the gut wall by reducing nutrient flow [81].
In monogastric animals, the feed efficiency can be reduced by 10–30% as compared with that of non-tannin sorghums [82]. The negative impact on feed intake has been attributed to the astringent taste of tannins [79,83].
Almost all cultured sorghum varieties contain minor amounts of tannins. There is evidence that tannins can also have significant health advantages [84]. Studies have revealed that sorghum phenolic compounds have potent antioxidant activity, and consumption of sorghum whole grain can improve gut health, and lower the risk of heart disease [30], due to the sorghum anti-inflammatory and anti-colon cancer activities [84].
4. Sorghum in Animal Nutrition
Sorghum has a long history in animal nutrition, and approximately half of the global sorghum production is used for animal feed [56,85]. Sorghum has been used in poultry feed as a corn replacement. This grain has approximately 95% of the feeding value of corn [60], while the price of sorghum in many areas is 10–15% lower than that of corn [12]. Mabelebele et al. [86] reported that whole sorghum inclusion does not negatively influence feed intake or weight gain in growing broiler chickens.
Sorghum is also grown for forage or silage, and dried leaves and stems are a useful carbohydrate ingredient for feeds for ruminants and other grazing species [81]. Whole sorghum grains can be given to sheep and pigs; however, they are usually ground to improve the feed conversion efficiency [71,87].
4.1. Aquatic Livestock
Limited studies are available regarding the application of sorghum as an aquafeed ingredient, although the potential of the grain has been examined extensively in poultry and cattle [88]. Available data, while limited, show that omnivorous fish such as carp and tilapia can digest and metabolize sorghum-based diets. In common carp, the nutrient digestibility of sorghum has been compared with that of wheat bran and rye. Protein digestibility in rye (91.89%) is higher than that of sorghum (71.86%) and wheat bran (80.64%), and fat digestibility estimates are 79.84%, 76.71%, and 82.01% in rye meal, sorghum meal, and wheat bran, respectively [89]. In another study, sorghum or pellets containing 25% protein were used to feed common carp in ponds. The results show that feeding on sorghum led to lower growth and higher body fat as compared with feeding on the pellet diet [17] (Figure 3).
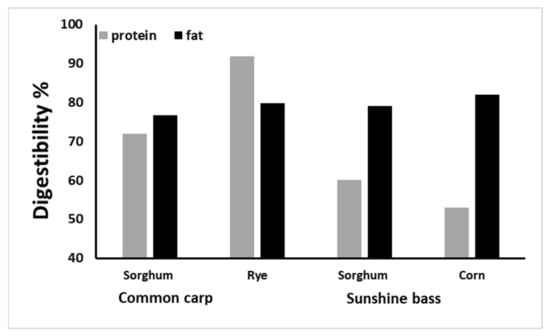
Figure 3.
A comparison between sorghum and corn and rye nutrient digestibility in common carp [88] and Sunshine bass [89].
In Sunshine bass, however, sorghum protein and fat digestibility were 60% and 79%, respectively, which are similar to those seen with corn [90]. Indigestible sorghum carbohydrates, such as lignin pentosans and cellulose, are hypothesized to be responsible for the relatively lower digestion efficiency of protein and fat in Sunshine bass. Fish species’ differences have a critical role in sorghum digestibility and performance, and omnivorous species such as carp digested sorghum better than a fish such as Sunshine bass. Sorghum apparent digestibility has also been evaluated in Florida pompano as a carnivorous species. Digestibility of the dry matter and energy of the sorghum diet are 45.4% and 63.9%, respectively, which are lower and higher than that of corn (58.1% and 71.4%) and wheat middlings (24.4% and 58.6%). The relatively lower digestibility of sorghum has been attributed to the source or type of starch, which needs higher temperatures for gelatinization [91].
Previous studies evaluating the use of sorghum products in tilapia have yielded some promising but inconclusive results. A comparison of five grain sources (corn, wheat, barley, sorghum, and rice) revealed that tilapia fed a diet containing 25% sorghum showed maximum growth performance and superior protein retention efficiency. The addition of sorghum also increased body fat content and reduces body moisture in tilapia [18].
In another study, Yones and Metwalli [92] demonstrated that sorghum starch can be used as the main carbohydrate source at inclusion rates of up to 30% in juvenile tilapia without any negative impacts on growth or digestibility. In a newer study, the growth performance and feed conversion of tilapia fed sorghum hominy at two inclusion rates (15% and 25%) were shown to be similar to that of tilapia fed a commercial diet [93]. These results confirm the ability of tilapia to use sorghum as the main dietary component at inclusion rates of 25% in custom formulated diets.
Sorghum is also considered a suitable replacement for corn in fish diets. Competition for use in human nutrition and other animal feeds has raised the corn market price and limited its availability [94]. It appears that sorghum as a source of starch has an immense potential to replace corn or cassava in omnivorous species. Replacement of cornmeal with up to 50% sorghum in both raw and fermented meals does not change growth-related parameters in juvenile Clarias gariepinus [5]. The growth performance in pangasius is not affected when cassava and corn are replaced with sorghum [95]. In addition, a sorghum diet does not change the fillet color in pangasius or the physical properties of the feed pellets (density and floatability). In silver catfish, the sorghum dry matter and protein digestibility are higher than corn [96], suggesting that sorghum is a favorable ingredient in catfish feed. Recent results reported by Rodrigues and Sanchez [97] reveal that corn can be replaced entirely by low-tannin sorghum in silver catfish diets without a negative impact on growth or efficacy.
4.2. Supplementation Strategies for Improving Sorghum Performance in Aquafeed
The relatively low protein, high fiber, and anti-nutritional content of sorghum grain restricts its inclusion ratio in high-value aquafeeds for higher trophic species. Improving digestibility and increasing the protein content through modification approaches could improve the ability of sorghum to serve as an alternative feed ingredient in aquaculture. Fungal fermentation increased sorghum hominy protein content by 21% and improved the desirable amino acid ratio. However, tilapia showed a lower feed efficiency on diets that contained bioprocessed sorghum with Trichoderma reesei compared to the commercial control diet [98]. Moreover, the resulting fillets from market-sized fish fed bioprocessed feed sometimes contained an off-flavor, which is highly undesirable for consumer choice. Final product quality also must be considered along with the bioprocessing results.
A combination of sorghum and probiotic has been introduced as a solution to increase the sorghum content of the fish feed. Nile tilapia fed diets containing 33% sorghum plus lactobacillus probiotic showed significantly larger growth and feed utilization than those fed corn-based diets [99]. An improved feed conversion ratio may suggest that a probiotic stimulates sorghum digestion, thereby increasing growth.
Prebiotic inulin has been used to protect the immune system against ectoparasite infection in tilapia fed sorghum-based diets. Supplementation of inulin and sorghum (2.5 g/kg and 15%, respectively) improved fish health and increased resistance to ectoparasites in Nile tilapia due to enhancement of the immune system [100]. The application of probiotics with prebiotics will improve fish growth, innate immune system and protection against bacteria [101].
Sorghum-based distilled soluble dried grain (DDGS) is a byproduct of the bio-fuel production industry and contains 28.7–32.9% crude protein, 8–13% fat, and 34.7–51.1% fiber [66,102,103]. There is interest in using this ingredient in aquafeed owing to its increased supply and low cost. The efficiency of extruded and pelleted sorghum-based DDGS diets has been assessed in two growth studies in Litopenaeus vannamei [104]. The results indicate that up to 40% of this new protein source can be used in feed formulations without affecting the performance of L. vannamei. Similar results have also been observed following the addition of up to 40% corn DDGS to feed for catfish, rainbow trout, and channel catfish [105,106,107].
Replacement of rice bran with sorghum distillery residue does not affect growth performance in grey mullet (Mugil cephalus); however, dietary intake of this ingredient, which has strong antioxidant activity, reduced hydroperoxide formation in fish gills [108]. The authors suggested that the presence of polyphenols in sorghum, which are able to inhibit hydroperoxide formation in the gill, is useful for stress resistance against changes in the marine or aquatic habitat [108].
A comparison between high- and low-tannin sorghum revealed that tilapia fed low-tannin sorghum silage had a larger weight gain and feed intake as compared with those fed either a high-tannin sorghum silage or corn diet, the latter two of which were similar [109].
Enzyme supplementation has been used as an alternative to increasing sorghum use in aquafeed. Phytase supplementation (1.500 FTU·kg−1) improved the digestibility coefficient of energy (3%) and phosphorous (7%) of sorghum grain in silver catfish as compared with those without enzyme supplementation [96]. Full replacement of corn with sorghum has been achieved using a combination of low-tannin sorghum and phytase addition to silver catfish diets. Phytase addition (1.500 FTU·kg−1) also improved feed conversion and protein efficacy in silver catfish when corn was replaced with low-tannin sorghum (50% or 100%) [110].
4.3. Reducing the Impact of Anti-Nutritional Factors
Fermentation, germination, enzymatic hydrolysis, heating, and chemical treatments have been employed as solutions to reduce the effect of the anti-nutritional factors [111,112].
In general, fermentation makes the feed ingredients easier to digest and the nutrients easier to absorb [113]. Fermentation reduced the sorghum crude protein content (12.25 vs. 10.70%) but improved the in vitro protein digestibility of sorghum flour (18 vs. 23%) [11]. Total polyphenols and phytates were decreased during the fermentation process of sorghum flour (8.1 vs. 6.6 and 317.6 vs. 247.9 mg/100 g, respectively); however, the tannin content was not affected. Soaking in water reduced the tannin content by 56–66% and 98–99% during 72 h in low- and high-tannin cultivars, respectively. In vitro protein digestibility was significantly increased by 15% after soaking in water only in high-tannin sorghum [114].
A combination of cooking and fermentation improved the nutrient quality and drastically reduces the anti-nutritional factors to safe levels (1.9 g and 3.2 ppm for tannin and cyanide, respectively), which is much better than any of the other processing methods tested [10]. The fungal fermentation process was also found to be an effective alternative to enhance the protein content of the sorghum hominy and increase the sorghum protein content by 20% [98].
Processing treatments of sorghum such as extrusion have a positive impact on tannin concentration. Awika and Dykes [115] observed that extrusion of tannin sorghum caused an 85% decrease in polymeric tannins, while the lower molecular weight tannins increased by 29–478%. Cooked sorghum protein is less digestible than the other grains [46]. The protein of moist-cooked sorghum has lower digestibility than that of uncooked sorghum [46,116,117] and proteins of other similarly cooked grains such as wheat and corn [47,118]. Moist cooking considerably alters the solubility properties of the sorghum protein as compared with those of corn, thereby lowering digestibility [58]; however, through proper processing, this problem can be overcome [82]. Some methods of processing sorghum can also improve protein digestibility. The inclusion of reducing agents seems to prevent the formation of protein polymers linked by disulfide bonds and can compensate for the decline in protein digestibility during the cooking process [58].
5. Improving Sorghum Digestibility
The low digestibility of sorghum protein (kafirins) and starch coupled with the low content of essential amino acids, lysine, and threonine, are the main obstacles to its further use in animal diets [119]. Genetic engineering tools may be alternatives to enhance kafirin digestibility and improve the nutritional value of sorghum grain [38,120]. A number of alternatives have been suggested to improve sorghum feed and food efficiency.
5.1. Fermentation
Fermentation has long been used as a way to improve protein digestibility [121,122,123]. A substantial increase in sorghum digestibility (51.8% to 75.6%) was observed after 24 h of fermentation [121], highlighting that enhanced protein digestibility may be attributed to the fractional degradation of complex storage proteins into simpler and more soluble products. In addition to digestibility, fermentation also influences functional properties, i.e., the pH shift in sorghum protein solubility. Water- and oil-binding capacities of sorghum flours decreased and increased, respectively, as a result of fermentation [123]. These modifications are due to proteolysis, during which monomers are generated from complicated large polymer molecules.
5.2. Chemical Modification
Sorghum protein conjugated to dextran or galactomannan has been applied to improve its functional properties [124]. Conjugation improves protein solubility at all pH levels and emulsification capacity doubles. Reducing agents, such as sodium metabisulphite and glutathione, have also been used to improve protein and starch digestibility [123,125], since these agents can break down the protein matrix [116].
5.3. Enzymatic Hydrolysis
Enzymatic methods are safer and cheaper than chemical methods and also do not produce toxic substances. Proteases have mainly been used to detach sorghum starch to improve the digestibility of animal feeds and increase the hydrolysis of starch for alcohol production. Treating sorghum starch with proteases reduced the protein content from 0.7–1.1% to 0.5–0.6% [126]. Pepsin pretreatment also increased sorghum starch digestibility [127].
Protease enzymes appear to have the potential to improve the digestibility and functionality of sorghum protein residue. Due to the protein barrier surrounding the starch granule, the enzymatic extraction of starch is not effortless. Thus, enzymatic hydrolysis of the protein matrix would significantly enhance the rate of starch hydrolysis by increasing surface area and improving starch-α amylase and amyloglucosidase interaction [128]. Zhang and Hamaker [125] used pepsin for sorghum and observed similar results. Sorghum has a high content of starch, which may reduce the efficiency of enzymatic hydrolysis due to the gelatin formation after heating [46].
5.4. Thermo-Mechanical Treatment
Sorghum protein digestibility can also be improved by extrusion technology. Fapojuwo and Maga [129] showed that extrusion improves the in vitro protein digestibility of sorghum by up to 30%. In another study, the digestibility of the sorghum flour protein was increased (18%) by extrusion [130].
6. Conclusions
Increasing food demand and shortages of natural resources (i.e., water and land) will result in a greater opportunity for the incorporation of sorghum into the food and feed industries. This highly photosynthetic-efficient crop can not only be used as a main source of grain but also for biomass production to produce high-quality food such as meat and milk. Increased use of sustainable plant ingredients to replace the long-used cereal crops such as corn and wheat in animal feed may also alleviate the conflict of human food security. Sorghum is also an ingredient opportunity for aquaculture and can easily replace corn in aquafeed. Herbivorous and omnivorous species can consume sorghum as the main feed ingredient without negative impacts on growth or digestibility. Improvements in sorghum nutritional value can increase its use in the aquafeed market as a new emerging substitute for fish meals. Sorghum protein content is between 8 and 15%, with different types of proteins including water-soluble, salt-soluble, alcohol-soluble, and reducing agent-soluble proteins. Sorghum protein usually contains high levels of Ala, Asp, Glu, Leu, Ile, Phe, Tyr, and Val, and low levels of Arg, Gly, His, and sulfur amino acids. Thus, in order to balance the animal diets, other protein sources should be used with sorghum. Protein modifications including thermal processing, enzymatic hydrolysis, and fermentation can improve sorghum protein functionality and digestibility; therefore, future research should focus on developing a suitable economical and safe method for the extraction of protein, starch, or even phenolic compounds that are useful for human health.
Author Contributions
Conceptualization, M.Z., A.K.A., M.H.S. and R.O.; investigation, M.Z., A.K.A. and R.O.; resources, R.O. and M.H.S.; writing—original draft preparation, R.O., A.K.A. and M.Z.; writing—review and editing, R.O., M.H.S., W.M.S. and J.T.T.; visualization, R.O.; supervision, M.Z. and R.O.; project administration, M.H.S.; funding acquisition, M.H.S. and R.O. All authors have read and agreed to the published version of the manuscript.
Funding
This project was financially supported by the United Sorghum Checkoff Program RG001-20.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Steve Urick and Ethan McAlhaney for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. FAOSTAT Statistical Database; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2020. [Google Scholar]
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food security: The challenge of feeding 9 billion people. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Valin, H.; Sands, R.D.; Van der Mensbrugghe, D.; Nelson, G.C.; Ahammad, H.; Blanc, E.; Bodirsky, B.; Fujimori, S.; Hasegawa, T.; Havlik, P. The future of food demand: Understanding differences in global economic models. Agric. Econ. 2014, 45, 51–67. [Google Scholar] [CrossRef]
- Nyachoti, C.; Atkinson, J.; Leeson, S. Sorghum tannins: A review. World’s Poult. Sci. J. 1997, 53, 5–21. [Google Scholar] [CrossRef]
- Aderolu, A.Z.; Kuton, M.P.; Odu-Onikosi, S.G. Substitution effect of sorghum meal for maize meal in the diet of catfish (Clarias gariepinus, Burchell, 1822) juvenile. Res. J. Fis. Hyd 2009, 4, 41–45. [Google Scholar]
- Stamenković, O.S.; Siliveru, K.; Veljković, V.B.; Banković-Ilić, I.B.; Tasić, M.B.; Ciampitti, I.A.; Đalović, I.G.; Mitrović, P.M.; Sikora, V.Š.; Prasad, P.V. Production of biofuels from sorghum. Renew. Sustain. Energy Rev. 2020, 124, 109769. [Google Scholar] [CrossRef]
- Bean, S.; Wilson, J.; Moreau, R.; Galant, A.; Awika, J.; Kaufman, R.; Adrianos, S.; Ioerger, B. Structure and composition of the sorghum grain. In Sorghum: State of the Art and Future Perspectives; American Society of Agronomy: Madison, WI, USA, 2016. [Google Scholar]
- USDA-NASS. Crop Production, 2019 Summary. Available online: https://downloads.usda.library.cornell.edu/usda-esmis/files/k3569432s/sj139j59z/1257b842j/cropan20.pdf (accessed on 13 May 2021).
- Jiddere, G.; Filli, K.B. The effect of feed moisture and barrel temperature on the essential amino acids profile of sorghum malt and bambara groundnut based extrudates. J. Food Processing Technol. 2015, 6, 1000448. [Google Scholar]
- Obizoba, I.C.; Atii, J. Effect of soaking, sprouting, fermentation and cooking on nutrient composition and some anti-nutritional factors of sorghum (Guinesia) seeds. Plant Foods Hum. Nutr. 1991, 41, 203–212. [Google Scholar] [CrossRef]
- Mohammed, N.A.; Ahmed, I.A.M.; Babiker, E.E. Nutritional evaluation of sorghum flour (Sorghum bicolor L. Moench) during processing of injera. World Acad. Sci. Eng. Technol. 2011, 51, 99–103. [Google Scholar]
- Aruna, C.; Visarada, K.; Bhat, B.V.; Tonapi, V.A. Breeding Sorghum for Diverse end Uses; Woodhead Publishing: Cambridge, MA, USA, 2018. [Google Scholar]
- Wang, L.; Weller, C.L.; Schlegel, V.L.; Carr, T.P.; Cuppett, S.L. Supercritical CO2 extraction of lipids from grain sorghum dried distillers grains with solubles. Bioresour. Technol. 2008, 99, 1373–1382. [Google Scholar] [CrossRef]
- Van Amerongen, A. Sorghum Surges. Available online: http://ethanolproducer.com/articles/6338/sorghum-surges (accessed on 13 May 2021).
- Cui, X.; Kavvada, O.; Huntington, T.; Scown, C.D. Strategies for near-term scale-up of cellulosic biofuel production using sorghum and crop residues in the US. Environ. Res. Lett. 2018, 13, 124002. [Google Scholar] [CrossRef]
- Baral, N.R.; Dahlberg, J.; Putnam, D.; Mortimer, J.C.; Scown, C.D. Supply cost and life-cycle greenhouse gas footprint of dry and ensiled biomass sorghum for biofuel production. ACS Sustain. Chem. Eng. 2020, 8, 15855–15864. [Google Scholar] [CrossRef]
- Hepher, B. Nutrition of pond fishes; Cambridge University Press: Cambridge, UK, 1988. [Google Scholar]
- Al-Ogaily, S.; Al-Asgah, N.; Ali, A. Effect of feeding different grain sources on the growth performance and body composition of tilapia, Oreochromis niloticus (L.). Aquac. Res. 1996, 27, 523–529. [Google Scholar] [CrossRef]
- Alavi, S.; Mazumdar, S.D.; Taylor, J.R. Modern convenient sorghum and millet food, beverage and animal feed products, and their technologies. In Sorghum and Millets; Elsevier: Amsterdam, The Netherlands, 2019; pp. 293–329. [Google Scholar]
- Froehlich, H.E.; Runge, C.A.; Gentry, R.R.; Gaines, S.D.; Halpern, B.S. Comparative terrestrial feed and land use of an aquaculture-dominant world. Proc. Natl. Acad. Sci. USA 2018, 115, 5295–5300. [Google Scholar] [CrossRef]
- Kraugerud, O.F.; Jørgensen, H.Y.; Svihus, B. Physical properties of extruded fish feed with inclusion of different plant (legumes, oilseeds, or cereals) meals. Anim. Feed Sci. Technol. 2011, 163, 244–254. [Google Scholar] [CrossRef]
- Lemlioglu-Austin, D. Sorghum: Obliging alternative and ancient grain. Cereal Foods World 2014, 59, 12–20. [Google Scholar] [CrossRef]
- Damodaran, S. Amino acids, peptides and proteins. Fennema’s Food Chem. 2008, 4, 425–439. [Google Scholar]
- Thain, M.; Hickman, M. Penguin Dictionary of Biology; Penguin Books: New York, NY, USA, 2004. [Google Scholar]
- Hahn, D.; Rooney, L. Effect of genotype on tannins and phenols of sorghum. Cereal Chem 1986, 63, 4–8. [Google Scholar]
- Price, M.L.; Van Scoyoc, S.; Butler, L.G. A critical evaluation of the vanillin reaction as an assay for tannin in sorghum grain. J. Agric. Food Chem. 1978, 26, 1214–1218. [Google Scholar] [CrossRef]
- Khoddami, A.; Messina, V.; Vadabalija Venkata, K.; Farahnaky, A.; Blanchard, C.L.; Roberts, T.H. Sorghum in foods: Functionality and potential in innovative products. Crit. Rev. Food Sci. Nutr. 2021, 1–17. [Google Scholar] [CrossRef]
- Tuinstra, M.R. Food-grade sorghum varieties and production considerations: A review. J. Plant Interact. 2008, 3, 69–72. [Google Scholar] [CrossRef]
- Stefoska-Needham, A.; Beck, E.J.; Johnson, S.K.; Tapsell, L.C. Sorghum: An underutilized cereal whole grain with the potential to assist in the prevention of chronic disease. Food Rev. Int. 2015, 31, 401–437. [Google Scholar] [CrossRef]
- Xiong, Y.; Zhang, P.; Warner, R.D.; Fang, Z. Sorghum grain: From genotype, nutrition, and phenolic profile to its health benefits and food applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 2025–2046. [Google Scholar] [CrossRef]
- Wall, J.; Blessin, C. Composition and structure of sorghum grains. Cereal Sci. Today 1969, 14, 264–268. [Google Scholar]
- Taylor, J.; Duodu, K.G. Sorghum and Millets: Chemistry, Technology, and Nutritional Attributes; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Gassem, M.A.; Osman, M.A. Proximate composition and the content of sugars, amino acids and anti-nutritional factors of three sorghum varieties. Agric. Res. Cent. King Saud Univ. Res. Bull. 2003, 125, 5–19. [Google Scholar]
- Pontieri, P.; Troisi, J.; Calcagnile, M.; Bean, S.R.; Tilley, M.; Aramouni, F.; Boffa, A.; Pepe, G.; Campiglia, P.; Del Giudice, F. Chemical composition, fatty acid and mineral content of food-grade white, red and black sorghum varieties grown in the mediterranean environment. Foods 2022, 11, 436. [Google Scholar] [CrossRef]
- Udachan, I.S.; Sahoo, A.; Hend, G. Extraction and characterization of sorghum (Sorghum bicolor L. Moench) starch. Int. Food Res. J. 2012, 19, 315–319. [Google Scholar]
- Douglas, J.; Sullivan, T.; Bond, P.; Struwe, F. Nutrient composition and metabolizable energy values of selected grain sorghum varieties and yellow corn. Poult. Sci. 1990, 69, 1147–1155. [Google Scholar] [CrossRef]
- Miller, D. Minerals. In Food Chemistry; Fennema Marcel Dekker: New York, NY, USA, 1996; pp. 617–649. [Google Scholar]
- Wong, J.H.; Marx, D.B.; Wilson, J.D.; Buchanan, B.B.; Lemaux, P.G.; Pedersen, J.F. Principal component analysis and biochemical characterization of protein and starch reveal primary targets for improving sorghum grain. Plant Sci. 2010, 179, 598–611. [Google Scholar] [CrossRef]
- Murty, D.; Renard, C. Sorghum. Crops in Tropical Africa; Raemaekers, R.H., Ed.; Directorate General for International Cooperation. Ministry of Foreign Affairs, External Trade and International Cooperation: Brussels, Belgium, 2001; pp. 68–96. [Google Scholar]
- Virupaksha, T.; Sastry, L. Protein content and amino acid composition of some varieties of grain sorghum. J. Agric. Food Chem. 1968, 16, 199–203. [Google Scholar] [CrossRef]
- Waggle, D.; Deyoe, C. Relationship between protein level and amino acid composition of sorghum grain. Feedstuffs 1966, 38, 18. [Google Scholar]
- Mossé, J.; Huet, J.; Baudet, J. The amino acid composition of wheat grain as a function of nitrogen content. J. Cereal Sci. 1985, 3, 115–130. [Google Scholar] [CrossRef]
- Portz, L.; Cyrino, J.E.P. Digestibility of nutrients and amino acids of different protein sources in practical diets by largemouth bass Micropterus salmoides (Lacepéde, 1802). Aquac. Res. 2004, 35, 312–320. [Google Scholar] [CrossRef]
- Breuer, L.H., Jr.; Dohm, C.K. Comparative nutritive value of several sorghum grain varieties and hybrids. J. Agric. Food Chem. 1972, 20, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Sikka, K.C.; Johari, R.P. Comparative nutritive value and amino acid content of different varieties of sorghum and effect of lysine fortification. J. Agric. Food Chem. 1979, 27, 962–965. [Google Scholar] [CrossRef]
- Duodu, K.; Nunes, A.; Delgadillo, I.; Parker, M.; Mills, E.; Belton, P.; Taylor, J. Effect of grain structure and cooking on sorghum and maize in vitro protein digestibility. J. Cereal Sci. 2002, 35, 161–174. [Google Scholar] [CrossRef]
- Hamaker, B.R.; Kirleis, A.W.; Mertz, E.T.; Axtell, J.D. Effect of cooking on the protein profiles and in vitro digestibility of sorghum and maize. J. Agric. Food Chem. 1986, 34, 647–649. [Google Scholar] [CrossRef]
- De Cindio, B.; Gabriele, D.; Pollini, C.M.; Peressini, D.; Sensidoni, A. Filled snack production by co-extrusion-cooking: 2. Effect of processing on cereal mixtures. J. Food Eng. 2002, 54, 63–73. [Google Scholar] [CrossRef]
- Glennie, C. Endosperm cell wall modification in sorghum grain during germination. Cereal Chem. 1984, 61, 285–289. [Google Scholar]
- Knudsen, K.B.; Munck, L. Dietary fibre contents and compositions of sorghum and sorghum-based foods. J. Cereal Sci. 1985, 3, 153–164. [Google Scholar] [CrossRef]
- Gram, N.H. The ultrastructure of germinating barley seeds. II. Breakdown of starch granules and cell walls of the endosperm in three barley varieties. Carlsberg Res. Commun. 1982, 47, 173. [Google Scholar] [CrossRef]
- Shull, J.M.; Watterson, J.J.; Kirleis, A. Purification and immunocytochemical localization of kafirins in Sorghum bicolor (L. Moench) endosperm. Protoplasma 1992, 171, 64–74. [Google Scholar] [CrossRef]
- Shewry, P.R.; Tatham, A.S. The prolamin storage proteins of cereal seeds: Structure and evolution. Biochem. J. 1990, 267, 1–12. [Google Scholar] [CrossRef]
- Belton, P.; Delgadillo, I.; Halford, N.; Shewry, P. Kafirin structure and functionality. J. Cereal Sci. 2006, 44, 272–286. [Google Scholar] [CrossRef]
- Watterson, J.; Shull, J.; Kirleis, A. and Opaque Endosperm of Sorghum bicolor. Cereal Chem 1970, 70, 452–457. [Google Scholar]
- Dowling, L.; Arndt, C.; Hamaker, B. Economic viability of high digestibility sorghum as feed for market broilers. Agron. J. 2002, 94, 1050–1058. [Google Scholar] [CrossRef]
- Laidlaw, H.; Mace, E.; Williams, S.; Sakrewski, K.; Mudge, A.; Prentis, P.; Jordan, D.; Godwin, I. Allelic variation of the β-, γ-and δ-kafirin genes in diverse Sorghum genotypes. Theor. Appl. Genet. 2010, 121, 1227–1237. [Google Scholar] [CrossRef]
- Hamaker, B.; Kirleis, A.; Butler, L.; Axtell, J.; Mertz, E. Improving the in vitro protein digestibility of sorghum with reducing agents. Proc. Natl. Acad. Sci. 1987, 84, 626–628. [Google Scholar] [CrossRef]
- Da Silva, L.S.; Taylor, J.; Taylor, J.R. Transgenic sorghum with altered kafirin synthesis: Kafirin solubility, polymerization, and protein digestion. J. Agric. Food Chem. 2011, 59, 9265–9270. [Google Scholar] [CrossRef]
- Rooney, L. Processing methods to improve nutritional value of sorghum for livestock. In Proceedings of the Sorghum Nutritional Quality Conference, Purdue University, West Lafayette, ID, USA, 26 February–1 March 1990; pp. 206–210. [Google Scholar]
- Baldwin, A.; Sniegowski, M. Fatty acid compositions of lipids from corn and grain sorghum kernels. J. Am. Oil Chem. Soc. 1951, 28, 24–27. [Google Scholar] [CrossRef]
- Osman, R.; Abd El-Gelil, F.; El-Noamany, H.; Dawood, M.G. Oil content and fatty acid composition of some varieties of barley and sorghum grains. Grasas Y Aceites 2000, 51, 157–162. [Google Scholar] [CrossRef]
- Kamath, V.; Niketh, S.; Chandrashekar, A.; Rajini, P. Chymotryptic hydrolysates of α-kafirin, the storage protein of sorghum (Sorghum bicolor) exhibited angiotensin converting enzyme inhibitory activity. Food Chem. 2007, 100, 306–311. [Google Scholar] [CrossRef]
- Kulamarva, A.G.; Sosle, V.R.; Raghavan, G.V. Nutritional and rheological properties of sorghum. Int. J. Food Prop. 2009, 12, 55–69. [Google Scholar] [CrossRef]
- Jambunathan, R.; Subramanian, V. Grain quality and utilization of sorghum and pearl millet. In Proceedings of the International Biotechnology Workshop, ICRISAT Center, Hyderabad, India, 12–15 January 1987; pp. 133–139. [Google Scholar]
- Lodge, S.; Stock, R.; Klopfenstein, T.; Shain, D.; Herold, D. Evaluation of corn and sorghum distillers byproducts. J. Anim. Sci. 1997, 75, 37–43. [Google Scholar] [CrossRef]
- McNeill, J.; Potter, G.; Riggs, J.; Rooney, L. Chemical and physical properties of processed sorghum grain carbohydrates. J. Anim. Sci. 1975, 40, 335–341. [Google Scholar] [CrossRef]
- Hibberd, C.; Wagner, D.; Schemm, R.; Mitchell, E., Jr.; Weibel, D.; Hintz, R. Digestibility characteristics of isolated starch from sorghum and corn grain. J. Anim. Sci. 1982, 55, 1490–1497. [Google Scholar] [CrossRef]
- Dreher, M.L.; Dreher, C.J.; Berry, J.W.; Fleming, S.E. Starch digestibility of foods: A nutritional perspective. Crit. Rev. Food Sci. Nutr. 1984, 20, 47–71. [Google Scholar] [CrossRef]
- Davis, A.; Hoseney, R. Grain sorghum condensed tannins. I. Isolation, estimation, and selective adsorption by starch. Cereal Chem 1979, 56, 310–314. [Google Scholar]
- Atteh, J. Principles and Practice of Livestock Feed Manufacturing; ADLEK Printers: Ilorin, Nigeria, 2002; pp. 52–58. [Google Scholar]
- Doggett, S.W.; Green, J.P.; Cantril, S.T. Efficacy of radiation therapy alone for limited squamous cell carcinoma of the anal canal. Int. J. Radiat. Oncol. Biol. Phys. 1988, 15, 1069–1072. [Google Scholar] [CrossRef]
- Purseglove, J.W. Tropical Crops. Dicotyledons 1 and 2; Cambridge University Press: Cambridge, UK, 1968. [Google Scholar]
- Kumar, R. Anti-nutritional factors, the potential risks of toxicity and methods to alleviate them. In Legume Trees and Other Fodder Trees as Protein Sources for Livestock; FAO Corporate Document Repository: Kuala Lumpur, Malaysia, 1992; Volume 102, pp. 145–160. [Google Scholar]
- Myer, R.; Gorbet, D. Waxy and normal grain sorghums with varying tannin contents in diets for young pigs. Anim. Feed. Sci. Technol. 1985, 12, 179–186. [Google Scholar] [CrossRef]
- Nelson, T.S.; Stephenson, E.L.; Burgos, A.; Floyd, J.; York, J.O. Effect of tannin content and dry matter digestion on energy utilization and average amino acid availability of hybrid sorghum grains. Poult. Sci. 1975, 54, 1620–1623. [Google Scholar] [CrossRef]
- Dykes, L.; Rooney, L.W. Sorghum and millet phenols and antioxidants. J. Cereal Sci. 2006, 44, 236–251. [Google Scholar] [CrossRef]
- Dykes, L. Sorghum phytochemicals and their potential impact on human health. Sorghum 2019, 1931, 121–140. [Google Scholar]
- Treviño, J.; Ortiz, L.; Centeno, C. Effect of tannins from faba beans (Vicia faba) on the digestion of starch by growing chicks. Anim. Feed Sci. Technol. 1992, 37, 345–349. [Google Scholar] [CrossRef]
- Elkin, R.G.; Freed, M.B.; Hamaker, B.R.; Zhang, Y.; Parsons, C.M. Condensed tannins are only partially responsible for variations in nutrient digestibilities of sorghum grain cultivars. J. Agric. Food Chem. 1996, 44, 848–853. [Google Scholar] [CrossRef]
- Etuk, E.; Ifeduba, A.; Okata, U.; Chiaka, I.; Okoli, I.C.; Okeudo, N.; Esonu, B.; Udedibie, A.; Moreki, J. Nutrient composition and feeding value of sorghum for livestock and poultry: A review. J. Anim. Sci. Adv. 2012, 2, 510–524. [Google Scholar]
- Hancock, J.; Bramel-Cox, P. Use of sorghum grain for feeding livestock and poultry. In Expected Impacts of Sorghum Farm Program Target Price Policies and Other Factors Affecting Demand: Completion Report for the Grain Sorghum Federation; Texas Agricultural Experiment Station: College Station, TX, USA, 1992. [Google Scholar]
- Butler, L.G. Sorghum polyphenols. Toxic. Plant Orig. 1989, 4, 95–121. [Google Scholar]
- Awika, J.M.; Rooney, L.W. Sorghum phytochemicals and their potential impact on human health. Phytochemistry 2004, 65, 1199–1221. [Google Scholar] [CrossRef]
- Iren, L. Sorghum and millets, in cultivated plants, primarily as food sources. Encycl. Life Support Syst. 2004, 1–7. [Google Scholar]
- Mabelebele, M.; Gous, R.; O’Neil, H.M.; Iji, P. Whole sorghum inclusion and feed form on performance and nutrient digestibility of broiler chickens. J. Appl. Anim. Nutr. 2018, 6. [Google Scholar] [CrossRef]
- Carter, P. Spectrophotometric determination of serum iron at the submicrogram level with a new reagent (ferrozine). Anal. Biochem. 1971, 40, 450–458. [Google Scholar] [CrossRef]
- D’mello, J. Antinutritional factors and mycotoxins. In Farm Animal Metabolism and Nutrition; CABI Publishing: Wallingford, UK, 2000; pp. 383–403. [Google Scholar]
- Degani, G. Digestible energy in dietary sorghum, wheat bran, and rye in the common carp (Cyprinus carpio L.). Isr. J. Aquac. 2006, 58, 71–77. [Google Scholar] [CrossRef]
- Rawles, S.D.; Gatlin III, D.M. Nutrient digestibility of common feedstuffs in extruded diets for sunshine bass Morone chrysops♀×M. saxatilis♂. J. World Aquac. Soc. 2000, 31, 570–579. [Google Scholar] [CrossRef]
- González-Félix, M.L.; Davis, D.A.; Rossi, W., Jr.; Perez-Velazquez, M. Evaluation of apparent digestibility coefficient of energy of various vegetable feed ingredients in Florida pompano, Trachinotus carolinus. Aquaculture 2010, 310, 240–243. [Google Scholar] [CrossRef]
- Yones, A.; Metwalli, A.A. Dietary sorghum starch influences growth performance, apparent digestibility coefficient and some hepatic enzyme activities of carbohydrate metabolism in Hypridered tilapia (Oreochromis mossambicus × O. niloticus) fingerlings. Int. J. Fish. Aquac. Res. 2016, 1, 1–16. [Google Scholar]
- Bruce, T.; Harris, H.W. Expanding Utilization and Increasing Value of Sorghum Fractions via Microbial Conversion; United Sorghum Checkoff Program (USCP): Lubbock, TX, USA, 2017; pp. 1–36. [Google Scholar]
- Balogun, A.; Fagbenro, O. Use of macadamia presscake as a protein feedstuff in practical diets for tilapia, Oreochromis niloticus (L.). Aquac. Res. 1995, 26, 371–377. [Google Scholar] [CrossRef]
- US Grains Council. Sorghum as Source of Ingredient to Replace Cassava for Feeding Pangasius in Vietnam; US Grains Council: Washington, DC, USA, 2017. [Google Scholar]
- Signor, A.; Lewandowski, V.; Silva, R.A.d.; Fries, E.M.; Schuller, J.M. Effect of phytase on digestibility of corn, sorghum and wheat bran by silver catfish (Rhamdia voulezi). Acta Scientiarum. Anim. Sci. 2016, 38, 355–359. [Google Scholar] [CrossRef]
- Lins Rodrigues, M.; Souza dos Santos Sanchez, M.; Ernzen Pessini, J.; Weiler, K.A.; Deparis, A.; Boscolo, W.R.; Bittencourt, F.; Signor, A. Replacement of corn by sorghum and phytase supplementation in silver catfish (Rhamdia quelen) diets: Growth performance, physiological variables and bone mineralization. J. Appl. Anim. Res. 2020, 48, 142–150. [Google Scholar] [CrossRef]
- Zahler, J.D. Improving the Nutritional Characteristics of Plant Feedstuff By-Products Using Fungal Metabolism; South Dakota State University: Brookings, SD, USA, 2018. [Google Scholar]
- Hussein, E.E.S.; Dabrowski, K.; El-Saidy, D.M.; Lee, B.J. Enhancing the growth of Nile tilapia larvae/juveniles by replacing plant (gluten) protein with algae protein. Aquac. Res. 2013, 44, 937–949. [Google Scholar] [CrossRef]
- Eissa, A.E.; Attia, M.M.; Elgendy, M.Y.; Ismail, G.A.; Sabry, N.M.; Prince, A.; Mahmoud, M.A.; El-Demerdash, G.O.; Abdelsalam, M.; Derwa, H.I. Streptococcus, Centrocestus formosanus and Myxobolus tilapiae concurrent infections in farmed Nile tilapia (Oreochromis niloticus). Microb. Pathog. 2021, 158, 105084. [Google Scholar] [CrossRef]
- Cavalcante, R.B.; Telli, G.S.; Tachibana, L.; de Carla Dias, D.; Oshiro, E.; Natori, M.M.; da Silva, W.F.; Ranzani-Paiva, M.J. Probiotics, Prebiotics and Synbiotics for Nile tilapia: Growth performance and protection against Aeromonas hydrophila infection. Aquac. Rep. 2020, 17, 100343. [Google Scholar] [CrossRef]
- Urriola, P.; Hoehler, D.; Pedersen, C.; Stein, H.; Shurson, G. Amino acid digestibility of distillers dried grains with solubles, produced from sorghum, a sorghum-corn blend, and corn fed to growing pigs. J. Anim. Sci. 2009, 87, 2574–2580. [Google Scholar] [CrossRef]
- Barekatain, M.; Antipatis, C.; Choct, M.; Iji, P. Interaction between protease and xylanase in broiler chicken diets containing sorghum distillers’ dried grains with solubles. Anim. Feed Sci. Technol. 2013, 182, 71–81. [Google Scholar] [CrossRef]
- Adedeji, O.E.; Jegede, D.E.; Abdulsalam, K.O.; Umeohia, U.E.; Ajayi, O.A.; Iboyi, J.E. Effect of processing treatments on the proximate, functional and sensory properties of soy-sorghum-roselle complementary food. Br. J. Appl. Sci. Technol. 2015, 6, 635. [Google Scholar] [CrossRef]
- Li, M.; Robinson, E.; Oberle, D.; Lucas, P. Effects of various corn distillers by-products on growth, feed efficiency, and body composition of channel catfish, Ictalurus punctatus. Aquac. Nutr. 2010, 16, 188–193. [Google Scholar] [CrossRef]
- Cheng, Z.J.; Hardy, R.W. Effects of microbial phytase supplementation in corn distiller’s dried grain with solubles on nutrient digestibility and growth performance of rainbow trout, Oncorhynchus mykiss. J. Appl. Aquac. 2004, 15, 83–100. [Google Scholar] [CrossRef]
- Webster, C. Evaluation of distillers’ grains with solubles in prepared channel catfish diets. Trans. Ky. Acad. Sci. 1991, 51, 135–138. [Google Scholar]
- Lee, S.M.; Pan, B.S. Effect of dietary sorghum distillery residue on hematological characteristics of cultured grey mullet (Mugil cephalus)—An animal model for prescreening antioxidant and blood thinning activities. J. Food Biochem. 2003, 27, 1–18. [Google Scholar] [CrossRef]
- Furuya, W.M.; Furlan, A.C.; Rossetto, V.; Furuya, B. Sorghum a potential energy source in tilapia feed. Glob. Aquac. Advocate 2003, 1–4. [Google Scholar]
- Rodrigues, S.R.; Dalmoro, V.; dos Santos, J.H. An evaluation of Acacia mearnsii tannin as an aluminum corrosion inhibitor in acid, alkaline, and neutral media. Mater. Corros. 2020, 71, 1160–1174. [Google Scholar] [CrossRef]
- Osman, M.A. Changes in sorghum enzyme inhibitors, phytic acid, tannins and in vitro protein digestibility occurring during Khamir (local bread) fermentation. Food Chem. 2004, 88, 129–134. [Google Scholar] [CrossRef]
- Dicko, M.H.; Gruppen, H.; Traoré, A.S.; van Berkel, W.J.; Voragen, A.G. Evaluation of the effect of germination on phenolic compounds and antioxidant activities in sorghum varieties. J. Agric. Food Chem. 2005, 53, 2581–2588. [Google Scholar] [CrossRef] [PubMed]
- Dirar, H. The Indigenous Fermented Foods and Beverages of Sudan: A Study in African Food and Nutrition; Cambridge University Press: Cambridge, UK, 1991; pp. 23–40. [Google Scholar]
- Elmaki, H.B.; Babiker, E.; El Tinay, A.H. Changes in chemical composition, grain malting, starch and tannin contents and protein digestibility during germination of sorghum cultivars. Food Chem. 1999, 64, 331–336. [Google Scholar] [CrossRef]
- Awika, J.M.; Dykes, L.; Gu, L.; Rooney, L.W.; Prior, R.L. Processing of sorghum (Sorghum bicolor) and sorghum products alters procyanidin oligomer and polymer distribution and content. J. Agric. Food Chem. 2003, 51, 5516–5521. [Google Scholar] [CrossRef] [PubMed]
- Rom, D.; Shull, J.; Chandrashekar, A.; Kirleis, A. on In Vitro Protein Digestibility and Microstructure of Sorghum Flour. Cereal Chem 1992, 69, 178–181. [Google Scholar]
- Oria, M.; Hamaker, B.; Schull, J. In vitro protein digestibility of developing and mature sorghum grain in relation to α-, β-, and γ-kafirin disulfide crosslinking. J. Cereal Sci. 1995, 22, 85–93. [Google Scholar] [CrossRef]
- Maclean, W.C., Jr.; RomaÑa, G.L.d.; Placko, R.P.; Graham, G.G. Protein quality and digestibility of sorghum in preschool children: Balance studies and plasma free amino acids. J. Nutr. 1981, 111, 1928–1936. [Google Scholar] [CrossRef]
- Mudge, S.R.; Campbell, B.C.; Mustapha, N.B.; Godwin, I.D. Genomic approaches for improving grain quality of sorghum. In The Sorghum Genome; Springer: Berlin/Heidelberg, Germany, 2016; pp. 189–205. [Google Scholar]
- Mehlo, L.; Mbambo, Z.; Bado, S.; Lin, J.; Moagi, S.M.; Buthelezi, S.; Stoychev, S.; Chikwamba, R. Induced protein polymorphisms and nutritional quality of gamma irradiation mutants of sorghum. Mutat. Res. 2013, 749, 66–72. [Google Scholar] [CrossRef]
- Yousif, N.E.; El Tinay, A.H. Effect of fermentation on sorghum protein fractions and in vitro protein digestibility. Plant Foods Hum. Nutr. 2001, 56, 175–182. [Google Scholar] [CrossRef]
- Taylor, J.; Belton, P.S. Sorghum. In Pseudocereals and Less Common Cereals; Springer: Berlin/Heidelberg, Germany, 2002; pp. 25–91. [Google Scholar]
- Elkhalifa, A.E.O.; Bernhardt, R.; Bonomi, F.; Iametti, S.; Pagani, M.A.; Zardi, M. Fermentation modifies protein/protein and protein/starch interactions in sorghum dough. Eur. Food Res. Technol. 2006, 222, 559–564. [Google Scholar] [CrossRef]
- Babiker, E.; Kato, A. Improvement of the functional properties of sorghum protein by protein-polysaccharide and protein-protein complexes. Food Nahr. 1998, 42, 286–289. [Google Scholar] [CrossRef]
- Zhang, G.; Hamaker, B.R. Low α-amylase starch digestibility of cooked sorghum flours and the effect of protein. Cereal Chem. 1998, 75, 710–713. [Google Scholar] [CrossRef]
- Yang, P.; Seib, P.A. Low-input wet-milling of grain sorghum for readily accessible starch and animal feed. Cereal Chem. 1995, 72, 498–503. [Google Scholar]
- Xu, X. In vitro digestibility of starch in sorghum differing in endosperm hardness and flour particle size. Ph.D. Thesis, Kansas State University, Manhattan, KS, USA, 2008. [Google Scholar]
- Rooney, L.; Pflugfelder, R. Factors affecting starch digestibility with special emphasis on sorghum and corn. J. Anim. Sci. 1986, 63, 1607–1623. [Google Scholar] [CrossRef]
- Fapojuwo, O.; Maga, J.; Jansen, G. Effect of extrusion cooking on in vitro protein digestibility of sorghum. J. Food Sci. 1987, 52, 218–219. [Google Scholar] [CrossRef]
- Hamaker, B.; Mertz, E.; Axtell, J. Effect of extrusion on sorghum kafirin solubility. Cereal Chem. 1994, 71, 515. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).