The Genetic Assessment of South African Nguni Sheep Breeds Using the Ovine 50K Chip
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampled Populations and Sample Collection
2.2. Laboratory Techniques
2.3. Statistical Analysis
2.3.1. Quality Control
2.3.2. Estimates of Within-Breed Genetic Diversity
2.3.3. Genetic Differentiation
2.4. Principal Component Analysis and Population Structure Analysis
2.5. Construction of Phylogenetic Trees and Ancestry Graphs
3. Results
3.1. SNP Polymorphism and Within-Breed Genetic Diversity
3.2. Analysis of Molecular Variances and Fst
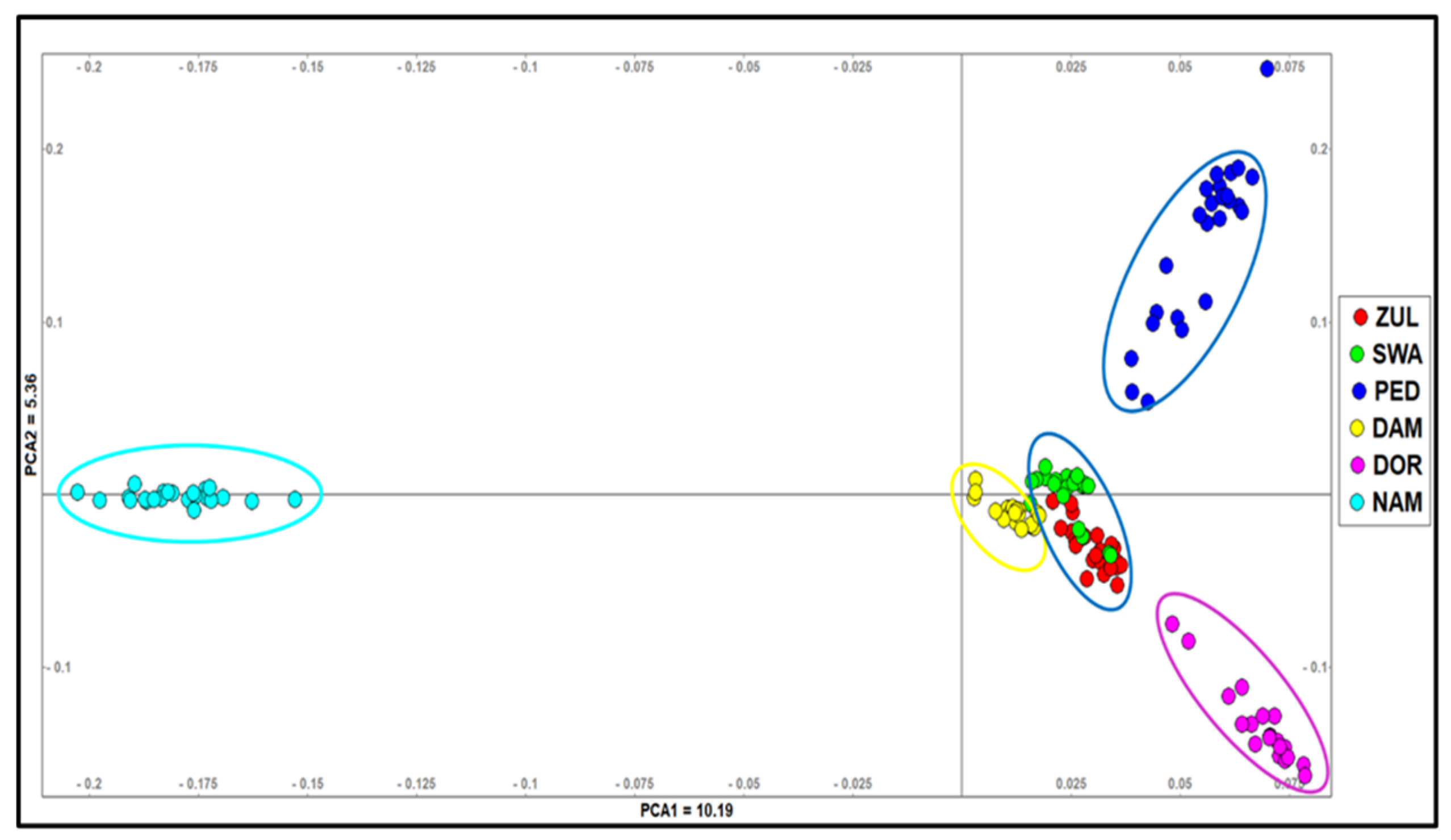
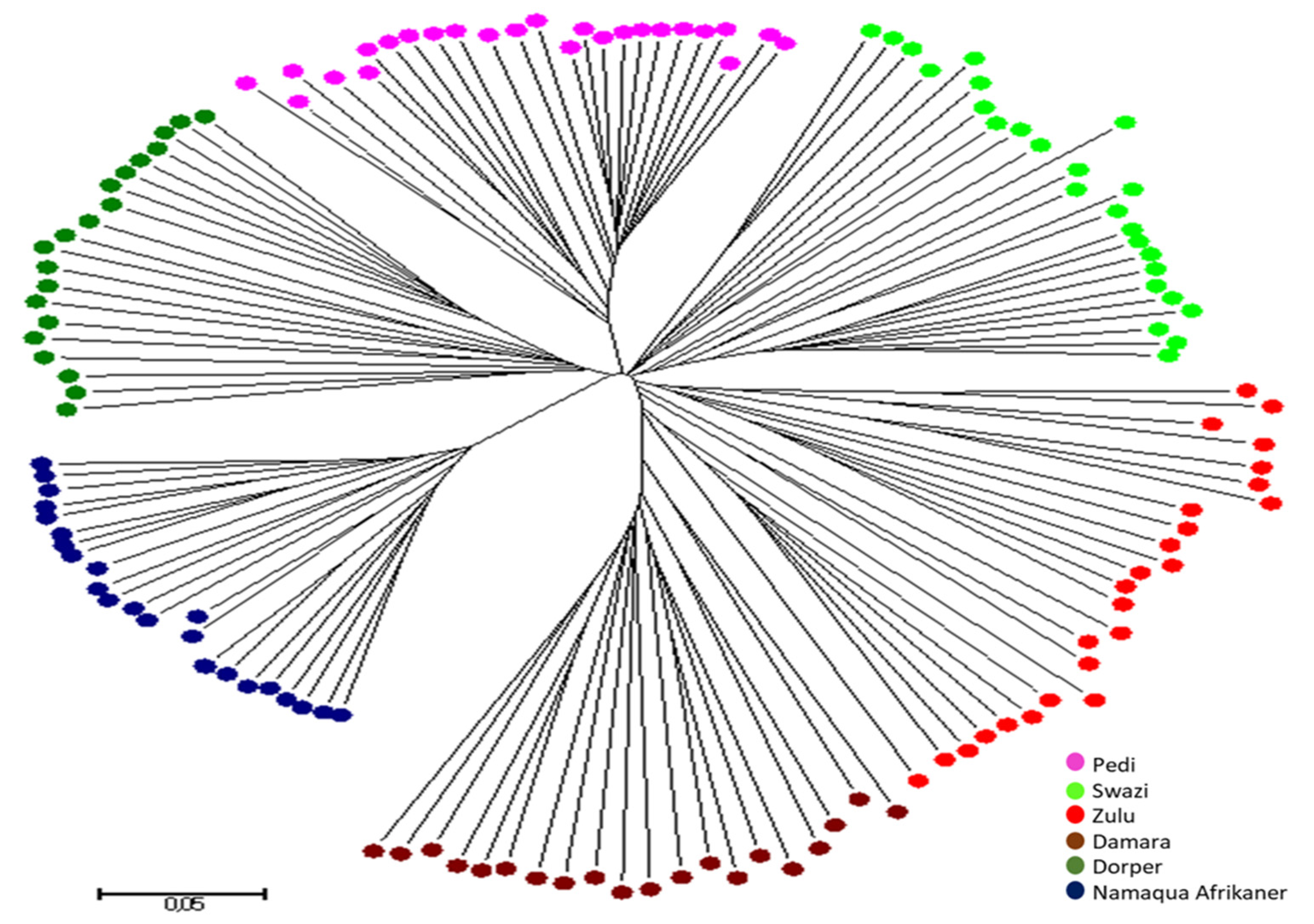
3.3. Population Structure and Relationships
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selepe, M.M.; Ceccobelli, S.; Lasagna, E.; Kunene, N.W. Genetic structure of South African Nguni (Zulu) sheep populations reveals admixture with exotic breeds. PLoS ONE 2018, 13, e0196276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gwala, P.E.; Kunene, N.W.; Bezuidenhout, C.C.; Mavule, B.S. Genetic and phenotypic variation among four Nguni sheep breeds using random amplified polymorphic DNA (RAPD) and morphological features. Trop. Anim. Health Prod. 2015, 47, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Cumbula, D.; Taela, M. Animal genetic resources (AnGR) in Mozambique. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020. [Google Scholar]
- Peters, F.; Kotze, A.; van der Bank, F.; Soma, P.; Grobler, J. Genetic profile of the locally developed Meatmaster sheep breed in South Africa based on microsatellite analysis. Small Rumin. Res. 2010, 90, 101–108. [Google Scholar] [CrossRef]
- Ramey, H.R.; Decker, J.E.; McKay, S.D.; Rolf, M.M.; Schnabel, R.D.; Taylor, J.F. Detection of selective sweeps in cattle using genome-wide SNP data. BMC Genom. 2013, 14, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kunene, N.; Bezuidenhout, C.; Nsahlai, I. Genetic and phenotypic diversity in Zulu sheep populations: Implications for exploitation and conservation. Small Rumin. Res. 2009, 84, 100–107. [Google Scholar] [CrossRef]
- Mwai, O.; Hanotte, O.; Kwon, Y.-J.; Cho, S. African Indigenous Cattle: Unique Genetic Resources in a Rapidly Changing World. Asian-Australas. J. Anim. Sci. 2015, 28, 911–921. [Google Scholar] [CrossRef] [Green Version]
- Qwabe, S.O.; van Marle-Köster, E.; Visser, C. Genetic diversity and population structure of the endangered Namaqua Afrikaner sheep. Trop. Anim. Health Prod. 2012, 45, 511–516. [Google Scholar] [CrossRef] [Green Version]
- Kunene, N.W.; Ceccobelli, S.; Di Lorenzo, P.; Hlophe, S.R.; Bezuidenhout, C.C.; Lasagna, E. Genetic Diversity in Four Populations of Nguni (Zulu) Sheep Assessed by Microsatellite Analysis. Ital. J. Anim. Sci. 2014, 13, 3083. [Google Scholar] [CrossRef]
- Steyn, G.J.; Tapson, D.R. Farming systems research and extension (FSR/E) approach to livestock development in parts of sub-Saharan Africa. Dev. S. Afr. 1993, 10, 411–419. [Google Scholar] [CrossRef]
- Senda, T.S.; Robinson, L.W.; Gachene, C.K.; Kironchi, G. Formalization of communal land tenure and expectations for pastoralist livelihoods. Land Use Policy 2021, 114, 105961. [Google Scholar] [CrossRef]
- Legesse, G.; Abebe, G.; Siegmund-Schultze, M.; Zárate, A.V. Small Ruminant Production in Two Mixed-Farming Systems of Southern Ethiopia: Status and Prospects for Improvement. Exp. Agric. 2008, 44, 399–412. [Google Scholar] [CrossRef]
- Nyamushamba, G.B.; Mapiye, C.; Tada, O.; Halimani, T.E.; Muchenje, V. Conservation of indigenous cattle genetic resources in Southern Africa’s smallholder areas: Turning threats into opportunities—A review. Asian-Australas. J. Anim. Sci. 2016, 30, 603–621. [Google Scholar] [CrossRef] [PubMed]
- Mavule, B.S.; Sarti, F.M.; Lasagna, E.; Kunene, N.W. Morphological differentiation amongst Zulu sheep populations in KwaZulu-Natal, South Africa, as revealed by multivariate analysis. Small Rumin. Res. 2016, 140, 50–56. [Google Scholar] [CrossRef] [Green Version]
- Sheriff, O.; Alemayehu, K. Genetic diversity studies using microsatellite markers and their contribution in supporting sustainable sheep breeding programs: A review. Cogent Food Agric. 2018, 4, 1459062. [Google Scholar] [CrossRef]
- Gama, L.; Bressan, M. Biotechnology applications for the sustainable management of goat genetic resources. Small Rumin. Res. 2011, 98, 133–146. [Google Scholar] [CrossRef]
- Shirasawa, K.; Ishii, K.; Kim, C.; Ban, T.; Suzuki, M.; Ito, T.; Muranaka, T.; Kobayashi, M.; Nagata, N.; Isobe, S.; et al. Development of Capsicum EST–SSR markers for species identification and in silico mapping onto the tomato genome sequence. Mol. Breed. 2012, 31, 101–110. [Google Scholar] [CrossRef] [Green Version]
- Edea, Z.; Dadi, H.; Kim, S.-W.; Dessie, T.; Lee, T.; Kim, H.; Kim, J.-J.; Kim, K.-S. Genetic diversity, population structure and relationships in indigenous cattle populations of Ethiopia and Korean Hanwoo breeds using SNP markers. Front. Genet. 2013, 4, 35. [Google Scholar] [CrossRef] [Green Version]
- Decker, J.E.; McKay, S.D.; Rolf, M.M.; Kim, J.; Alcalá, A.M.; Sonstegard, T.S.; Hanotte, O.; Götherström, A.; Seabury, C.M.; Praharani, L.; et al. Worldwide Patterns of Ancestry, Divergence, and Admixture in Domesticated Cattle. PLoS Genet. 2014, 10, e1004254. [Google Scholar] [CrossRef] [Green Version]
- Rezvannejad, E.; Nanaei, H.A.; Esmailizadeh, A. Detection of candidate genes affecting milk production traits in sheep using whole-genome sequencing analysis. Veter Med. Sci. 2022, 8, 1–8. [Google Scholar] [CrossRef]
- Illumina, I. Infinium® HD Assay: Ultra Protocol Guide; © Illumina, Inc.: San Diego, CA, USA, 2009. [Google Scholar]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A Tool Set for Whole-Genome Association and Population-Based Linkage Analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [Green Version]
- Manel, S.; Gaggiotti, O.; Waples, R.S. Assignment methods: Matching biological questions with appropriate techniques. Trends Ecol. Evol. 2005, 20, 136–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caballero, A.; Toro, M.A. Analysis of genetic diversity for the management of conserved subdivided populations. Conserv. Genet. 2002, 3, 289–299. [Google Scholar] [CrossRef]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evol. Bioinform. 2005, 1, 47–50. [Google Scholar] [CrossRef] [Green Version]
- Lischer, H.E.L.; Excoffier, L. PGDSpider: An automated data conversion tool for connecting population genetics and genomics programs. Bioinformatics 2011, 28, 298–299. [Google Scholar] [CrossRef] [Green Version]
- Shlens, J. A tutorial on principal component analysis. arXiv 2014, arXiv:1404.1100. [Google Scholar]
- Buchmann, R.; Hazelhurst, S. Genesis Manual [Internet]; University of Witwatersrand: Johannesburg, South Africa, 2014. [Google Scholar]
- Alexander, D.H.; Novembre, J.; Lange, K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009, 19, 1655–1664. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Pickrell, J.K.; Pritchard, J.K. User Manual for TreeMix v1. 2012. Available online: http://docplayer.net/31797577-User-manual-for-treemix-v1-0-joseph-k-pickrell-jonathan-k-pritchard.html (accessed on 12 March 2020).
- Abdelkader, A.A.; Ata, N.; Benyoucef, M.T.; Djaout, A.; Azzi, N.; Yilmaz, O.; Cemal, I.; Gaouar, S.B.S. New genetic identification and characterisation of 12 Algerian sheep breeds by microsatellite markers. Ital. J. Anim. Sci. 2017, 17, 38–48. [Google Scholar] [CrossRef]
- Liu, S.; He, S.; Chen, L.; Li, W.; Di, J.; Liu, M. Estimates of linkage disequilibrium and effective population sizes in Chinese Merino (Xinjiang type) sheep by genome-wide SNPs. Genes Genom. 2017, 39, 733–745. [Google Scholar] [CrossRef] [Green Version]
- Tosser-Klopp, G.; Bardou, P.; Bouchez, O.; Cabau, C.; Crooijmans, R.; Dong, Y.; Donnadieu-Tonon, C.; Eggen, A.; Heuven, H.C.M.; Jamli, S.; et al. Design and Characterization of a 52K SNP Chip for Goats. PLoS ONE 2014, 9, e86227. [Google Scholar] [CrossRef]
- Deniskova, T.E.; Okhlopkov, I.M.; Sermyagin, A.A.; Gladyr, E.; Bagirov, V.A.; Sölkner, J.; Mamaev, N.V.; Brem, G.; Zinov’Eva, N.A. Whole genome SNP scanning of snow sheep (Ovis nivicola). Dokl. Biochem. Biophys. 2016, 469, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Hlophe, S.R. Genetic variation between and within six selected South African sheep breeds using random amplified polymorphic DNA and protein markers. Unizulu Inst. Repos. 2011. Available online: http://hdl.handle.net/10530/611 (accessed on 18 September 2021).
- Hoffmann, I.; Scherf, B. Animal genetic resources-time to worry. Livest. Rep. 2006, 2006, 57–74. [Google Scholar]
- Buduram, P. Genetic Characterization of Southern African Sheep Breeds Using DNA Markers. Doctoral Dissertation, University of the Free State, Bloemfontein, South Africa, 2004. [Google Scholar]
- Oosthuizen, P.A. Distribution, Phenotype and Factors Influencing the Production Potential of Nguni Sheep. Doctoral Dissertation, University of KwaZulu-Natal, Durban, South Africa, 2018. [Google Scholar]
- Gaouar, S.B.S.; Kdidi, S.; Ouragh, L. Estimating population structure and genetic diversity of five Moroccan sheep breeds by microsatellite markers. Small Rumin. Res. 2016, 144, 23–27. [Google Scholar] [CrossRef]


| Polymorphic Loci (MAF > 0.05) | Ho (SD) | He (SD) | MAF (SD) | Fis | p-Value | |
|---|---|---|---|---|---|---|
| Pedi | 42,078 | 0.28 (0.22) | 0.25 (0.18) | 0.18 (0.16) | −0.104 | 0.988 |
| Swazi | 43,546 | 0.27 (0.16) | 0.38 (0.19) | 0.20 (0.16) | 0.003 | 0.460 |
| Zulu | 44,306 | 0.32 (1.77) | 0.32 (1.60) | 0.24 (0.15) | 0.028 | 0.237 |
| Damara | 44,709 | 0.42 (0.18) | 0.40 (0.14) | 0.35 (0.16) | 0.017 | 0.343 |
| Dorper | 45,939 | 0.32 (0.17) | 0.33 (0.16) | 0.25 (0.15) | 0.042 | 0.171 |
| Namaqua Afrikaner | 37,473 | 0.26 (0.21) | 0.25 (0.19) | 0.18 (0.16) | −0.017 | 0.591 |
| Merged | 48,429 | 0.32 (0.16) | 0.33 (0.17) | 0.27 (0.15) |
| Source of Variation | Sum of Squares | Variance Components | Percentage of Variation |
|---|---|---|---|
| Among | 208,055.192 | 489.84 | 5.62 |
| groups | |||
| Among | |||
| populations | 84,735.08 | 704.85 | 8.08 |
| within | |||
| groups | |||
| Within populations | 2,080,828.92 | 7528.56 | 86.30 |
| Total | 2,373,619 | 8723.26 |
| Source of Variation | d.f. | Sum Squares | Variance of Components | Percentage of Variation |
|---|---|---|---|---|
| Among populations | 2 | 48,837.13 | 284.64 | 2.72 |
| Within populations | 147 | 1,497,428 | 10,186.58 | 97.28 |
| Total | 149 | 1,546,265 | 10,471.22 |
| Population | Ped | Swa | Zul | Dam | Dor | Nam |
|---|---|---|---|---|---|---|
| Ped | *** | |||||
| Swa | 0.11 | *** | ||||
| Zul | 0.11 | 0.04 | *** | |||
| Dam | 0.13 | 0.07 | 0.06 | *** | ||
| Dor | 0.16 | 0.11 | 0.08 | 0.12 | *** | |
| Nam | 0.22 | 0.16 | 0.15 | 0.17 | 0.19 | *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nxumalo, K.S.; Grobler, P.; Ehlers, K.; Nesengani, L.T.; Mapholi, N.O. The Genetic Assessment of South African Nguni Sheep Breeds Using the Ovine 50K Chip. Agriculture 2022, 12, 663. https://doi.org/10.3390/agriculture12050663
Nxumalo KS, Grobler P, Ehlers K, Nesengani LT, Mapholi NO. The Genetic Assessment of South African Nguni Sheep Breeds Using the Ovine 50K Chip. Agriculture. 2022; 12(5):663. https://doi.org/10.3390/agriculture12050663
Chicago/Turabian StyleNxumalo, Khanyisani S., Paul Grobler, Karen Ehlers, Lucky T. Nesengani, and Ntanganedzeni O. Mapholi. 2022. "The Genetic Assessment of South African Nguni Sheep Breeds Using the Ovine 50K Chip" Agriculture 12, no. 5: 663. https://doi.org/10.3390/agriculture12050663
APA StyleNxumalo, K. S., Grobler, P., Ehlers, K., Nesengani, L. T., & Mapholi, N. O. (2022). The Genetic Assessment of South African Nguni Sheep Breeds Using the Ovine 50K Chip. Agriculture, 12(5), 663. https://doi.org/10.3390/agriculture12050663




