Mechanism of Reduction of Drought-Induced Oxidative Stress in Maize Plants by Fertilizer Seed Coating
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Pots Experiment
2.2. Reactive Oxygen Species Accumulation
2.3. SOD, CAT, and GPOX Activity
2.4. Total Phenolic Content Assay
2.5. Determination of Antioxidant Activity
2.6. Statistical Analysis
3. Results and Discussion
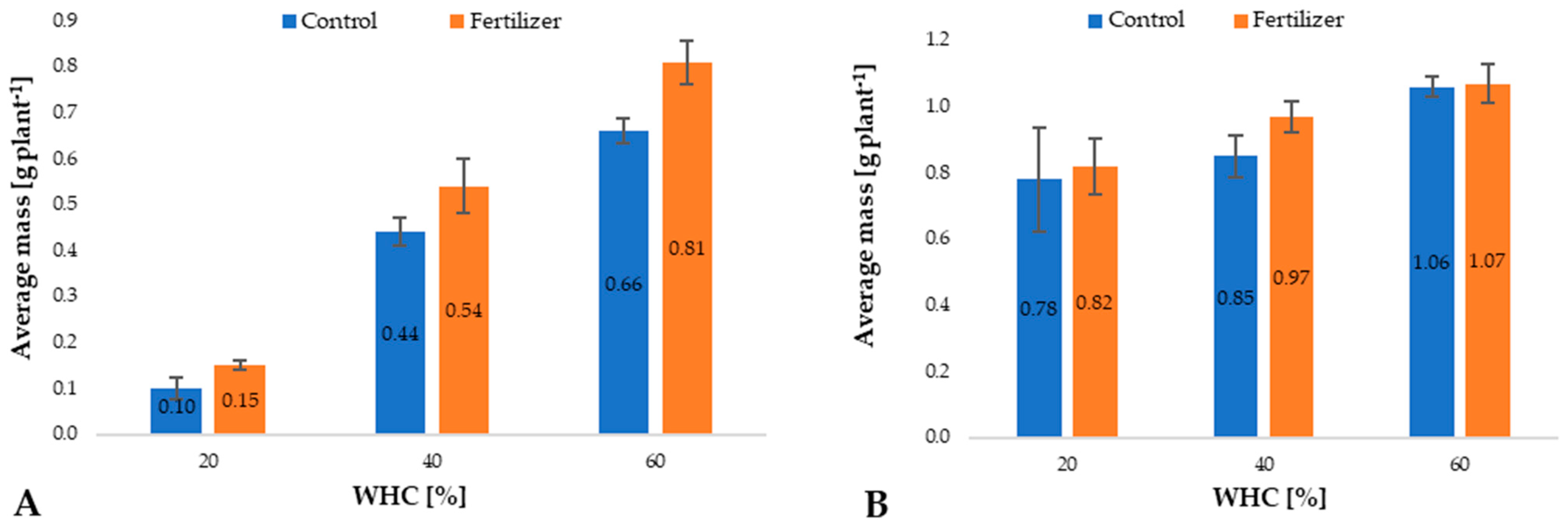
3.1. Maize Plant Mass
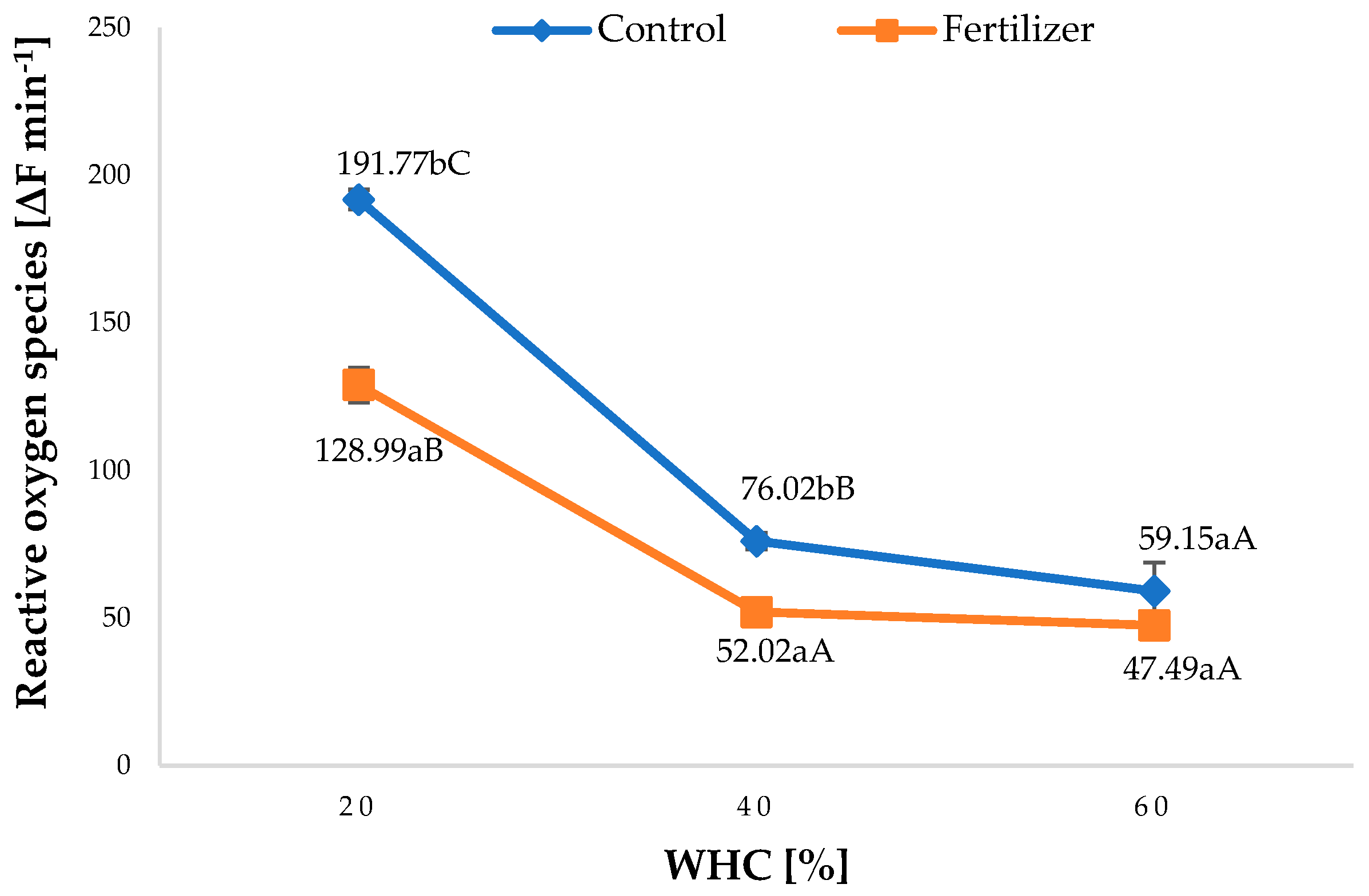
3.2. Reactive Oxygen Species Accumulation
3.3. Antioxidant Enzymes Activity
3.4. Antioxidant Activity and Total Phenolic Content
3.4.1. Antioxidant Activity
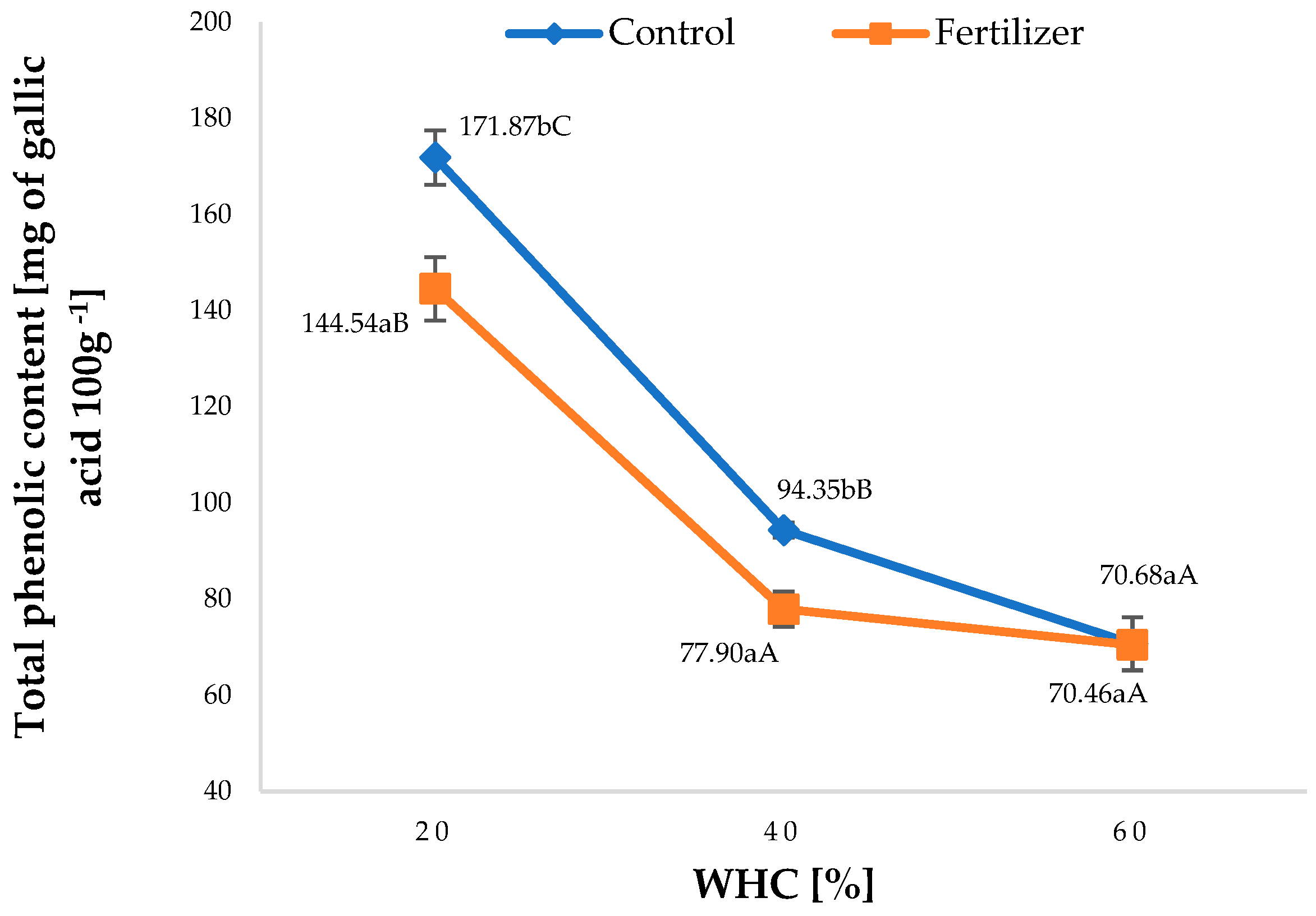
3.4.2. Total Phenolic Content
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balawejder, M.; Szostek, M.; Gorzelany, J.; Antos, P.; Witek, G.; Matłok, N. A Study on the Potential Fertilization Effects of Microgranule Fertilizer Based on the Protein and Calcined Bones in Maize Cultivation. Sustainability 2020, 12, 1343. [Google Scholar] [CrossRef]
- Balawejder, M.; Matłok, N.; Gorzelany, J.; Pieniążek, M.; Antos, P.; Witek, G.; Szostek, M. Foliar Fertilizer Based on Calcined Bones, Boron and Molybdenum—A Study on the Development and Potential Effects on Maize Grain Production. Sustainability 2019, 11, 5287. [Google Scholar] [CrossRef]
- Huma, B.; Hussain, M.; Ning, C.; Yuesuo, Y. Human benefits from maize. Sch. J. Appl. Sci. Res. 2019, 2, 4–7. [Google Scholar]
- Matlok, N.; Szostek, M.; Antos, P.; Gajdek, G.; Gorzelany, J.; Bobrecka-Jamro, D.; Balawejder, M. Effect of Foliar and Soil Fertilization with New Products Based on Calcinated Bones on Selected Physiological Parameters of Maize Plants. Appl. Sci. 2020, 10, 2579. [Google Scholar] [CrossRef]
- Baroni, D.F.; Vieira, H.D. Coating seeds with fertilizer: A promising technique for forage crop seeds. Agric. Sci. 2020, 44, 44. [Google Scholar] [CrossRef]
- Rosa, C.; Bell, R.W.; White, P.F. Phosphorus seed coating and soaking for improving seedling growth of Oryza sativa (rice) cv. IR66. Seed Sci. Technol. 2000, 28, 201–211. [Google Scholar]
- Wiatrak, P. Influence of Seed Coating with Micronutrients on Growth and Yield of Winter Wheat in Southeastern Coastal Plains. Am. J. Agric. Biol. Sci. 2013, 8, 230–238. [Google Scholar] [CrossRef][Green Version]
- Scott, J.; Jessop, R.; Steer, R.; McLachlan, D.G. Effect of nutrient seed coating on the emergence of wheat and oats. Fertil. Res. 1987, 14, 205–217. [Google Scholar] [CrossRef]
- Kamara, A.; Menkir, A.; Badu-Apraku, B.; Ibikunle, O. The influence of drought stress on growth, yield and yield components of selected maize genotypes. J. Agric. Sci. 2003, 141, 43–50. [Google Scholar] [CrossRef]
- Hahn, A.; Kilian, J.; Mohrholz, A.; Ladwig, F.; Peschke, F.; Dautel, R.; Harter, K.; Berendzen, K.W.; Wanke, D. Plant core environmental stress response genes are systemically coordinated during abiotic stresses. Int. J. Mol. Sci. 2013, 14, 7617–7641. [Google Scholar] [CrossRef]
- Punia, H.; Tokas, J.; Malik, A.; Bajguz, A.; El-Sheikh, M.A.; Ahmad, P. Ascorbate–Glutathione Oxidant Scavengers, Metabolome Analysis and Adaptation Mechanisms of Ion Exclusion in Sorghum under Salt Stress. Int. J. Mol. Sci. 2021, 22, 13249. [Google Scholar] [CrossRef] [PubMed]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Piechowiak, T.; Skóra, B.; Grzelak-Błaszczyk, K.; Sójka, M. Extraction of Antioxidant Compounds from Blueberry Fruit Waste and Evaluation of Their In Vitro Biological Activity in Human Keratinocytes (HaCaT). Food Anal. Methods 2021, 14, 2317–2327. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Nxelea, X.; Klein, A.; Ndimba, B.K. Drought and salinity stress alters ROS accumulation, water retention, and osmolyte content in sorghum plants. S. Afr. J. Bot. 2017, 18, 261–266. [Google Scholar] [CrossRef]
- Janas, R.; Szafirowska, A.; Kołosowki, S. Wpływ nawozów donasiennych na porażenie nasion warzyw przez grzyby patogeniczne. Prog. Plant Prot. 2001, 41, 679–682. [Google Scholar]
- Gorzelany, J.; Matłok, N.; Dziura, M.; Witek, G.; Migut, D. Impact of Dr Green Prime Seed Fertilizer on the Germination Energy and Ability of Wheat Seeds. ECONTECHMOD Int. Q. J. Econ. Technol. Model. Processes 2017, 4, 33–40. [Google Scholar]
- Piechowiak, T.; Antos, P.; Kosowski, P.; Skrobacz, K.; Józefczyk, R.; Balawejder, M. Impact of ozonation process on the microbiological and antioxidant status of raspberry (Rubus ideaeus L.) fruit during storage at room temperature. Agric. Food Sci. 2019, 28, 35–44. [Google Scholar] [CrossRef]
- Uarrota, V.G.; Moresco, R.; Schmidt, E.C.; Bouzon, Z.L.; da Costa Nunes, E.; de Oliveira Neubert, E.; Peruch, L.A.M.; Rocha, M.; Maraschin, M. The role of ascorbate peroxidase, guaiacol peroxidase, and polysaccharides in cassava (Manihot esculenta Crantz) roots under postharvest physiological deterioration. Food Chem. 2016, 197, 737–746. [Google Scholar] [CrossRef]
- Matłok, N.; Gorzelany, J.; Stępień, A.E.; Figiel, A.; Balawejder, M. Effect of Fertilization in Selected Phytometric Features and Contents of Bioactive Compounds in Dry Matter of Two Varieties of Basil (Ocimum basilicum L.). Sustainability 2019, 11, 6590. [Google Scholar] [CrossRef]
- Matłok, N.; Stępień, A.E.; Gorzelany, J.; Wojnarowska-Nowak, R.; Balawejder, M. Effects of Organic and Mineral Fertilization on Yield and Selected Quality Parameters for Dried Herbs of Two Varieties of Oregano (Origanum vulgare L.). Appl. Sci. 2020, 10, 5503. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Fujita, M. Drought Stress Responses in Plants, Oxidative Stress, and Antioxidant Defense. Clim. Chang. Plant Abiotic Stress Toler. 2013, 209–250. [Google Scholar] [CrossRef]
- Sampath-Wiley, P.; Neefus, C.D.; Jahnke, L.S. Seasonal effects of sun exposure and emersion on intertidal seaweed physiology: Fluctuations in antioxidant contents, photosynthetic pigments and photosynthetic efficiency in the red alga Porphyra umbilicalis Kützing (Rhodophyta, Bangiales). J. Exp. Mar. Biol. Ecol. 2008, 361, 83–91. [Google Scholar] [CrossRef]
- Gabryś, H.; Kasperska-Lewak, A.; Kopcewicz, J.; Krzymowska, M.; Lewak, S.; Rychter, A.; Starck, Z.; Strzałka, K.; Szymańska, M.; Tretyn, A.; et al. Lewak. Fizjologia Roślin; PWN: Warszawa, Poland, 2016. [Google Scholar]
- Porcel, R.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal influence on leaf water potential, solute accumulation, and oxidative stress in soybean plants subjected to drought stress. J. Exp. Bot. 2004, 55, 1743–1750. [Google Scholar] [CrossRef]
- Abo Sedera, F.A.; Amany, A.; Abd El-Latif, L.A.A.; Bader Rezk, S.M. Effect of NPK mineral fertilizer levels and foliar application with humic and amino acids on yield and quality of strawberry. Egyp. J. Appl. Sci. 2010, 25, 154–169. [Google Scholar]
- Denisov, E.T.; Afanas’ev, I.B. Oxidation and Antioxidants in Organic Chemistry and Biology; CRC Taylor & Francis Group: Boca Raton, FL, USA, 2005; p. 1024. [Google Scholar]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Gill, S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2000, 48, 909–930. [Google Scholar] [CrossRef]
- Banu, M.N.A.; Hoque, M.A.; Watanabe-Sugimoto, M.; Matsuoka, K.; Nakamura, Y.; Shimoishi, Y.; Murata, Y. Proline and glycinebetaine induce antioxidant defense gene expression and suppress cell death in cultured tobacco cells under salt stress. J. Plant Physiol. 2009, 166, 146–156. [Google Scholar] [CrossRef]
- Hozayn, M.; Qados, A.A. Magnetic water application for improving wheat (Triticum aestivum L.) crop production. Agric. Biol. J. N. Am. 2010, 1, 677–682. [Google Scholar]
- Rahimizadeh, M.; Habibi, D.; Madani, H.; Mohammadi, G.N.; Mehraban, A.; Sabet, A.M. The effect of micronutrients on antioxidant enzymes metabolism in sunflower (Helianthus annus L.) under drought stress. Helia 2007, 30, 167–174. [Google Scholar] [CrossRef]
- Cakmak, I. Possible roles of zinc in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 85–200. [Google Scholar] [CrossRef] [PubMed]
- Jubany-Marí, T.; Munné-Bosch, S.; López-Carbonell, M.; Alegre, L. Hydrogen peroxide is involved in the acclimation of the Mediterranean shrub, Cistus albidus L., to summer drought. J. Exp. Bot. 2009, 60, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Waszczak, C.; Carmody, M.; Kangasjärvi, J. Reactive Oxygen Species in Plant Signaling. Annu. Rev. Plant Biol. 2018, 69, 209–236. [Google Scholar] [CrossRef] [PubMed]
- Zarabi, M.; Allah Dadi, A.; Akbari, G.; Irannejad, H.; Akbari, G. Reduction in effects of drought stress on yield and yield components of maize grain using a combination of biological and phosphorus fertilizers. Plant Breed. 2010, 12, 37–50. [Google Scholar]
- Adebayo, M.; Menkir, A. Assessment of hybrids of drought tolerant maize (Zea mays L.) inbred lines for grain yield and other traits under stress managed conditions. Nigerian J. Genet. 2015, 28, 19–23. [Google Scholar] [CrossRef]
- Farooq, M.; Basra, S.M.A.; Wahid, A.; Ahmad, N.; Saleem, B.A. Improving the Drought Tolerance in Rice (Oryza sativa L.) by Exogenous Application of Salicylic Acid. J. Agron. Crop Sci. 2009, 195, 262–269. [Google Scholar] [CrossRef]
- Dehnavi, M.M.; Sanavi, A.M.M.; Soroushzadae, A.; Jalali, M. Effect foliar application of zinc and manganese on yield and yield components of three safflower cultivars under drought stress in Isfahan. In Proceedings of the 8th Congress of Agronomy and Plant Breeding, Karaj, Iran, 8–11 December 2002. [Google Scholar]
- Tittal, M.; Mir, R.A.; Jatav, K.S.; Agarwal, R.M. Supplementation of potassium alleviates water stress-induced changes in Sorghum bicolor L. Physiol. Plant. 2021, 172, 1149–1161. [Google Scholar] [CrossRef]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Talebi, M.; Matkowski, A. The effect of drought stress on polyphenolic compounds and expression of flavonoid biosynthesis related genes in Achillea pachycephala Rech.f. Phytochemistry 2019, 162, 90–98. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matłok, N.; Piechowiak, T.; Królikowski, K.; Balawejder, M. Mechanism of Reduction of Drought-Induced Oxidative Stress in Maize Plants by Fertilizer Seed Coating. Agriculture 2022, 12, 662. https://doi.org/10.3390/agriculture12050662
Matłok N, Piechowiak T, Królikowski K, Balawejder M. Mechanism of Reduction of Drought-Induced Oxidative Stress in Maize Plants by Fertilizer Seed Coating. Agriculture. 2022; 12(5):662. https://doi.org/10.3390/agriculture12050662
Chicago/Turabian StyleMatłok, Natalia, Tomasz Piechowiak, Kamil Królikowski, and Maciej Balawejder. 2022. "Mechanism of Reduction of Drought-Induced Oxidative Stress in Maize Plants by Fertilizer Seed Coating" Agriculture 12, no. 5: 662. https://doi.org/10.3390/agriculture12050662
APA StyleMatłok, N., Piechowiak, T., Królikowski, K., & Balawejder, M. (2022). Mechanism of Reduction of Drought-Induced Oxidative Stress in Maize Plants by Fertilizer Seed Coating. Agriculture, 12(5), 662. https://doi.org/10.3390/agriculture12050662







