Forever Young? Late Shoot Pruning Affects Phenological Development, Physiology, Yield and Wine Quality of Vitis vinifera cv. Malbec
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Site, Plant Material and Meteorological Conditions
2.2. Experimental Design
2.3. Pruning Treatments
2.4. Standard Winter Pruning Method
2.5. Late Shoot Pruning Method
2.6. Phenology, Canopy Evaluation and Meteorological Data
2.7. Water Relations and Gas Exchange Measurements
2.8. Yield Components and Fruit Quality
2.9. Wine Vinification
2.10. Must and Wine Analysis
2.11. Wine Tasting
2.12. Statistical Analyses
3. Results
3.1. Seasonal Phenological Course
3.2. Seasonal Vegetative Development
3.3. Water Relations and Plant Physiology
3.4. Crop Yield and Its Components
3.5. Must Composition
3.6. Wine Color and Polyphenolics Analysis
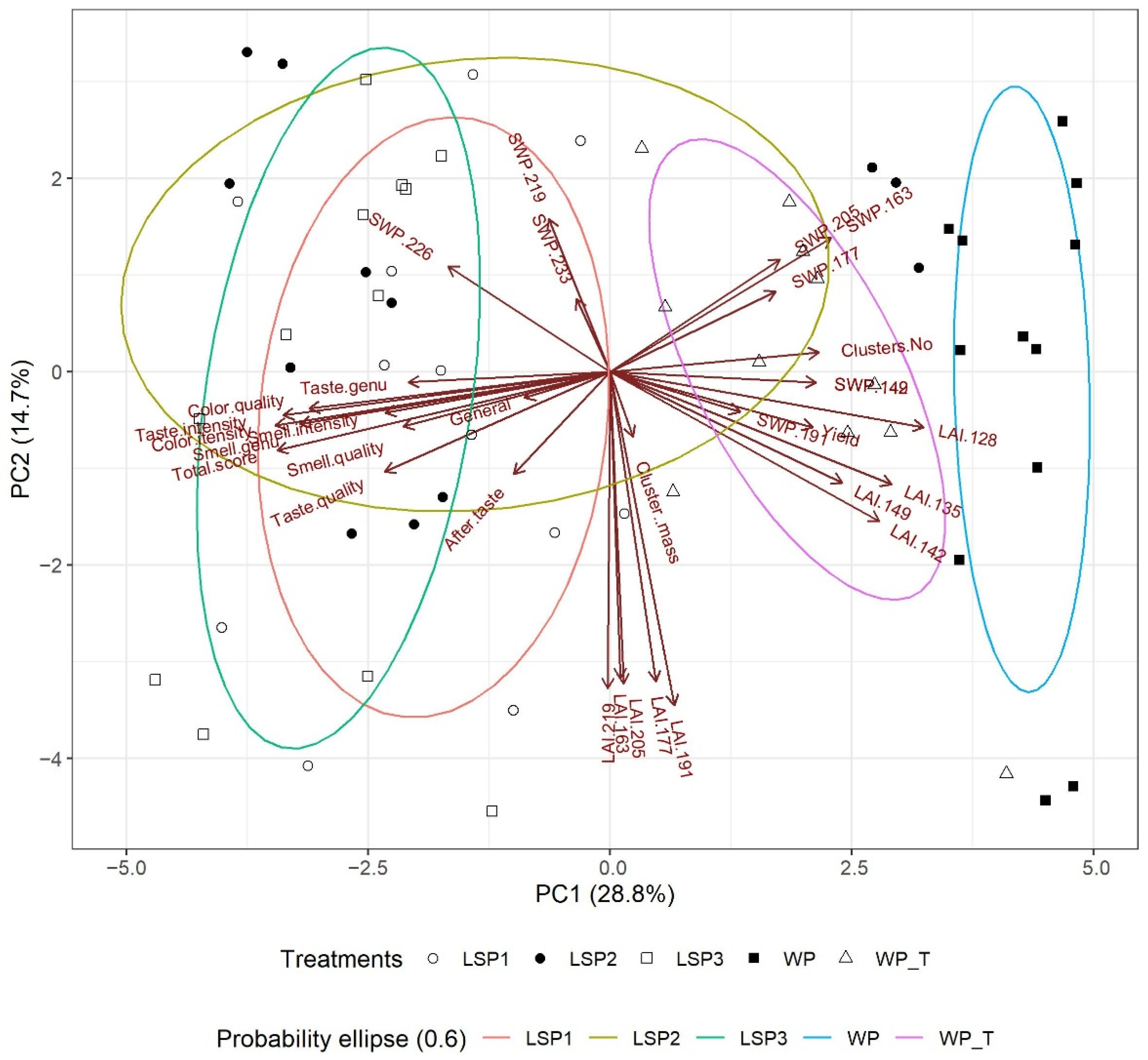
3.7. Statistical Analyses—PCA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Winkler, A.J.; Cook, J.A.; Kliewer, W.M.; Lider, L.A. General Viticulture, 2nd ed.; University of California Press: Berkeley, CA, USA, 1974. [Google Scholar]
- Palliotti, A.; Gatti, M.; Lanari, V.; Silvestroni, O.; Tombesi, S.; Poni, S.; Silvestroni, O.; Lanari, V.; Gatti, M.; Poni, S. Changes in vineyard establishment and canopy management urged by earlier climate-related grape ripening: A review. Sci. Hortic. 2014, 178, 43–54. [Google Scholar] [CrossRef]
- Frioni, T.; Pirez, F.J.; Diti, I.; Ronney, L.; Poni, S.; Gatti, M. Post-budbreak pruning changes intra-spur phenology dynamics, vine productivity and berry ripening parameters in Vitis vinifera L. cv. ‘Pinot Noir’. Sci. Hortic. 2019, 256, 108584. [Google Scholar] [CrossRef]
- Bonada, M.; Sadras, V.; Moran, M.; Fuentes, S. Elevated temperature and water stress accelerate mesocarp cell death and shrivelling, and decouple sensory traits in Shiraz berries. Irrig. Sci. 2013, 31, 1317–1331. [Google Scholar] [CrossRef]
- Jones, G.; Davis, R. Climate influences on grapevine phenology, grape composition, and wine production and quality for Bordeaux, France. Am. J. Enol. Vitic. 2000, 51, 249–261. [Google Scholar] [CrossRef]
- Kizildeniz, T.; Mekni, I.; Santesteban, H.; Pascual, I.; Morales, F.; Irigoyen, J.J. Effects of climate change including elevated CO2 concentration, temperature and water deficit on growth, water status, and yield quality of grapevine (Vitis vinifera L.) cultivars. Agric. Water Manag. 2015, 159, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Lüscher, J.; Kizildeniz, T.; Vucetic, V.; Dai, Z.; Luedeling, E.; van Leeuwen, C.; Gomès, E.; Pascual, I.; Irigoyen, J.J.; Morales, F.; et al. Sensitivity of grapevine phenology to water availability, temperature and CO2 concentration. Front. Environ. Sci. 2016, 4, 48. [Google Scholar] [CrossRef]
- Parra, C.S.; Aguirreolea, J.; Sánchez-Díaz, M.; Irigoyen, J.J.; Morales, F. Effects of climate change scenarios on Tempranillo grapevine (Vitis vinifera L.) ripening: Response to a combination of elevated CO2 and temperature, and moderate drought. Plant Soil 2010, 337, 179–191. [Google Scholar] [CrossRef]
- Petrie, P.R.; Brooke, S.J.; Moran, M.A.; Sadras, V.O. Pruning after budburst to delay and spread grape maturity. Aust. J. Grape Wine Res. 2017, 23, 378–389. [Google Scholar] [CrossRef]
- Martínez-Moreno, A.; Sanz, F.; Yeves, A.; Gil-Muñoz, R.; Martínez, V.; Intrigliolo, D.S.; Buesa, I. Forcing bud growth by double-pruning as a technique to improve grape composition of Vitis vinifera L. cv. Tempranillo in a semi-arid Mediterranean climate. Sci. Hortic. 2019, 256, 108614. [Google Scholar] [CrossRef]
- Cus, F.; Korosec Koruza, Z.; Koruza, B.; Lavrencic, P. Influence of crop load and cultivar’s dependent canopy management on grape must quality of “sipon”, “zametovka”, and “rebula”. Acta Hortic. 2004, 652, 141–146. [Google Scholar] [CrossRef]
- Senthilkumar, S.; Vijayakumar, R.; Soorianathasundaram, K.; Durga Devi, D. Effect of Pruning Severity on V egetative, Physiological, Yield and Quality Attributes in Grape (Vitis vinifera L.): A Review. Curr. Agric. Res. J. 2015, 3, 42–54. [Google Scholar] [CrossRef]
- Bravdo, B.; Hepner, Y.; Loinger, C.; Cohen, S.; Tabacman, H. Effect of Crop Level and Crop Load on Growth, Yield, Must and Wine Composition, and Quality of Cabernet Sauvignon. Am. J. Enol. Vitic. 1985, 36, 125–131. [Google Scholar]
- Dai, Z.W.; Vivin, P.; Robert, T.; Milin, S.; Li, S.H.; Ǵnard, M. Model-based analysis of sugar accumulation in response to sourcesink ratio and water supply in grape (Vitis vinifera) berries. Funct. Plant Biol. 2009, 36, 527–540. [Google Scholar] [CrossRef]
- Munitz, S.; Netzer, Y.; Schwartz, A. Sustained and regulated deficit irrigation of field grown Merlot grapevines. Aust. J. Grape Wine Res. 2017, 23, 87–94. [Google Scholar] [CrossRef]
- Netzer, Y.; Munitz, S.; Shtein, I.; Schwartz, A. Structural memory in grapevines: Early season water availability affects late season drought stress severity. Eur. J. Agron. 2019, 105, 96–103. [Google Scholar] [CrossRef]
- Shtein, I.; Hayat, Y.; Munitz, S.; Harcavi, E.; Akerman, M.; Drori, E.; Schwartz, A.; Netzer, Y. From structural constraints to hydraulic function in three Vitis rootstocks. Trees Struct. Funct. 2017, 31, 851–861. [Google Scholar] [CrossRef]
- Gerzon, E.; Biton, I.; Yaniv, Y.; Zemach, H.; Netzer, Y.; Schwartz, A.; Fait, A.; Ben-Ari, G. Grapevine anatomy as a possible determinant of isohydric or anisohydric behavior. Am. J. Enol. Vitic. 2015, 66, 340–347. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Tregoat, O.; Chone, X.; Bois, B.; Pernet, D.; Gaudillere, J.P. Vine water status is a key factor in grape ripening and vintage quality for red bordeaux wine. How can it be assessed for vineyard management purposes? J. Int. Des. Sci. La Vigne Du Vin 2009, 43, 121–134. [Google Scholar] [CrossRef]
- Drori, E.; Rahimi, O.; Henig, Y.; Lorenzi, S.; Brauner, H.; Marrano, A.; Amar, Z.; Netzer, Y.; Failla, O.; Grando, M.S.S. Ampelographic and genetic characterization of an initial Israeli grapevine germplasm collection. Vitis J. Grapevine Res. 2015, 54, 107–110. [Google Scholar]
- Cavagnaro, J.B.; Ponce, M.T.; Guzmán, J.; Cirrincione, M.A. Argentinean cultivars of Vitis vinifera grow better than European ones when cultured in vitro under salinity. Biocell 2006, 30, 1–7. [Google Scholar] [CrossRef]
- Vivier, M.A.; Pretorius, I.S. Genetically tailored grapevines for the wine industry. Trends Biotechnol. 2002, 20, 472–478. [Google Scholar] [CrossRef]
- Keller, M.; Mills, L.J.; Wample, R.L.; Spayd, S.E. Cluster Thinning Effects on Three Deficit-Irrigated Vitis vinifera Cultivars. Am. J. Enol. Vitic. 2005, 2, 91–103. [Google Scholar]
- Naor, A.; Gal, Y.; Bravdo, B. Crop load affects assimilation rate, stomatal conductance, stem water potential and water relations of field-grown Sauvignon blanc grapevines. J. Exp. Bot. 1997, 48, 1675–1680. [Google Scholar] [CrossRef] [Green Version]
- Guidoni, S.; Allara, P.; Schubert, A. Effect of cluster thinning on berry skin anthocyanin composition of Vitis vinifera cv. Nebbiolo. Am. J. Enol. Vitic. 2002, 53, 224–226. [Google Scholar]
- Petrie, P.R.; Clingeleffer, P.R. Crop thinning (hand versus mechanical), grape maturity and anthocyanin concentration: Outcomes from irrigated Cabernet Sauvignon (Vitis vinifera L.) in a warm climate. Aust. J. Grape Wine Res. 2006, 12, 21–29. [Google Scholar] [CrossRef]
- Xi, X.J.; Zha, Q.; Jiang, A.L.; Tian, Y.H. Stimulatory effect of bunch thinning on sugar accumulation and anthocyanin biosynthesis in Shenhua grape berry (Vitis vinifera × V. labrusca). Aust. J. Grape Wine Res. 2018, 24, 158–165. [Google Scholar] [CrossRef]
- Kliewer, W.M.; Dokoozlian, N.K. Leaf Area/Crop Weight Ratios of Grapevines: Influence on Fruit Composition and Wine Quality. Am. J. Enol. Vitic. 2005, 2, 170–181. [Google Scholar]
- Jackson, D.I.; Lombard, P.B.; Kabinett, L.Q. Environmental and management practices affecting grape composition and wine quality—A review. Am. J. Enol. Vitic. 1993, 44, 409–430. [Google Scholar]
- Reynolds, A.G.; Edwards, C.G.; Wardle, D.A.; Webster, D.; Dever, M. Shoot density affects “Riesling” grapevines. II.Wine composition and sensory response. J. Am. Soc. Hortic. Sci. 1994, 119, 881–892. [Google Scholar] [CrossRef] [Green Version]
- Sinton, T.H.; Ough, C.S.; Kissler, J.J.; Kasimatis, A.N. Grape Juice Indicators for Prediction of Potential Wine Quality. I. Relationship Between Crop Level, Juice and Wine Composition, and Wine Sensory Ratings and Scores. Am. J. Enol. Vitic. 1978, 29, 267–271. [Google Scholar]
- Bravdo, B.; Hepner, Y.; Cohen, S.; Tabacman, H. Effect of crop level on growth, yield and wine quality of a high yielding Carignane vineyard. Am. J. Enol. Vitic. 1984, 35, 247–252. [Google Scholar]
- Bindon, K.A.; Dry, P.R.; Loveys, B.R.; Osmond, G.; Campus, W.; Unit, H.; Rooyen, V. The Interactive Effect of Pruning Level and Irrigation Strategy on Water Use Efficiency of. Development 2008, 29, 59–70. [Google Scholar]
- Holt, H.E.; Francis, I.L.; Field, J.; Herderich, M.J.; Iland, P.G. Relationships between berry size, berry phenolic composition and wine quality scores for Cabernet Sauvignon (Vitis vinifera L.) from different pruning treatments and different vintages. Aust. J. Grape Wine Res. 2008, 14, 191–202. [Google Scholar] [CrossRef]
- Nir, G. The effects of pruning date on bud break and yield of 4 wine grape varieties in 4 seasons at the Southern inner plain of Israel. Alon Hanotea 2019, 6, 50–56. [Google Scholar]
- Svoray, T.; Hassid, I.; Atkinson, P.M.; Moebius-Clune, B.N.; van Es, H.M. Mapping Soil Health over Large Agriculturally Important Areas. Soil Sci. Soc. Am. J. 2015, 79, 1420. [Google Scholar] [CrossRef]
- Moran, M.A.; Sadras, V.O.; Petrie, P.R. Late pruning and carry-over effects on phenology, yield components and berry traits in Shiraz. Aust. J. Grape Wine Res. 2017, 23, 390–398. [Google Scholar] [CrossRef]
- Frioni, T.; Tombesi, S.; Silvestroni, O.; Lanari, V.; Bellincontro, A.; Sabbatini, P.; Gatti, M.; Poni, S.; Palliotti, A. Postbudburst spur pruning reduces yield and delays fruit sugar accumulation in sangiovese in central Italy. Am. J. Enol. Vitic. 2016, 67, 419–425. [Google Scholar] [CrossRef]
- Parkin, R. Late pruning improves wine quality. Agric. Gaz. N. South Wales 1980, 91, 36–37. [Google Scholar]
- Friend, A.P.; Trought, M.C.T. Delayed winter spur-pruning in New Zealand can alter yield components of Merlot grapevines. Aust. J. Grape Wine Res. 2007, 13, 157–164. [Google Scholar] [CrossRef]
- van Es, H.M.; Gomes, C.P.; Sellmann, M.; van Es, C.L. Spatially-Balanced Complete Block designs for field experiments. Geoderma 2007, 140, 346–352. [Google Scholar] [CrossRef]
- Lorenz, D.H.; Eichorn, K.W.; Bleiholder, H.; Klose, R.; Meir, U.; Weber, E. Growth Stages of the Grapevine: Phenological growth stages of the grapevine (Vitis vinifera L. ssp. vinifera)—Codes and descriptions according to the extended BBCH scale. Aust. J. Grape Wine Res. 1995, 1, 100–103. [Google Scholar] [CrossRef]
- Munitz, S.; Schwartz, A.; Netzer, Y. The effect of irrigation initiation timing on vegetative growth, physiology and yield parameters in “Cabernet Sauvignon” grapevines. Aust. J. Grape Wine Res. 2020, 26, 220–232. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine; Organisation, I. OIV Standard for International Wine Competitions and Spiritous Beverages of Vitivinicultural Origin (OIV-Concours 332A-2009). Available online: https://www.oiv.int/public/medias/7895/oiv-patronage-competition-norme-ed-2021.pdf (accessed on 13 February 2022).
- Hotelling, H. Analysis of a complex of statistical variables into principal components. J. Educ. Psychol. 1933, 24, 417–441. [Google Scholar] [CrossRef]
- Jolliffe, I. Principal Component Analysis. In International Encyclopedia of Statistical Science; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1094–1096. [Google Scholar]
- Gabriel, K.R. The biplot graphic display of matrices with application to principal component analysis. Biometrika 1971, 58, 453–467. [Google Scholar] [CrossRef]
- Silvestroni, O.; Lanari, V.; Lattanzi, T.; Palliotti, A. Delaying winter pruning, after pre-pruning, alters budburst, leaf area, photosynthesis, yield and berry composition in Sangiovese (Vitis vinifera L.). Aust. J. Grape Wine Res. 2018, 24, 478–486. [Google Scholar] [CrossRef]
- Gatti, M.; Pirez, F.J.; Chiari, G.; Tombesi, S.; Palliotti, A.; Merli, M.C.; Poni, S. Phenology, Canopy Aging and Seasonal Carbon Balance as Related to Delayed Winter Pruning of Vitis vinifera L. cv. Sangiovese Grapevines. Front. Plant Sci. 2016, 7, 659. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; García, J.; Balda, P.; De Toda, F.M. Effects of late winter pruning at different phenological stages on vine yield components and berry composition in la Rioja, north-central Spain. J. Int. Des. Sci. La Vigne Du Vin 2017, 51, 363–372. [Google Scholar] [CrossRef]
- Gatti, M.; Pirez, F.J.; Frioni, T.; Squeri, C.; Poni, S. Calibrated, delayed-cane winter pruning controls yield and significantly postpones berry ripening parameters in Vitis vinifera L. cv. Pinot Noir. Aust. J. Grape Wine Res. 2018, 24, 305–316. [Google Scholar] [CrossRef]
- Friend, A.P.; Trought, M.C.T.; Stushnoff, C.; Wells, G.H. Effect of delaying budburst on shoot development and yield of Vitis vinifera L. Chardonnay “Mendoza” after a spring freeze event. Aust. J. Grape Wine Res. 2011, 17, 378–382. [Google Scholar] [CrossRef]
- Howell, G.S.; Wolpert, J.A. Nodes per cane, primary bud phenology, and spring freeze damage to concord grapevines: A preliminary note. Am. J. Enol. Vitic. 1978, 29, 229–232. [Google Scholar]
- Munitz, S.; Schwartz, A.; Netzer, Y. Water consumption, crop coefficient and leaf area relations of a Vitis vinifera cv. “Cabernet Sauvignon” vineyard. Agric. Water Manag. 2019, 219, 86–94. [Google Scholar] [CrossRef]
- Ohana-Levi, N.; Munitz, S.; Ben-Gal, A.; Schwartz, A.; Peeters, A.; Netzer, Y. Multiseasonal grapevine water consumption—Drivers and forecasting. Agric. For. Meteorol. 2020, 280, 107796. [Google Scholar] [CrossRef]
- Poni, S.; Intrieri, C.; Silvestroni, O. Interactions of LeafAge, Fruiting, and Exogenous Cytokinins in Sangiovese Grapevines Under Non-Irrigated Conditions. I. Gas Exchange. Am. J. Enol. Vitic. 1994, 45, 71–78. [Google Scholar]
- Kriedemann, P.; Kliewer, W.; Harris, J. Leaf age and photosynthesis in Vitis vinifera L. Vitis 1970, 104, 97–104. [Google Scholar]
- Khanduja, S.D.; Balasubrahmanyam, V.R. Fruitfulness of grape vine buds. Econ. Bot. 1972, 26, 280–294. [Google Scholar] [CrossRef]
- Matus, J.T.; Loyola, R.; Vega, A.; Peña-Neira, A.; Bordeu, E.; Arce-Johnson, P.; Alcalde, J.A. Post-veraison sunlight exposure induces MYB-mediated transcriptional regulation of anthocyanin and flavonol synthesis in berry skins of Vitis vinifera. J. Exp. Bot. 2009, 60, 853–867. [Google Scholar] [CrossRef] [Green Version]
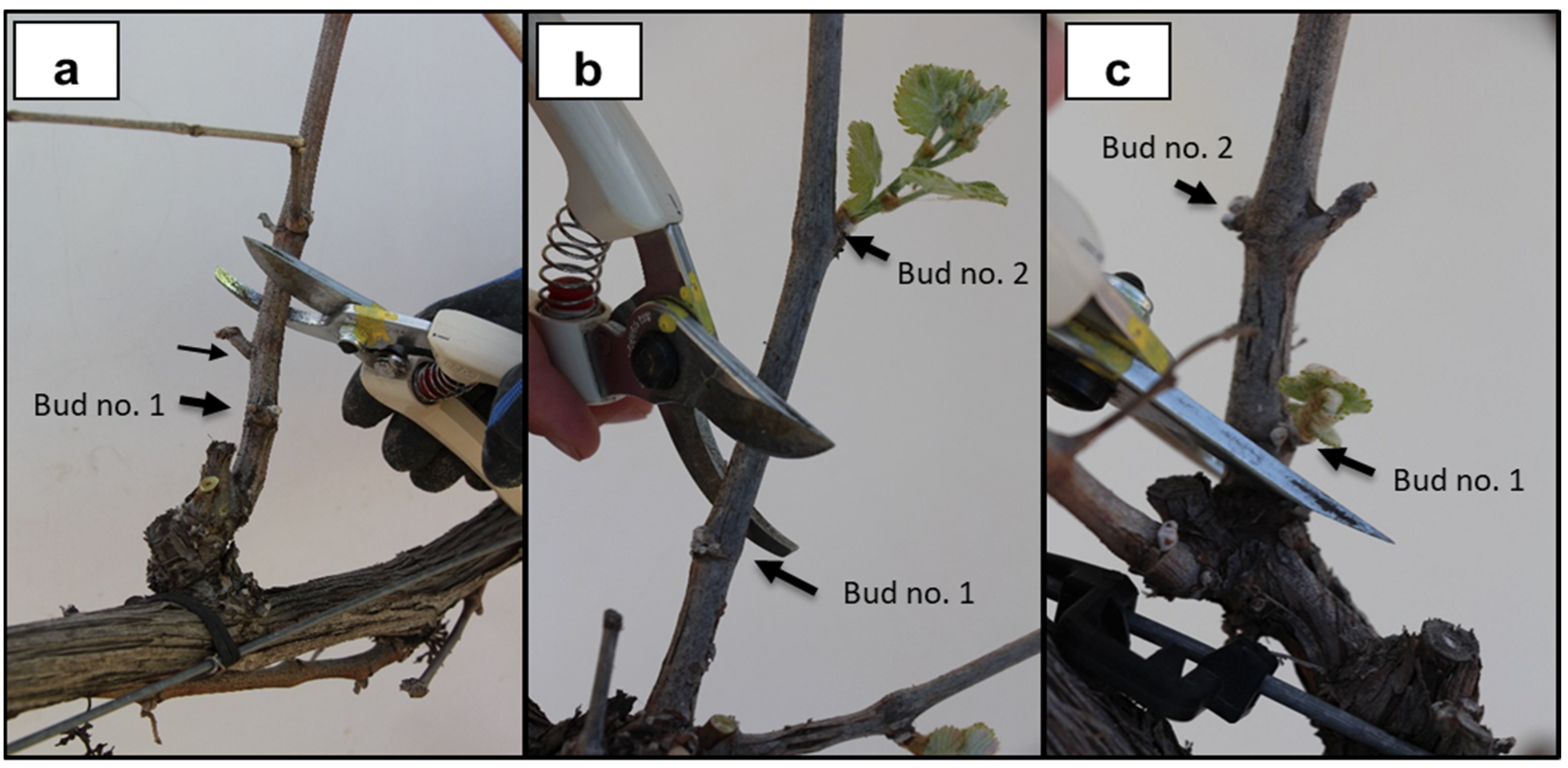
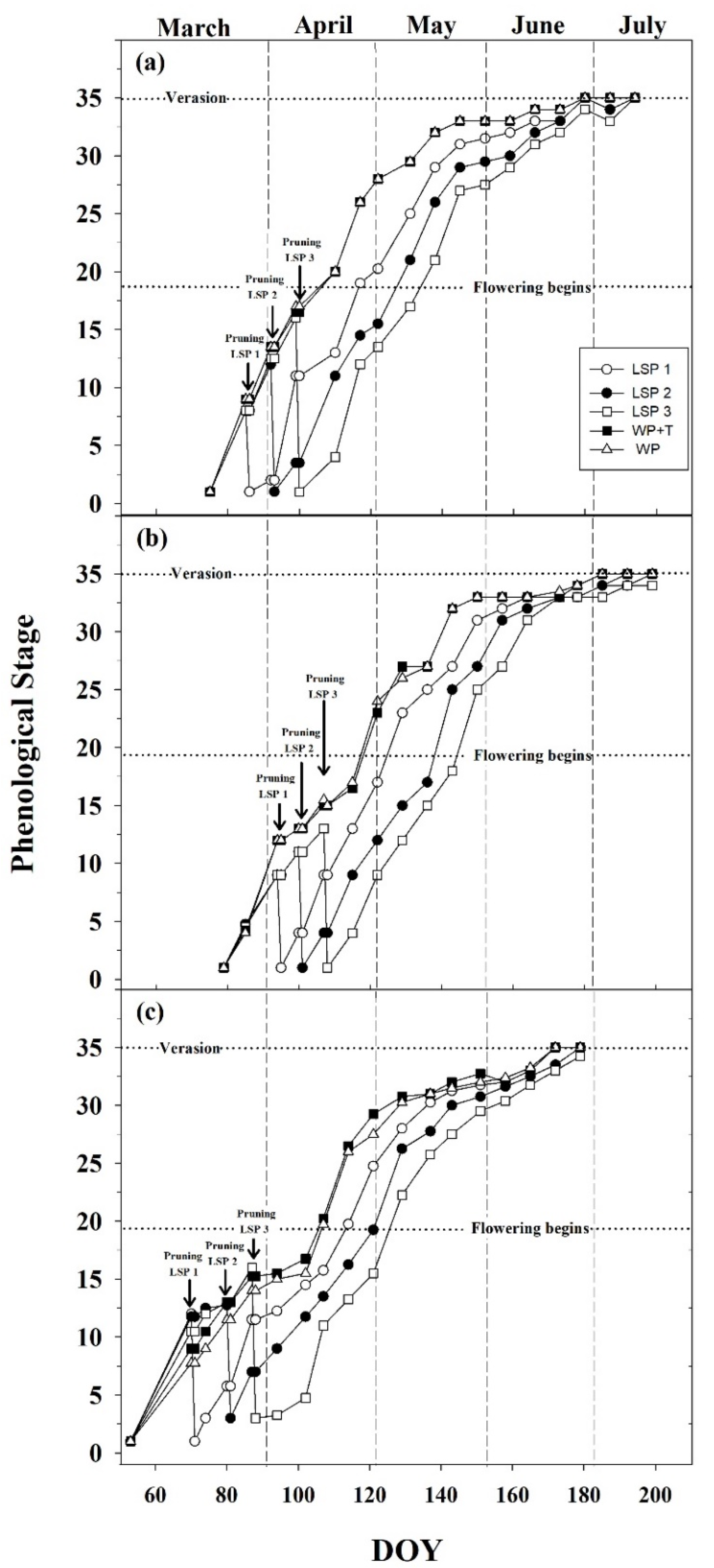

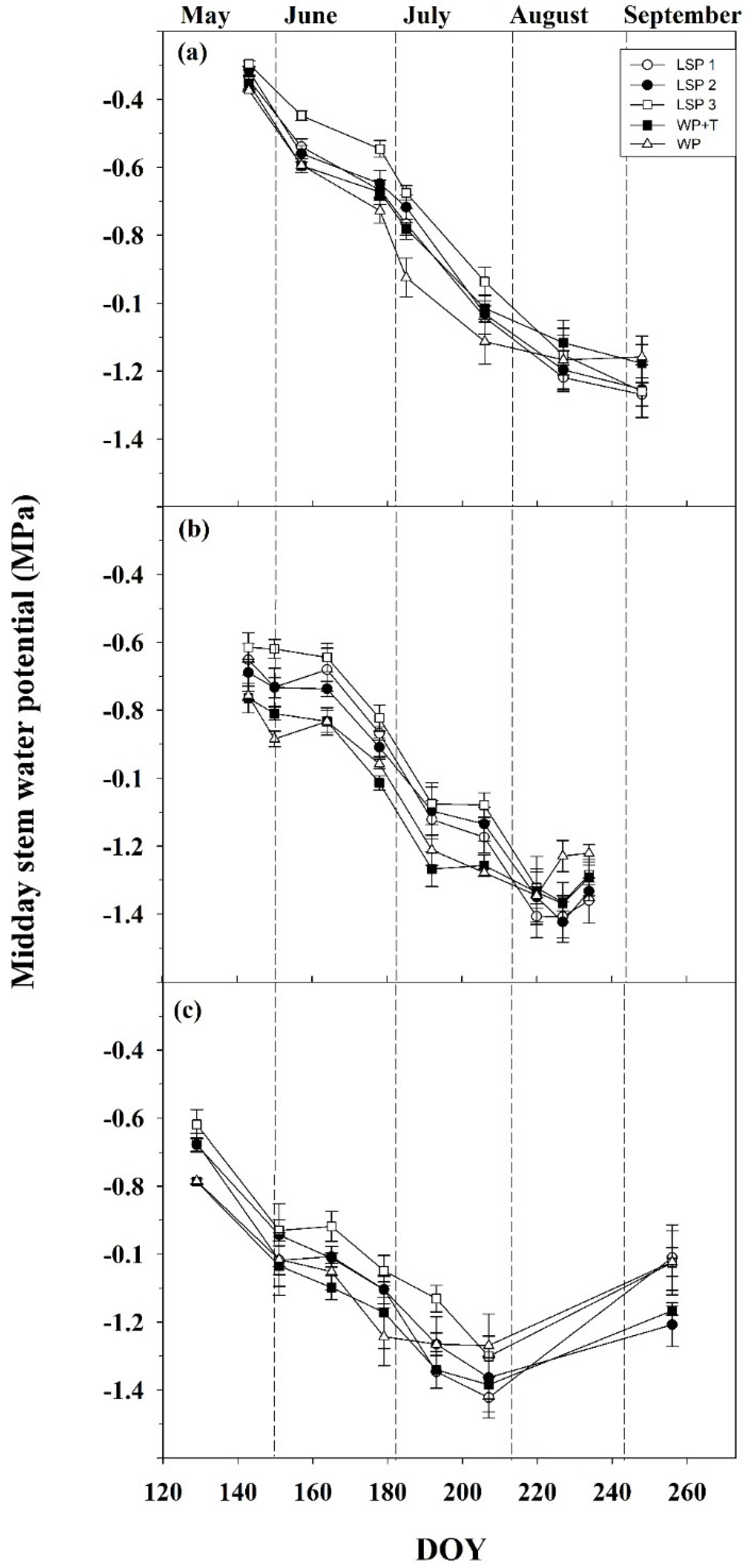
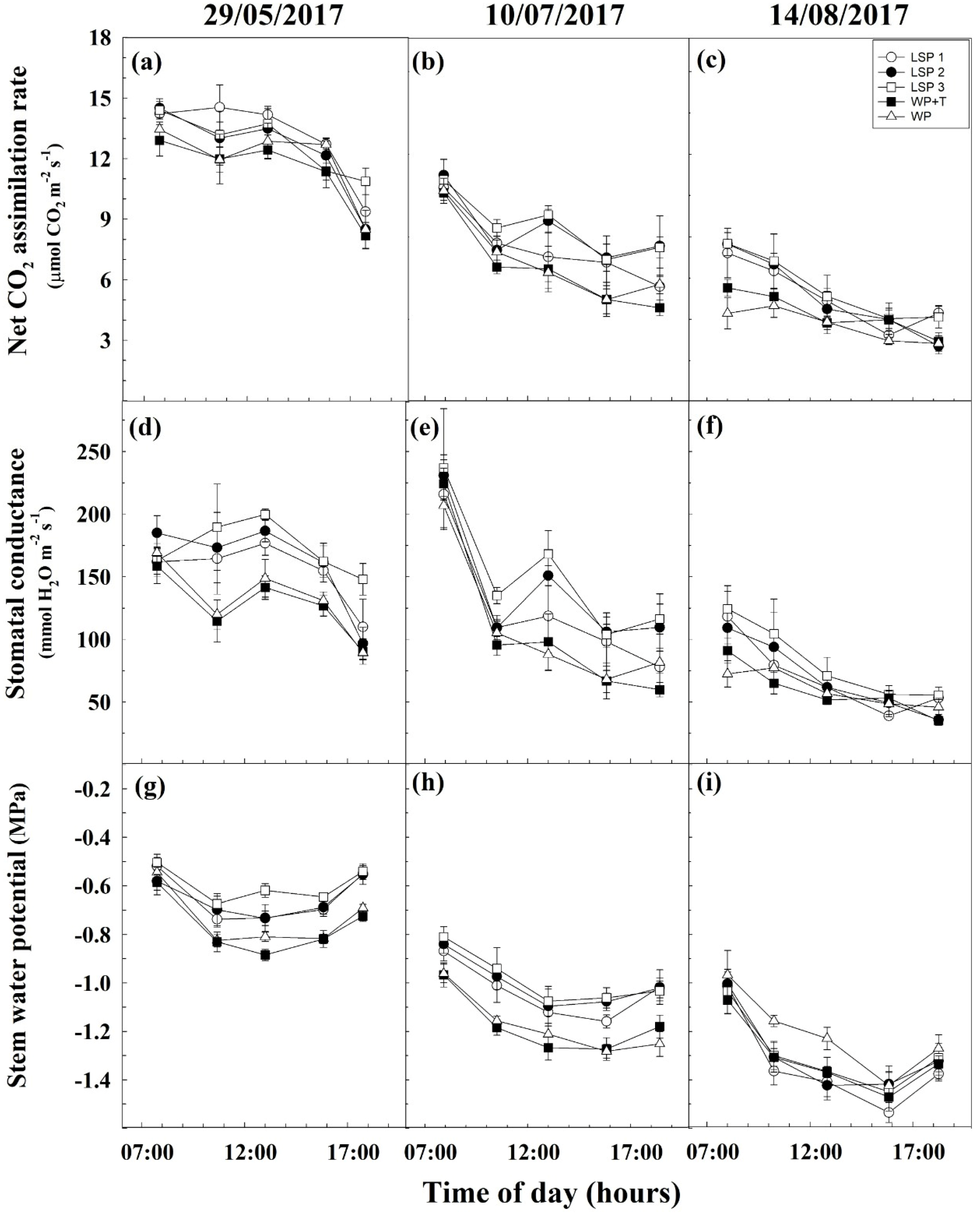
| Treatments | Abbreviation | Date of Pruning 2016 (DOY) | Date of Pruning 2017 (DOY) | Date of Pruning 2018 (DOY) |
|---|---|---|---|---|
| Late shoot pruning 1 (one week after bud break) | LSP1 | 27 March 2016 (86) | 4 April 2017 (95) | 15 March 2018 (74) |
| Late shoot pruning 2 (two weeks after bud break) | LSP2 | 3 April 2016 (93) | 10 April 2017 (101) | 22 March 2018 (81) |
| Late shoot pruning 3 (three weeks after bud break) | LSP3 | 10 April 2016 (100) | 17 April 2017 (108) | 29 March 2018 (88) |
| Standard winter pruning and shoot and cluster thinning | WP + T | 17 February 2016 (48) | 19 February 2017 (50) | 20 February 2018 (51) |
| Standard winter pruning | WP | 17 February 2016 (48) | 19 February 2017 (50) | 20 February 2018 (51) |
| Treatments | Yield (kg per Vine) | Clusters (No per Vine) | Cluster Mass (g) | Berry Mass (g) | Berries (Per Cluster) |
|---|---|---|---|---|---|
| 2016 | |||||
| LSP1 | 7.7 b | 46.5 b | 168.2 a | 1.37 a | 123.0 ab |
| LSP2 | 5.4 bc | 36.7 bc | 144.2 a | 1.27 ab | 114.8 ab |
| LSP3 | 4.2 c | 30.5 c | 139.2 a | 1.40 a | 100.7 b |
| WP + T | 7.5 b | 51.0 b | 148.2 a | 1.22 ab | 123.1 ab |
| WP | 14.4 a | 87.7 a | 164.2 a | 1.17 b | 140.8 a |
| 2017 | |||||
| LSP1 | 7.43 ab | 62.5 b | 120.1 b | 1.46 a | 82.5 b |
| LSP2 | 5.07 b | 46.5 bc | 108.6 b | 1.44 a | 75.5 b |
| LSP3 | 5.10 b | 49.3 bc | 104.2 b | 1.29 a | 81.0 b |
| WP + T | 7.88 a | 44.1 c | 182.1 a | 1.66 a | 109.0 a |
| WP | 9.26 a | 84.2 a | 109.9 b | 1.51 a | 75.0 b |
| 2018 | |||||
| LSP1 | 7.53 a | 63 ab | 120.2 b | 1.43 ab | 84 ab |
| LSP2 | 6.28 ab | 55 bc | 116 b | 1.5 ab | 77 b |
| LSP3 | 4.73 b | 38 c | 123.2 b | 1.57 a | 79 b |
| WP + T | 7.76 a | 55 bc | 142.2 a | 1.34 ab | 110 a |
| WP | 7.23 ab | 76 a | 94.6 b | 1.26 b | 76 b |
| Treatments | Date of First Harvest | Date of Last Harvest | TSS (°Brix) | pH | Titratable Acidity (g L−1) | Absorbance Units (518 nm) |
|---|---|---|---|---|---|---|
| 2016 | ||||||
| LSP1 | 25 August (238) | - | 23.2 a | 3.51 bc | 5.6 a | 23.30 b |
| LSP2 | 25 August (238) | - | 22.7 a | 3.49 c | 5.8 a | 25.40 ab |
| LSP3 | 1 September (245) | - | 24.0 a | 3.65 ab | 5.5 a | 28.50 a |
| WP + T | 18 August (231) | - | 23.6 a | 3.68 a | 4.0 b | 17.35 c |
| WP | 1 September (245) | - | 22.4 a | 3.72 a | 3.8 b | 17.03 c |
| 2017 | ||||||
| LSP1 | 31 August (243) | 26 September (269) | 22.5 a | 3.45 b | 5.4 cb | 12.79 ab |
| LSP2 | 24 August (236) | 31 August (243) | 23.4 a | 3.50 b | 5.9 b | 15.12 a |
| LSP3 | - | 26 September (269) | 20.3 b | 3.40 b | 6.9 a | 14.42 ab |
| WP + T | 24 August (236) | 26 September (269) | 23.4 a | 3.7 a | 4.1 d | 13.91 ab |
| WP | - | 26 September (269) | 19.0 b | 3.46 a | 4.8 c | 9.24 b |
| 2018 | ||||||
| LSP1 | 22 August (234) | 29 August (241) | 23.14 ab | 3.54 a | - | - |
| LSP2 | 15 August (227) | 29 August (241) | 23.54 a | 3.54 a | - | - |
| LSP3 | 15 August (227) | 29 August (241) | 24.08 a | 3.60 a | - | - |
| WP + T | 22 August (234) | 5 September (248) | 22.94 ab | 3.58 a | - | - |
| WP | 22 August (234) | 5 September (248) | 21.50 b | 3.57 a | - | - |
| Treatment | 2016 | 2017 | 2018 |
|---|---|---|---|
| LSP1 | 87.3 | 86.5 a | 86.5 a |
| LSP2 | 87.5 | 84.8 ab | 85.3 ab |
| LSP3 | 86.0 | 85.8 ab | 87.0 a |
| WP + T | 84.2 | 84.0 bc | 85.2 ab |
| WP | 80.0 | 81.7 c | 84.3 b |
| Treatment | Yellow, AU | Red, AU | Blue, AU | CI, AU | CH | Total Phenolics, (as mg Gallic Acid/L) |
|---|---|---|---|---|---|---|
| 2016 | ||||||
| LSP1 | 5.82 | 8.96 | 2.41 | 17.9 | 0.65 | 1618 |
| LSP2 | 7.10 | 11.51 | 3.16 | 21.77 | 0.62 | 1749 |
| LSP3 | 7.71 | 12.71 | 3.54 | 23.96 | 0.61 | 1657 |
| WP + T | 4.50 | 6.41 | 1.84 | 12.75 | 0.70 | 973 |
| WP | 4.32 | 6.82 | 1.71 | 12.84 | 0.63 | 938 |
| 2017 | ||||||
| LSP1 | 2.58 ab | 3.67 ab | 0.83 bc | 7.08 bc | 0.70 a | 1265 ab |
| LSP2 | 3.47 a | 4.95 a | 1.58 a | 10.01 a | 0.71 a | 1419 ab |
| LSP3 | 2.87 a | 3.95 ab | 0.97 b | 7.78 ab | 0.72 a | 1536 a |
| WP + T | 2.88 a | 4.43 a | 1.01 b | 8.33 ab | 0.65 a | 1109 bc |
| WP | 1.84 b | 2.67 b | 0.48 c | 4.99 c | 0.69 a | 797 c |
| 2018 | ||||||
| LSP1 | 3.79 a | 6.46 a | 1.46 ab | 11.72 ab | 0.59 a | 1290 a |
| LSP2 | 4.07 a | 7.04 a | 1.62 ab | 12.73 ab | 0.71 a | 1362 a |
| LSP3 | 4.31 a | 7.19 a | 1.80 a | 13.3 a | 0.72 a | 1436 a |
| WP + T | 3.61 a | 6.17 a | 1.41 ab | 11.19 ab | 0.65 a | 1321 a |
| WP | 3.32 b | 5.37 a | 1.19 b | 9.9 b | 0.69 a | 1249 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Netzer, Y.; Suued, Y.; Harel, M.; Ferman-Mintz, D.; Drori, E.; Munitz, S.; Stanevsky, M.; Grünzweig, J.M.; Fait, A.; Ohana-Levi, N.; et al. Forever Young? Late Shoot Pruning Affects Phenological Development, Physiology, Yield and Wine Quality of Vitis vinifera cv. Malbec. Agriculture 2022, 12, 605. https://doi.org/10.3390/agriculture12050605
Netzer Y, Suued Y, Harel M, Ferman-Mintz D, Drori E, Munitz S, Stanevsky M, Grünzweig JM, Fait A, Ohana-Levi N, et al. Forever Young? Late Shoot Pruning Affects Phenological Development, Physiology, Yield and Wine Quality of Vitis vinifera cv. Malbec. Agriculture. 2022; 12(5):605. https://doi.org/10.3390/agriculture12050605
Chicago/Turabian StyleNetzer, Yishai, Yedidya Suued, Matanya Harel, Danielle Ferman-Mintz, Elyashiv Drori, Sarel Munitz, Maria Stanevsky, José M. Grünzweig, Aaron Fait, Noa Ohana-Levi, and et al. 2022. "Forever Young? Late Shoot Pruning Affects Phenological Development, Physiology, Yield and Wine Quality of Vitis vinifera cv. Malbec" Agriculture 12, no. 5: 605. https://doi.org/10.3390/agriculture12050605
APA StyleNetzer, Y., Suued, Y., Harel, M., Ferman-Mintz, D., Drori, E., Munitz, S., Stanevsky, M., Grünzweig, J. M., Fait, A., Ohana-Levi, N., Nir, G., & Harari, G. (2022). Forever Young? Late Shoot Pruning Affects Phenological Development, Physiology, Yield and Wine Quality of Vitis vinifera cv. Malbec. Agriculture, 12(5), 605. https://doi.org/10.3390/agriculture12050605






