Abstract
Biodegradable liquid film (BLF) improves soil structure and increases plant freezing tolerance after spraying on the surface of soil and plant. In this study, the effects of BLF on grape composition and volatile compounds in Cabernet sauvignon (Vitis vinifera L.) grapes were determined by spraying BLF during the dormant periods over three years. The aim of the study was to evaluate the potential impact of BLF as an overwintering protection measure on grape fruit quality. In 2020 and 2021, BLF spraying increased reducing sugar content and 100-berry weight, decreased titratable acid content, and improved the maturity factor. Compared with the vines not sprayed with BLF, the content of total phenols and total anthocyanins in grape skins showed an increase over the three-year period, with the largest increases of 31.92% and 48.38%, respectively, and the content of total tannins and total flavan-3-ols increased in 2020 and 2021. BLF treatment also increased the total phenolic content in seeds for all three years, reaching a significant level in 2019, 16.38% higher than control treatment (CK). HPLC analysis showed that BLF treatment affected the content and composition of monomeric anthocyanins in grape skins, especially in 2021, BLF treatment significantly increased the content of nine monomeric anthocyanins, and the proportion of acetylated and coumaroylated anthocyanins. However, GC-MS analysis indicated that BLF had little effect on volatile compounds. These results suggest that BLF can be used as an overwintering protection measure in cold regions to promote the accumulation of sugars and polyphenolics, thereby improving overall grape quality.
1. Introduction
As one of the most important abiotic stresses, low temperature has many adverse effects on plant growth and development, including reduced yield and quality, growth retardation, tissue damage, and increased production costs, especially for perennial fruit trees such as wine grapes [1,2,3]. Most commercial grape varieties are sensitive to cold and are at risk of dying in areas where the minimum temperatures are below −15 °C [4,5]. Numerous strategies have been proposed to allow grape plants to safely overwinter, such as cross-breeding [6], agronomic practices [7,8], chemical treatments [9], and genetic engineering [10,11] The most widely used production practice is material mulching [12,13,14], especially traditional the soil-burial of vines. The burial of vines can effectively protect grapevines from the effects of freezing temperatures, but this practice increases labor intensity and costs, can cause cane damage and disease, restricts mechanized production, and can even damage the ecological environment [15,16,17]. Therefore, there is significant interest in the use of simple, mechanized, and environmentally friendly materials to protect vines during winter [18].
The biodegradable liquid film (BLF) used is a kind of humic acid degradable liquid film, which is mainly made of emulsified asphalt for agriculture, humic acid macromolecular substances, plus suspending agent, etc. This liquid emulsion was originally developed as an environmentally friendly soil structure conditioner. After being sprayed on the soil surface, its strong adhesion ability can connect soil particles to form aggregates, adjust soil structure and physical and chemical properties, and can also reducing soil wind erosion, which is beneficial to ecological environment protection [16,19,20]. Xue et al. found that BLF can be used as exogenous antifreeze for plants. After spraying BLF on the surface of vines, it solidified to form a film, which improved the survival rate of many varieties in winter without the need for soil burial [21]. Whether using BLF as overwintering protection material will affect the quality of grape berries aroused our interest. After all, the ultimate purpose of growing wine grapes is to obtain high-quality raw materials to produce wine; it should have enough sugar to produce alcohol, suitable acid to balance the flavor, sufficient polyphenolics to bring color and structure, and appropriate levels of pleasant aroma compounds [22,23,24]. However, there are fewer studies on the potential effects of using BLF as an overwintering protection material on grape berry quality. Xue et al. studied the effects of BLF on reducing sugar, titrated acid and polyphenols in grape fruit compared with soil burial [21]. In this study, naked overwintering was selected as the control, and the effects of BLF on aroma compounds and monomeric anthocyanins were further analyzed.
Compared with brewing, the raw materials used in wine-making contribute more to product quality, and the maturity and metabolites composition of wine grapes are important determinants of quality [23]. Maturity is generally expressed by the ratio of the sugar content to the acid content in grapes. Among the metabolites of grapes, polyphenols have received the most attention, with two main categories: flavonoids and non-flavonoids. Flavonoids include anthocyanins and their derivatives and flavanols, and non-flavonoids includes phenolic acids, stilbene, and other low-content phenolic substances [25]. These phenolic compounds are important contributors to the color and taste of grape and wine [26]. Different concentrations and combinations of volatiles impart different aromas to wine. The volatile characteristics of wine are not only related to the fermentation process, but also to the grapes [27,28], with multiple aspects of viticulture affecting the aroma potential of grapes and wines [24,29].
This study aimed to evaluate the effect of using BLF on the quality of wine grape cultivar ‘Cabernet sauvignon’ as an alternative overwintering measure for cold regions. Quality was assessed by measuring general composition and major secondary metabolites of grapes, including polyphenols and volatile compounds.
2. Materials and Methods
2.1. Plant Material and Field Trial
This study was conducted on 10-year-old ‘Vitis vinifera L. Cabernet sauvignon’ grapevines grown in a commercial vineyard in Xia County, Shanxi Province (lat. 35°24′ N, long. 111°17′ E, alt. 433 m), China. The monthly precipitation, peak sunshine hours, and average temperature data in Xia County are shown in Figure 1.
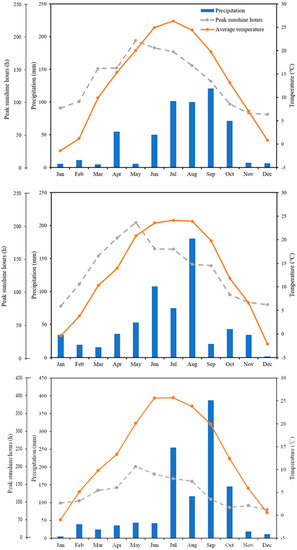
Figure 1.
Monthly precipitation, peak sunshine hours, and average temperature during 2019, 2020, and 2021.
Grapevines grown at this experimental site are at risk of low temperature damage in winter. Here, a biodegradable liquid film (BLF) was used as a protection measure for overwintering, and control plants (CK) were overwintered without treatment. The BLF is brown and creamy and forms a thin, brown, multi-molecular chemical protection film that wraps and encloses the branch surface. The amount of BLF sprayed per hectare was 150 kg, which was diluted at the ratio of BLF:water = 1:3 (v/v) when used, and a gasoline sprayer was used for spraying. The application of BLF protects the branches in winter and gradually degrades over 70–90 days [21].
Vine rows were oriented west–east with 1.0 × 2.7 m2 row vine spacing. Vines were trained using a crawled cordon training system. The vines were divided into two treatment groups: no treatment overwintering control (CK) and BLF-sprayed plants. In winter, BLF was sprayed on the trunk of the treatment group (BLF) and on the shoots that were to retain buds the following year, as well as on the ground within the row (Figure 2). The control group (CK) was not treated and overwintered naked. There is no difference in other cultivation management. A completely randomized design was adopted, with three replications, and 24 grapevine plants per experimental unit.

Figure 2.
Biodegradable liquid film (BLF) spraying treatment: spraying operation (a) and three months after spraying (b).
2.2. Investigation of Fruit Setting
During the inflorescence revealing period in 2021, 10 normally fruiting vines were randomly selected and the number of fruiting branches and the total number of annual branches were counted. The fruiting branch rate was calculated according to the following Formula (1):
Fruiting branch rate (%) = number of fruiting branches/total number of annual branches × 100%
In the fruit expansion period in 2021, 10 normal fruiting vines were randomly selected and the number of clusters on each fruiting branch was counted. The fruiting coefficient was calculated according to the following Formula (2):
Fruiting coefficient = total number of clusters/number of fruiting branches
2.3. Sample Collection and Analysis of the General Index
Trial treatments were conducted in the winters of 2018, 2019, and 2020 and grape samples were collected in autumn in 2019, 2020, and 2021. To obtain a representative sample, for each sampling, 300 grapes were randomly selected from each sample block. Grapevines berries were taken randomly from different parts of grapevines and different parts of clusters and analyzed during harvest. The collected grape samples were brought back to the laboratory with ice and divided into two parts, one part was used for the determination of general index on the day of harvest, and the other part was frozen in a −80 °C cryogenic freezer for the determination of other indicators.
On the day of harvest, a total of 100 grapes were randomly collected and the weight was measured. The grapes were manually crushed to obtain must, and the supernatant of the must was used to measure the total soluble solids (TSS), reducing sugar (RS), and titratable acid (TA). The TSS were measured using a pocket refractometer, and the RS and TA of the must samples were analyzed according to GB/T 150382006. These and all parameters were analyzed in triplicate.
2.4. Polyphenols Content Analysis
Polyphenols were extracted from the skins and seeds of grapes according to the method previously described by Xue et al. with some modifications [21]. Briefly, 100 grapes from those stored at −80 °C were randomly selected, the skins were peeled, and the seeds were removed. Liquid nitrogen was added to the separated skins and seeds, and then the material was ground in a mortar to a fine powder. The powder was put in a freeze dryer for 24 h, and then phenolic substances in the powder were extracted with methanol-HCl (60% methanol, 0.1% HCl) solution at a solvent-to-sample ratio of 20:1. After treatment for 30 min at 30 °C and 40 W in an ultrasonic irradiator, the liquid extract was separated from the solid material by centrifugation at 10,000 rpm for 10 min. The supernatant was collected into glass vials, and the above extraction steps were repeated three times before samples were stored in a −20 °C freezer. The above operations were performed with protection from light.
The total tannin content of the samples was determined by methyl cellulose precipitation [30]. An appropriate amount of extract (0.25 mL of grape skin extract, 0.1 mL of grape seed extract) was added to the centrifuge tube, 3 mL of 0.04% methylcellulose solution was added to the sample group, and 3 mL of distilled water was added to the control group. After mixing, the tubes were left to stand for 2–3 min. Next, 2 mL of saturated ammonium sulfate solution was added to each reaction tube, distilled water was used to make up to 10 mL, and all reagents were mixed. After 10 min of reaction at room temperature, the supernatant was separated by centrifugation at 1800× g for 5 min, and the absorbance was measured at 280 nm. The absorbance value of tannin in the sample is obtained by subtracting the value of the control tube and the sample tube, and the total tannin content is expressed as (+)-catechin equivalent.
The total flavan-3-ol content of the samples was determined using a p-DMACA-HCl method [31]. A total of 1 mL of grape skin extract was mixed with 3 mL of distilled water (or 1 mL of grape seed extract and 9 mL of distilled water) in the reaction tube. In total, 0.1 mL of the mixed solution was added to a 10 mL glass test tube, and then 3 mL of 0.1% p-DMACA in 1.0 mol/L hydrochloric acid methanol solution was added. After fully shaking and mixing, the reaction was performed at room temperature for 10 min, and the absorbance was measured at 640 nm, and each treatment was repeated three times. The total flavan-3-ol content was expressed as (+)-catechin equivalent.
The total flavonoid content of the samples was determined by spectrophotometric method [32]. A total of 0.1 mL of grape skin extract and 0.9 mL of methanol (or 0.05 mL of grape seed extract and 0.95 mL of methanol) were mixed in the reaction tube, and then 2.7 mL of 30% methanol was added. Next, 0.2 mL of 0.5 mol/L sodium nitrite solution was added to the tubes, then 0.2 mL of 0.3 mol/L aluminum chloride solution was added, which was shaken well and let stand for 5 min, after which 1 mL of 1 mol/L sodium hydroxide solution was added. The solution was mixed well after each addition of reagent. Absorbance was measured at 510 nm. Each treatment was repeated three times. The total flavonoid content was expressed as rutin equivalent.
The total phenol content in grapes was determined using a modified Folin– Ciocalteu colorimetric method [33]. First, 0.05 mL of the grape skin or grape seed extract, 5 mL of distilled water, and 0.5 mL of Folin-Schorca reagent were mixed in the reaction tube. After 2 min, 1.5 mL of saturated sodium carbonate solution was added, and finally, 2.95 mL of distilled water was added. The solution was mixed well after each addition of reagent. After 2 h of reaction in the dark, colorimetry was performed at a wavelength of 765 nm to measure the absorbance, and each treatment was repeated three times. The total phenol content was expressed as gallic acid equivalent.
The total anthocyanin content in grape skins was determined using the AOAC pH differential method [34]. First, 0.25 mL of grape skin extract was taken and diluted 20 times with pH 1.0 hydrochloric acid-sodium chloride buffer and pH 4.5 acetic acid-sodium acetate buffer. The absorbance of these two dilutions was then measured at 510 nm and 700 nm, respectively. The final absorbance (A) was calculated by Formula (3):
A = (A510 nm − A700 nm) pH 1.0 − (A510 nm − A700 nm) pH 4.5
The total anthocyanin content in grape skin was expressed as malvidin 3-O-glucoside (mg/g) and calculated by Formula (4):
where MW is the relative molecular weight of malvidin 3-O-glucoside (493.5), DF is the dilution factor, ε is the molar absorption coefficient (28,000), Ve is the total volume of the extract, and M is the sampling mass of grape peel dry powder.
Total anthocyanin content in peel/W (mg/g) = (A × MW × DF × Ve × 1000)/(ε × 1 × M)
Phenolic substances were detected by a spectrophotometer (Cary 60 UV-Vis; Agilent Technologies, Santa Clara, CA, USA).
2.5. HPLC Analysis of Monomeric Anthocyanins
The monomeric anthocyanins were determined by high performance liquid chromatography (HPLC-DAD) refer to He et al. [35]. In total, 0.5 g of grape skin dry powder (the preparation method is the same as Section 2.4) was taken into a centrifuge tube and 10 mL of methanol solution containing 2% formic acid was added, extracted by ultrasonic for 10 min in the dark (temperature is 30 °C, power is 40 kHz), and shaken (150 rpm) in the dark for 30 min. The supernatant was separated by centrifugation at 8000 rpm for 10 min and collected in a 50 mL centrifuge tube, and the above extraction steps were repeated three times. The supernatants were combined and passed through a 0.45 μm organic filter for HPLC analysis. Analysis was performed using a Shimadzu LC-20AT coupled with a photodiode array detector (Shimadzu, Japan) and a Synergi Hydro-RPC18 (4.6 × 250 mm2, 4 μm) chromatographic column. The chromatographic conditions were set as follows: mobile phase A was formic acid:acetonitrile:water = 32:4:1 (v/v/v); mobile phase B was formic acid:acetonitrile:water = 16:20:1 (v/v/v). The elution program was programmed as: ① 0~45 min, 0~35% B (mobile phase B); ② 45~46 min, 35~100% B; ③ 46~50 min, 100% B; ④ 50~51 min, 100~0% B; ⑤ 51~55 min, 0% B. The flow rate was 1 mL·min−1, the column temperature was 30 °C, the detection wavelength was 520 nm, and the injection volume was 20 μL. Quantitative determination was carried out by comparison with an external standard method of malvidin-3-Oglucoside [36].
2.6. HS-SPME-GC-MS Analysis of Volatile Compounds
The volatile compounds were analyzed using headspace–solid phase micro extraction–gas chromatography with a mass spectrometry (HS-SPME-GC-MS) [37].
Randomly selected whole grape samples (~100 g) stored at −80 °C were ground in a mortar under liquid nitrogen to a fine power. The seeds were removed before grinding. PVP (Polyvinylpyrrolidone) was added to the obtained powder to avoid polyphenol interference with the extraction of volatile compounds The mixture was allowed to macerate in the dark for 2.5 h at 25 °C and then centrifuged at 10,000 rpm for 20 min. The supernatant was used for volatile analysis. Before SPME-GC-MS analysis, 5 mL of extracted juice and 1.5 g NaCl were added to each SPME vial containing 10 μL of the internal standard solution (0.230 g/L 2-Octanol).
SPME-GC-MS analysis was performed using a GCMS-QP2010 Ultra (Shimadzu, Kyoto, Japan). Compounds were separated using a DB-Wax column (30 m × 0.25 mm inner diameter, 0.25 μm film thickness, Agilent, Palo Alto, CA, USA). The oven temperature was programmed to hold at 40 °C for 4 min, increase to 120 °C at a rate of 4 °C/min, then increase to 240 °C at a rate of 6 °C/min, and hold at 240 °C for 11 min. A constant helium flow of 17 mL/min was used. A column splitter was used at the end of the column, 1 mL/min column flow was introduced to the MS, while the other 1.5 mL/min was vented out. The ion source temperature was 230 °C. Electron impact mass spectrometric data from m/z 35–350 were collected, with an ionization voltage of 70 eV.
The volatile compounds were qualitatively analyzed according to the NIST 14 mass spectrometry database in the GC-MS analysis software (GCMS solution version 4.30). The relative concentrations of volatile compounds in the samples were calculated based on the amount of 2-octanol as the internal standard.
2.7. Instruments and Reagents
Main instruments: −80 °C cryogenic freezer (Meling DW-HL6785, Xi’an, China), pocket refractometer (PAL-1), freeze dryer (FD-1C-50, Xi’an, China), centrifugation (Eppendorf AG 22331, Hamburg, Germany), ultrasonic irradiator (KQ-250DE, Xi’an, China), spectrophotometer (Cary 60 UV-Vis; Agilent Technologies, CA, USA), HPLC (Shimadzu LC-20AT, Kyoto, Japan), GCMS-QP2010 Ultra (Shimadzu, Kyoto, Japan), and DB-Wax column (30 m × 0.25 mm inner diameter, 0.25 μm film thickness, Agilent, Palo Alto, CA, USA).
Reagents: Standards (gallic acid, catechin, 2-octanol, rutin, delphinidin-3-O-glucoside, cyanidin-3-O-glucoside, petunidin-3-O-glucoside, peonidin-3-O-glucoside, malavidin -3-O-glucoside, peonidin-3-O-(6-O-acetyl)-glucoside, malavidin-3-O-(6-O-acetyl)-glucoside, trans-Peonidin-3-O-(6-O-p-coumaryl)-glucoside, and trans-Malavidin-3-O-(6-O-p-coumaryl)-glucoside) were purchased from Sigma-Aldrich (Sigma-aldrich Shanghai Trading Co., Ltd., Shanghai, China). Methanol, sodium carbonate, ammonium sulfate, sodium hydroxide, potassium chloride, sodium acetate, sodium nitrite, and aluminum chloride were purchased from Jinhuada Company (Guangzhou Jinhuada Chemical Reagent Co., Ltd., Guangzhou, China). Folin–Ciocalteu reagent, methylcellulose, PVP, and p-DMACA were purchased from Solarbio (Beijing solarbio science & technology Co., Ltd., Beijing, China). Concentrated hydrochloric acid was purchased from Luoyang Chemical Reagent Factory (Luoyang, China). Formic acid and acetonitrile were purchased from Aladdin (Shanghai Aladdin Biochemical Technology Co., Ltd., Shanghai, China). Plant antifreeze liquid film (BLF used in the study) was purchased from Mingrui (Mingrui Ecological Technology Co., Ltd., Yangling, China).
2.8. Statistical Analysis
Statistical data processing was performed using the software SPSS 20.0 (IBM, Armonk, NY, USA). Statistical analyses of the data were performed using one-way analysis of variance (ANOVA) and Duncan’s test with significance at the p < 0.05 level. Principle component analysis (PCA) plots were prepared using Origin 2016 (OriginLab Corporation, Northampton, MA, USA). The Pearson’s coefficient of correlation analysis was determined between the levels of each indicator, correlation significance was defined at the 0.05 level. The graph was created by the genescloud tools, a free online platform for data analysis (https://www.genescloud.cn, accessed on 10 March 2022).
3. Results
3.1. General Composition of Grapes
The trends of the indicators were basically the same in 2020 and 2021 (Table 1). Among grapes harvested on the same date, the BLF-treated grapes showed higher RS and lower TA than the non-treated grapes, with higher M. The opposite was true in 2019. The TSS was higher with BLF treatment than that of CK in 2021, but this difference was not significant. In both 2020 and 2021, the BLF treatment increased the 100-berry weight, but no increase was seen in 2019.

Table 1.
General composition of ‘Cabernet sauvignon’ grapes from the two treatments (BLF, sprayed with biodegradable liquid film; CK, not treated) in 2019, 2020, and 2021.
3.2. Polyphenols of Grape Skins and Seeds
In all three years, BLF treatment significantly increased the total phenolic content in grape skins, with total phenol levels in 2019, 2020, and 2021 that were 12.22%, 1.50%, and 31.92% higher than those of CK, respectively (Table 2). Similarly, BLF treatment increased the total anthocyanin content of grape skins in the three years, with significant increases in 2019 and 2021 of 48.38% and 12.76%, respectively. However, the trends of total skin tannins, total flavonoids, and total flavan-3-ols contents were not consistent among the three vintages. BLF treatment increased the total tannin content of grape skins in both 2020 and 2021, but tannin content was decreased in 2019. BLF significantly reduced total flavonoids in grape skins in 2020, but there was no significant effect in the other two years. BLF treatment increased total flavan-3-ols in grape skins in 2020 and 2021, but levels were decreased in 2019. Additionally, as shown in Table 2 and Table 3 the levels of phenolic substances fluctuated greatly in the different years.

Table 2.
Concentrations (mg/g, mean ± standard deviation) of polyphenols in ‘Cabernet sauvignon’ grape skins from BLF and CK treatments in 2019, 2020, and 2021.

Table 3.
Concentrations (mg/g, mean ± standard deviation) of polyphenols in Cabernet sauvignon grape seeds from BLF and CK treatments in 2019, 2020, and 2021.
Similar to the results for grape skins, the trends of phenolic content in seeds from the three vintages were not completely consistent between the two treatments (Table 3). BLF treatment increased the total phenolic content in seeds for all three years, reaching a significant level in 2019, 16.38% higher than CK. BLF treatments significantly increased the levels of total tannins in seeds in 2019 and 2020, but levels were decreased in 2021. Except for 2020, the trend of total flavan-3-ol content in seeds between the two treatments was exactly the same as that of tannins. There was no clear trend in the content of total flavonoids in seeds between the two treatments for the three years.
3.3. Pearson Correlation Analysis
The fruiting branch rates of the BLF and CK treated vines were 95% and 97%, respectively, and the fruiting coefficients were 1.92 and 2.22, respectively (data not shown in the figures and tables). Pearson correlation analysis was used to calculate the correlations of general composition and the amounts of polyphenols of the grapes with fruiting branch rate and fruiting coefficient (Figure 3). The fruiting branch rate was significantly negatively correlated with the total phenol content of seeds, but was not significantly correlated with other indexes. The fruiting coefficient did exhibit strong influence, with a significant negative correlation with 100-berry weight, reducing sugar content, and maturity factor, and a significant positive correlation with titratable acid content, indicating that the fruiting coefficient significantly affects fruit maturation. The fruiting coefficient also exhibited significant negative correlation with the contents of polyphenols in the grape skin, but not in the seeds.
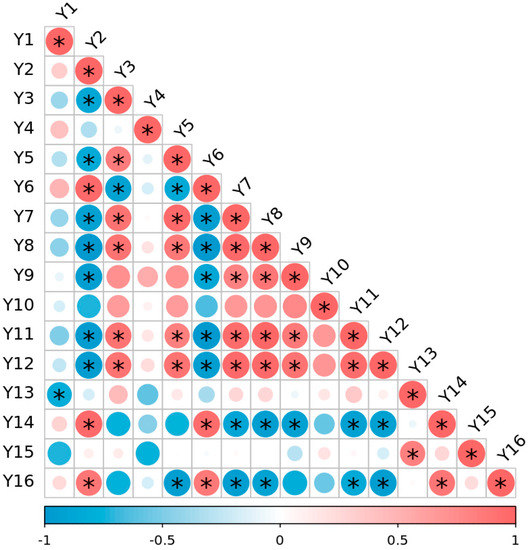
Figure 3.
Pearson correlation analysis of general composition of grapes, polyphenols, and fruiting status in 2021. Note: Y1: fruiting branch rate; Y2: fruiting coefficient; Y3: 100-berry weight; Y4: total soluble solid (TSS); Y5: reducing sugar (RS); Y6: titratable acid (TA); Y7: maturity factor; Y8: total phenol of skin; Y9: total tannin of skin; Y10: total flavonoid of skin; Y11: total flavan-3-ol of skin; Y12: total anthocyanin of skin; Y13: total phenol of seed; Y14: total tannin of seed; Y15: total flavonoid of seed; Y16: total flavan-3-ol of seed. ‘*’ indicates a significant correlation between the two factors at the 0.05 level. Red indicates a positive correlation between the two indicators, and blue indicates a negative correlation. ‘−1~1’ indicates the value of the correlation coefficient. The size of the circle represents the absolute value of the correlation coefficient; the larger the absolute value, the larger the circle and the darker the color.
3.4. Monomeric Anthocyanins in Grape Skins
The major flavonoids synthesized in the grapevine berry, anthocyanins and tannins (also known as proanthocyanidins), strongly impact the quality of red wines by affecting wine color and astringency [38,39]. The content of total anthocyanins in peel was determined by pH method, revealing significantly higher content in BLF-treated grape skins than that of CK (Table 2). To probe this more quantitatively, HPLC analysis was used to more accurately analyze the content of monomeric anthocyanins (Table 4).

Table 4.
Concentrations of monomeric anthocyanins in ‘Cabernet sauvignon’ grape skins from BLF and CK treatments in 2020 and 2021.
In 2020, the contents of cyanidin-3-O-glucoside and peonidin-3-O-glucoside were significantly higher in BLF-treated grape skins than those in CK, but there was no significant difference in the contents of the other monomeric anthocyanins or in the total number of anthocyanins. However, there was a significant difference between the two treatments in 2021. The levels of nine monomeric anthocyanins in BLF-treated grape skins were significantly higher than those in CK in 2021, and the total number of anthocyanins was also significantly higher than that in CK, with an increase of 18.42%. This is consistent with the previous analysis (Table 2). Among the nine monomeric anthocyanins detected, delphinidin and its derivatives accounted for the vast majority of the total, reaching more than 80%. Further analysis of the acylation degree of each monomeric anthocyanin showed that BLF treatment significantly increased the ratio of acetylated anthocyanins and coumaroylated anthocyanins in 2021.
3.5. Principal Component Analysis of Monomeric Anthocyanins
A 4 × 9 original data matrix was constructed for the content of each monomer anthocyanin in grape peel, and principal component analysis was performed (Origin 2016). Two principal components were extracted, and the cumulative contribution rate reached 96.2% (Figure 4). The results showed greater differences between grapes grown in different years than between grapes treated or not treated with BLF, with inconsistent results between the two sample years. The observed differences in grape anthocyanins may reflect differences in weather between the two sample years, with differences in temperature, light, precipitation, or other factors that may have contributed to differences in wines made from these grapes [40]. Thus, the effect of BLF on grape skin anthocyanins may vary from year to year.
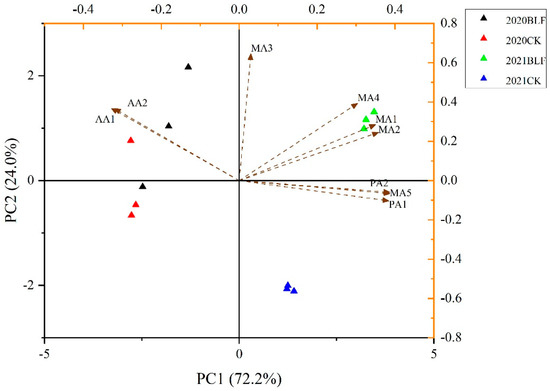
Figure 4.
Principal component analysis of anthocyanins in ‘Cabernet sauvignon’ grape skins from BLF and CK treatments in 2020 and 2021. MA1: Delphinidin-3-O-glucoside; MA2: cyanidin-3-O-glucoside; MA3: Petunidin-3-O-glucoside; MA4: Peonidin-3-O-glucoside; MA5: Malavidin-3-O-glucoside; AA1: Peonidin-3-O-(6-O-acetyl)-glucoside; AA2: Malavidin-3-O-(6-O-acetyl)-glucoside; PA1: Trans-Peonidin-3-O-(6-O-p-coumaryl)-glucoside; PA2: Trans-Malavidin-3-O-(6-O-p-coumaryl)-glucoside.
According to the eigenvalues, the scores S1 and S2 and the comprehensive score S were calculated for the two principal components. The higher the comprehensive score S, the better the anthocyanin quality of the grapes. The formula is as follows:
S1 = 0.350 × X1 + 0.357 × X2 + 0.030 × X3 + 0.304 × X4 + 0.387 × X5 − 0.328 × X6 − 0.320 × X7 + 0.384 × X8 + 0.386 × X9
S2 = 0.285 × X1 + 0.245 × X2 + 0.646 × X3 + 0.393 × X4 − 0.066 × X5 + 0.367 × X6 + 0.367 × X7 − 0.103 × X8 − 0.059 × X9
S = 0.722 × S1 + 0.240 × S2
The results showed that there was no significant difference in the scores of principal component 1 (S1) and principal component 2 (S2) and the comprehensive score (S) of the two treatments in 2020, but the scores of the BLF treatment were significantly higher than those of the CK in 2021. Overall, the results indicate that the anthocyanin quality of the 2021 grapes was generally better than that of the 2020 grapes (Figure 5).
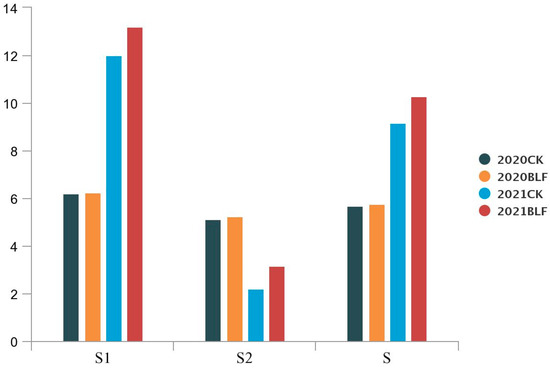
Figure 5.
Principal components for anthocyanins in ‘Cabernet sauvignon’ grape skins from BLF and CK treatments in 2020 and 2021. S1, Score of component 1; S2, Score of component 2; S, Synthetic score.
3.6. Volatile Compounds of Berries
The content of volatile compounds was determined by GC-MS instrument in 2019 grapes subjected to the two treatments (Table 5). A total of thirty-eight kinds of volatile compounds were detected, including nine kinds of alcohols, eleven kinds of aldehydes and ketones, three kinds of acids, four kinds of esters, and eleven other substances, with mainly floral, fruity, and green odors. The content of volatile compounds that mainly contribute to the aroma characteristics of grape berries has been listed in Table 5.

Table 5.
Relative concentrations of volatile compounds in ‘Cabernet sauvignon’ grapes from BLF and CK treatments in 2019.
The contents of aldehydes and ketones in grapes accounted for the largest proportion of the total volatile compounds, accounting for 63.99% and 60.72% of the total in CK and BLF treatments, respectively. The most abundant aldehydes detected are hexanal and (E)-2-hexenal, which are present at far greater levels than other volatile compounds and provide green, grass, and apple aromas. However, the two treatments showed no significant differences in the contents of the four aldehydes other than octanal and nonanal.
Many types of alcohols were detected. The content of (E)-2-hexen-1-ol and hexanol was higher in BLF-treated grapes than in CK, contributing aromas of green and grass. The opposite was true for 1-octen-3-ol, which confers a mushroom-like aroma, indicating that CK-treated grapes exhibited more mushroom aroma than BLF-treated grapes. Alcohols in grapes, especially saturated alcohols in the range of C7 to C10, may also contribute floral aroma [51].
Acetic and benzoic acid were detected, which may contribute to the unpleasant smell of vinegar and bitter almonds, but may help balance aromatic compounds. Esters are the most important odorants in wines, and impart abundant floral and tropical fruity aromas [52]. Methyl 2-methoxybenzoate and ethyl octanoate were the most abundant esters identified in the grape samples. Ethyl octanoate was detected in grapes treated with BLF, but not in CK-treated grapes, which may provide obvious fruity and pleasant floral aromas.
4. Discussion
Biodegradable liquid film (BLF) is a humic acid film originally developed as an environmentally friendly soil structure modifier. After being sprayed on the soil surface, BLF can quickly connect soil particles into agglomerates, which can improve soil structure, adjust soil physical and chemical properties, and promote crop growth and development [19,53,54]. In addition, the chelating effect of humic acid can significantly improve soil nutrients, and BLF mulching significantly increased total nitrogen, total phosphorus, and available potassium in vineyard soils [55]. Humic acid also significantly increases the number of soil microorganisms and microbial activities, as well as the activities of enzymes such as urease, phosphatase, invertase, and catalase [56].
4.1. BLF Influence on the General Composition of Grapes
Adjusting the load is an important way to regulate the balance between vegetative and reproductive growth [57]. Lower yield per unit area is typically good quality, while a higher yield provides lower quality [58]. Interestingly, despite the use of the same pruning strategy, the BLF-treated plants had lower fruiting coefficient than CK, implying lower per-plant loadings. The reducing sugar content of grapes from BLF-treated plants was also significantly higher than that of CK, while the titratable acid content exhibited the opposite trend (Table 1); both parameters were significantly correlated with the fruiting coefficients (Figure 3). Many previous studies have shown that low loading is beneficial to increase berry weight, sugar content, and sugar-acid ratio, and reduce acid content [59,60,61]. In addition, the growth-promoting effect of humic acid can also improve fruit quality [62]. Although the number of clusters per plant was reduced, there was not necessarily a large loss in yield due to the increase in the weight and proportion of high-quality fruit [61,62].
4.2. BLF Increased Phenolic Compounds, Especially Anthocyanin
Most of the phenolic substances in grapes are present in the skins and seeds. These compounds provide color, astringency, structure, and flavor balance to grapes and wines, play a key role in red wine quality, and also serve as the main nutrient providers, with antioxidant, anti-inflammatory, and metabolic disease curative properties [63]. Among them, flavonoids have attracted much attention and are often used to evaluate the quality and biological activity of grapes and wine [64]. In this study, BLF treatment increased the content of nearly all polyphenols in grape skins and increased the total phenol content in grape seeds (Table 2 and Table 3). This may be explained by the reduction of the fruiting coefficient (Figure 3), which increases the light exposure of the clusters and promotes grape ripening [59,61,65], resulting in higher content of skin polyphenols for BLF-treated plants. The effect of BLF to improve the soil likely also contributes to the observed changes in polyphenols. The use of BLF makes the soil structure looser and increases the air permeability. This is conducive to the growth of plants, and the increase in soil nitrogen content can affect the leaf curtain microenvironment of grapes [55], thus changing the levels of polyphenols [66,67,68,69].
Among several polyphenolic compounds that exhibited changes with treatment, we were particularly interested in the significant alterations in anthocyanins. BLF-treated plants had higher anthocyanin content than CK in all years, and further PCA analysis also indicated that BLF had a higher anthocyanin score (Figure 5). Sugar can stimulate the activity of enzymes related to anthocyanin biosynthesis to increase synthesis [70]. Thus, the high sugar content of BLF may stimulate anthocyanin synthesis (Figure 3). The stability of anthocyanins depends on their structure, and modifications of methoxylation, glycosylation, and acylation can increase the stability of anthocyanins [71]. The increased proportion of acetylated and coumaylated anthocyanins in BLF-treated grape skins increased the structural stability of anthocyanins, which results in better color stability of wine [72].
4.3. BLF Influence on Volatile Compounds
Aroma is one of the main determinants of the quality of wine. The volatile compounds in wine mainly come from the grapes, fermentation, and aging. The types of volatile compounds in wine grapes vary for different varieties, but the concentrations of specific volatile compounds are affected by the cultivation strategies [73]. Numerous studies have focused on best management practices from flowering to maturity, including fertilization [74], irrigation [29], crop covering [75], leaf removal [60], cluster and grape thinning [76], and time of harvest [28]. Relatively few studies have investigated the effects of management in other phenological stages [24,77]. Our study showed that the differences in overwintering protection measures had no significant effect on the total amount of volatile compounds (Table 5).
Different compounds in wine samples make different contributions to the overall aroma profile of wine [78]. C6 compounds accounted for the vast majority of volatile compounds detected in grapes, including Hexanol, (E)-2-Hexen-1-ol, (Z)-3-Hexen-1-ol, Hexanal, and (E)-2-Hexenal, consistent with the results of previous studies [42,79]. BLF treatment significantly increased the content of Hexanol and (E)-2-Hexen-1-ol, but not the other compounds, which may contribute more pungent and herbaceous notes to the aroma profile [42].
5. Conclusions
Overall, BLF treatment has a certain improvement effect on grape fruit quality. The basic physical and chemical indicators showed that BLF treatment increased the reducing sugar content, decreased the titratable acid content, improved the maturity coefficient, and increased the 100-berry weight in two of the three years. BLF treatment had the most significant effect on grape skin polyphenols, increasing the content of total phenol and total anthocyanin in grape skins in all tested years, with the largest increases of 31.92% and 48.38%, respectively. BLF treatment also increased the content of total tannin and total flavan-3-ol in grape skins in two of the three years. The effect of BLF treatment on grape seed polyphenols was mainly reflected in the increase of total phenolic content, while other grape seed polyphenols showed no obvious regularity. BLF specifically increased the content of total anthocyanins. Especially in 2021, BLF significantly increased the content of each anthocyanin component, and increased the proportion of acetylated and coumaylated anthocyanin. BLF had no significant effect on grape fruit volatile compounds and content. In conclusion, for a single vintage condition, the BLF-treated grapes were of higher quality than grapes from non-treated plants.
Author Contributions
All authors contributed significantly to this manuscript. This article was primarily organized by X.H., who participated in the entire process from experimental design to sample collection, indicators determination, data analysis, and article writing. F.Y., Y.W., X.D., T.X. and X.L. made contributions to the determination of indicators, and Z.W. and Y.L. made contributions to the experimental treatment and sample collection. H.W. and H.L. provided guidance on experimental design and revised the article. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ningxia Hui Nationality Autonomous Region Major Research and Development Project—Research and demonstration on key technology of wine style curing in the eastern foot of Helan Mountain in Ningxia (2020BCF01003) and Research and application of key technologies for sustainable development of wine industry (LYNJ202110).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to the two engineers Huaitang Zheng and Xinyi Dong of the Great Winery for their help and support in the field trials.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Alberdi, M.; Corcuera, L.J. Cold acclimation in plants. Phytochemistry 1991, 30, 3177–3184. [Google Scholar] [CrossRef]
- Pearce, R.S. Plant freezing and damage. Ann. Bot. 2001, 87, 417–424. [Google Scholar] [CrossRef]
- Snyder, R.L.; Melo-Abreu, J.P.D.; Matulich, S. Frost Protection: Fundamentals, Practice and Economics; Food and Agriculture Organization of the United Nations: Rome, Italy, 2005. [Google Scholar]
- Zhang, J.; Wu, X.; Niu, R.; Liu, Y.; Liu, N.; Xu, W.; Wang, Y. Cold-resistance evaluation in 25 wild grape species. Vitis 2012, 51, 153–160. [Google Scholar]
- Li, H.; Wang, H. Chinese Wine; NWSUAF Press: Xianyang, China, 2010. [Google Scholar]
- Wang, Z.-L.; Xue, T.-T.; Gao, F.-F.; Zhang, L.; Han, X.; Wang, Y.; Hui, M.; Wu, D.; Li, H.; Wang, H. Intraspecific recurrent selection in V. vinifera: An effective method for breeding of high quality, disease-, cold-, and drought-resistant grapes. Euphytica 2021, 217, 111. [Google Scholar] [CrossRef]
- Wolf, T.K. Crop yield effects on cold hardiness of ‘Cabernet Sauvignon’ dormant buds. Acta Hortic. 2004, 640, 177–187. [Google Scholar] [CrossRef]
- Kalkan, N.N.; Kaya, Ö.; Karadoğan, B.; Köse, C. Determination of cold damage and lipid peroxidation levels of Karaerik (Vitis vinifera L.) grape cultivar having different trunk height in winter buds. Alınteri J. Agric. Sci. 2017, 32, 2017–2028. [Google Scholar] [CrossRef]
- Habibi, G. Effects of soil- and foliar-applied silicon on the resistance of grapevine plants to freezing stress. Acta Biol. Szeged. 2015, 59, 109–117. [Google Scholar]
- Xiao, H.; Siddiqua, M.; Braybrook, S.; Nassuth, A. Three grape CBF/DREB1 genes respond to low temperature, drought and abscisic acid. Plant Cell Environ. 2006, 29, 1410–1421. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Wang, Z.-X.; Yang, Y.-M.; Liu, H.-S.; Shi, G.-L.; Ai, J. Analysis of the cold tolerance and physiological response differences of amur grape (Vitis amurensis) germplasms during overwintering. Sci. Hortic. 2020, 259, 108760. [Google Scholar] [CrossRef]
- Jolivet, Y.; Dubois, J.M.M.; Granberg, H. Évaluation du régime thermique du cépage Vitis vinifera Muscadet Melon durant la saison froide au Québec. J. Int. Sci. Vigne Vin 1998, 32, 51–58. [Google Scholar]
- Khanizadeh, S.; Rekika, D.; Levasseur, A.; Groleau, Y.; Richer, C.; Fisher, H. The effects of different cultural and environmental factors on grapevine growth, winter hardiness and performance, in three locations, in Canada. Small Fruits Rev. 2005, 4, 3–28. [Google Scholar] [CrossRef]
- Gao, M.Y.; Han, Y.; Zhao, W.; Ji, W. Effect of different cold protection measures on overwintering ability of grapevine. North. Hortic. 2019, 7, 17–26. [Google Scholar]
- Wang, S. Effect of Shoots Windbreak on Vineyard Ecotope in the Soil-Buried Cold-Proof Period; Northwest A&F University: Xianyang, China, 2015. [Google Scholar]
- Xue, T.T.; Han, X.; Zhang, H.J.; Li, H. Wind erosion prevention effect of different overwintering treatments in viticulture regions based on wind tunnel test. J. Sediment. Res. 2018, 43, 58–64. [Google Scholar] [CrossRef]
- Han, X.; Xue, T.; Liu, X.; Wang, Z.; Zhang, L.; Wang, Y.; Yao, F.; Wang, H.; Li, H. A sustainable viticulture method adapted to the cold climate zone in China. Horticulturae 2021, 7, 150. [Google Scholar] [CrossRef]
- Li, P.C.; Guo, S.J.; Li, M.; Su, X.D.; Wang, Q. Effects of different insulation materials for covering soil on Gobi ground grapevines over-wintering. Hubei Agric. Sci. 2014, 53, 2838–2840. [Google Scholar] [CrossRef]
- Wang, H.T.; Ma, X.F.; Zhang, J.Z. The application of humic acid in soil and fertilizer. Heilongjiang Sci. 2010, 1, 59–62. [Google Scholar]
- Wang, X.; Xu, G.B.; Ren, Z.G.; Zhang, Z.J.; Jian, Y.F.; Zhang, Y.M. Effects of environment-friendly degradable films on corn growth and soil environment. Chin. J. Eco-Agric. 2007, 15, 78–81. [Google Scholar]
- Xue, T.; Han, X.; Zhang, H.; Wang, Y.; Wang, H.; Li, H. Effects of a biodegradable liquid film on winter chill protection of winegrape cultivars. Sci. Hortic. 2019, 246, 398–406. [Google Scholar] [CrossRef]
- Li, Z.; Pan, Q.; Jin, Z.; Mu, L.; Duan, C. Comparison on phenolic compounds in Vitis vinifera cv. Cabernet Sauvignon wines from five wine-growing regions in China. Food Chem. 2011, 125, 77–83. [Google Scholar] [CrossRef]
- Kontoudakis, N.; Esteruelas, M.; Fort, F.; Canals, J.-M.; De Freitas, V.; Zamora, F. Influence of the heterogeneity of grape phenolic maturity on wine composition and quality. Food Chem. 2011, 124, 767–774. [Google Scholar] [CrossRef]
- Nan, L.J.; Liu, L.Y.; Zhao, X.H.; Qiu, S.; Wang, H.; Li, H. Effect of alternative new pruning system and harvesting times on aroma compounds of young wines from Ecolly (Vitis vinifera) in a new grape growing region of the Weibei plateau in China. Sci. Hortic. 2013, 162, 181–187. [Google Scholar] [CrossRef] [Green Version]
- Cheng, C.F.; Qi, M.Y.; Shi, Y.; Zhang, X.K.; Zhao, X. Phenolics in wines I: A review of categories, structures and detection methods. Food Sci. 2019, 40, 255–268. [Google Scholar] [CrossRef]
- Seddon, T.J.; Downey, M.O. Comparison of analytical methods for the determination of condensed tannins in grape skin. Aust. J. Grape Wine Res. 2008, 14, 54–61. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef]
- Zhao, T.; Wu, J.; Meng, J.; Shi, P.; Fang, Y.; Zhang, Z.; Sun, X. Harvesting at the right time: Maturity and its effects on the aromatic characteristics of Cabernet Sauvignon wine. Molecules 2019, 24, 2777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Shellie, K.C.; Wang, H.; Qian, M.C. Influence of deficit irrigation and kaolin particle film on grape composition and volatile compounds in Merlot grape (Vitis vinifera L.). Food Chem. 2012, 134, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Sarneckis, C.J.; Dambergs, R.G.; Jones, P.; Mercurio, M.; Herderich, M.J.; Smith, P.A. Quantification of condensed tannins by precipitation with methyl cellulose: Development and validation of an optimised tool for grape and wine analysis. Aust. J. Grape Wine Res. 2006, 12, 39–49. [Google Scholar] [CrossRef]
- Li, Y.-G.; Tanner, G.; Larkin, P. The DMACA-HCl Protocol and the threshold proanthocyanidin content for bloat safety in forage legumes. J. Sci. Food Agric. 1996, 70, 89–101. [Google Scholar] [CrossRef]
- Marinova, D.; Ribarova, F.; Atanassova, M. Total phenolics and flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall. 2005, 40, 255–260. [Google Scholar]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001, 73, 285–290. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E. Determination of total monomeric anthocyanin pigment content of fruit juices, beverages, natural colorants, and wines by the pH differential method: Collaborative study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, J.-J.; Liu, Y.-X.; Pan, Q.-H.; Cui, X.-Y.; Duan, C.-Q. Different anthocyanin profiles of the skin and the pulp of Yan73 (Muscat Hamburg × Alicante Bouschet) grape berries. Molecules 2010, 15, 1141–1153. [Google Scholar] [CrossRef]
- Yang, P.; Yuan, C.; Wang, H.; Han, F.; Liu, Y.; Wang, L.; Liu, Y. Stability of Anthocyanins and their degradation products from Cabernet Sauvignon red wine under gastrointestinal pH and temperature conditions. Molecules 2018, 23, 354. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xue, T.T.; Han, X.; Guan, L.X.; Zhang, L.; Wang, H.; Li, H. Kaolin particle film affects grapevine berry quality in cv. Meili in humid climate conditions. HortScience 2020, 55, 1987–2000. [Google Scholar] [CrossRef]
- Cheynier, V.; Duenas-Paton, M.; Salas, E.; Maury, C.; Souquet, J.-M.; Sarni-Manchado, P.; Fulcrand, H. Structure and properties of wine pigments and tannins. Am. J. Enol. Vitic. 2006, 57, 298–305. [Google Scholar]
- Kennedy, J.A.; Saucier, C.; Glories, Y. Grape and wine phenolics: History and perspective. Am. J. Enol. Vitic. 2006, 57, 239–248. [Google Scholar]
- Falcão, L.D.; de Revel, G.; Perello, M.C.; Moutsiou, A.; Zanus, M.C.; Bordignon-Luiz, M.T. A survey of seasonal temperatures and vineyard altitude influences on 2-methoxy-3-isobutylpyrazine, C13-norisoprenoids, and the sensory profile of Brazilian Cabernet Sauvignon wines. J. Agric. Food Chem. 2007, 55, 3605–3612. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.-T.; Wen, Y.; Tao, Y.-S.; Lan, Y.-Y. Modulating the formation of Meili wine aroma by prefermentative freezing process. J. Agric. Food Chem. 2013, 61, 1542–1553. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.Z.; Kang, W.H.; Xu, Y. Comparative analysis of contents of C6 aldehydes and alcohols responsible for the aroma of different varieties of wine grapes. Food Sci. 2010, 31, 252–256. [Google Scholar] [CrossRef]
- Longo, R.; Blackman, J.W.; Antalick, G.; Torley, P.J.; Rogiers, S.Y.; Schmidtke, L.M. Volatile and sensory profiling of Shiraz wine in response to alcohol management: Comparison of harvest timing versus technological approaches. Food Res. Int. 2018, 109, 561–571. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X.Y.; Wang, H.L.; Yan, A.L.; Zhang, G.J.; Ren, J.C.; Xu, H.Y. The influence of rootstocks on the growth and aromatic quality of two table grape varieties. Sci. Agric. Sin. 2021, 54, 4405–4420. [Google Scholar] [CrossRef]
- Peinado, R.A.; Moreno, J.; Bueno, J.E.; Moreno, J.A.; Mauricio, J.C. Comparative study of aromatic compounds in two young white wines subjected to pre-fermentative cryomaceration. Food Chem. 2004, 84, 585–590. [Google Scholar] [CrossRef]
- Fan, W.; Xu, Y.; Jiang, W.; Li, J. Identification and quantification of impact aroma compounds in 4 nonfloral Vitis vinifera varieties grapes. J. Food Sci. 2010, 75, S81–S88. [Google Scholar] [CrossRef]
- Šuklje, K.; Zhang, X.; Antalick, G.; Clark, A.C.; Deloire, A.; Schmidtke, L.M. Berry shriveling significantly alters Shiraz (Vitis vinifera L.) grape and wine chemical composition. J. Agric. Food Chem. 2016, 64, 870–880. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Tang, Y.; Wang, P.; Song, C.; Duan, B.; Zhang, Z.; Meng, J. Influence of natural variation in berry size on the volatile profiles of Vitis vinifera L. cv. Merlot and Cabernet Gernischt grapes. PLoS ONE 2018, 13, e0201374. [Google Scholar] [CrossRef]
- Buttery, R.G.; Turnbaugh, J.G.; Ling, L.C. Contribution of volatiles to rice aroma. J. Agric. Food Chem. 1988, 36, 1006–1009. [Google Scholar] [CrossRef]
- Jiang, B.; Xi, Z.; Luo, M.; Zhang, Z. Comparison on aroma compounds in Cabernet Sauvignon and Merlot wines from four wine grape-growing regions in China. Food Res. Int. 2013, 51, 482–489. [Google Scholar] [CrossRef]
- Zhao, Y.; Sun, Y.X.; Sun, Q.Y.; Han, A.Q.; Miao, L.P.; Zhao, X.J. Difference of volatile aroma compounds in ripen berries of wine grape (Cabernet Sauvignon) among production regions. North. Hortic. 2016, 4, 23–28. [Google Scholar] [CrossRef]
- Zhang, L.; Tao, Y.S.; Wen, Y.; Wang, H. Aroma evaluation of young Chinese merlot wines with denomination of origin. S. Afr. J. Enol. Vitic. 2013, 34, 307. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.; Fan, J.H. Study on physiological mechanism of humic acid to promote wheat growth. Humic Acid 1998, 3, 32–34. [Google Scholar] [CrossRef]
- Lan, Y.C.; Shen, L.X.; Li, R.F. Effects of different film mulching on soil temperature and moisture. Chin. Agric. Sci. Bull. 2013, 29, 120–126. [Google Scholar]
- Duan, X.Y.; Gao, F.F.; Han, X.; Guan, L.X.; Zhang, L.; Li, H.; Wang, H. Effects of mulching treatments on fruit quality of Vitis vinifera cv. Meili and physical and chemical indicators of topsoil. J. Northwest A F Univ. Nat. Sci. Ed. 2022, 50, 1–9. [Google Scholar] [CrossRef]
- Bi, J.; Xia, G.L.; Bi, Y.W.; Zhang, P.; Shi, G.F.; Zhu, G.L. Effect of humic bio-active fertilizer on winter wheat and soil microbial activity. Plant Nutr. Fertil. Sci. 2005, 11, 99–103. [Google Scholar]
- Morinaga, K.; Yakushiji, H.; Koshita, Y.; Imai, S. Effect of fruit load levels on root activity, vegetative growth and sugar accumulation in berries of grapevine. Acta Hortic. 2000, 512, 121–128. [Google Scholar] [CrossRef]
- Bravdo, B.; Hepner, Y.; Loinger, C.; Cohen, S.; Tabacman, H. Effect of crop level on growth, yield and wine quality of a high yielding Carignane vineyard. Am. J. Enol. Vitic. 1984, 35, 247–252. [Google Scholar]
- Gil, M.; Esteruelas, M.; González, E.; Kontoudakis, N.; Jiménez, J.; Fort, F.; Canals, J.-M.; Hermosín-Gutiérrez, I.; Zamora, F. Effect of two different treatments for reducing grape yield in Vitis vinifera cv Syrah on wine composition and quality: Berry thinning versus cluster thinning. J. Agric. Food Chem. 2013, 61, 4968–4978. [Google Scholar] [CrossRef]
- Song, C.-Z.; Wang, C.; Xie, S.; Zhang, Z.-W. Effects of leaf removal and cluster thinning on berry quality of Vitis vinifera cultivars in the region of Weibei Dryland in China. J. Integr. Agric. 2018, 17, 1620–1630. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Qi, J.S.; Liu, X.Y.; Cai, J.S.; Lv, Y.X.; Li, X.W. Effects of different single plant loading capacity on fruit growth and quality indexes of Cabernet Sauvignon. China Brew. 2021, 40, 70–75. [Google Scholar]
- Zhang, Z.H. Effects of Application of Humic Acid Fertilizer Combined with Straw on Grape Growth and Soil Fertility; Huazhong Agriculture University: Wuhan, China, 2017. [Google Scholar]
- Yang, C.; Shang, K.; Lin, C.; Wang, C.; Shi, X.; Wang, H.; Li, H. Processing technologies, phytochemical constituents, and biological activities of grape seed oil (GSO): A review. Trends Food Sci. Technol. 2021, 116, 1074–1083. [Google Scholar] [CrossRef]
- Javorský, J.; Král, M.; Šnirc, M.; Árvay, J.; Tremlová, B.; Dordević, D. Characterization of Moravian wines by selected chemical parameters. Separations 2021, 8, 89. [Google Scholar] [CrossRef]
- Chen, Y.L.; Zhao, X.F.; Zhang, J.X.; Zhang, Z.W. Effect of fruit yields on quality of ‘Cabernet Gernischet’ grape. North. Hortic. 2012, 23, 5–7. [Google Scholar]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural practice and environmental impacts on the flavonoid composition of grapes and wine: A review of recent research. Am. J. Enol. Vitic. 2006, 57, 257–268. [Google Scholar]
- Cohen, S.D.; Kennedy, J.A. Plant metabolism and the environment: Implications for managing phenolics. Crit. Rev. Food Sci. Nutr. 2010, 50, 620–643. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, R.P.; Scagel, C.F.; Lee, J. N, P, and K Supply to Pinot noir grapevines: Impact on berry phenolics and free amino acids. Am. J. Enol. Vitic. 2014, 65, 43–49. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Z.W. Analysis ob hillside soil nutrition and wine-grape berries quality. Acta Agric. Shanghai 2017, 33, 58–62. [Google Scholar] [CrossRef]
- Li, D.; Zhang, X.; Xu, Y.; Li, L.; Aghdam, M.S.; Luo, Z. Effect of exogenous sucrose on anthocyanin synthesis in postharvest strawberry fruit. Food Chem. 2019, 289, 112–120. [Google Scholar] [CrossRef]
- George, F.; Figueiredo, P.; Toki, K.; Tatsuzawa, F.; Saito, N.; Brouillard, R. Influence of trans-cis isomerisation of coumaric acid substituents on colour variance and stabilisation in anthocyanins. Phytochemistry 2001, 57, 791–795. [Google Scholar] [CrossRef]
- Jingjing, L. Effect of Exogenous Hormones and Soil Type of Grape Anthocyanin and Wine Color Stability; Qilu University of Technology: Ji’nan, China, 2020. [Google Scholar]
- Reynolds, A.G.; Heuvel, J.E.V. Influence of grapevine training systems on vine growth and fruit composition: A review. Am. J. Enol. Vitic. 2009, 60, 251–268. [Google Scholar]
- Gachons, C.P.D.; van Leeuwen, C.; Tominaga, T.; Soyer, J.-P.; Gaudillère, J.-P.; Dubourdieu, D. Influence of water and nitrogen deficit on fruit ripening and aroma potential of Vitis vinifera L. cv Sauvignon blanc in field conditions. J. Sci. Food Agric. 2005, 85, 73–85. [Google Scholar] [CrossRef]
- Xi, Z.-M.; Tao, Y.-S.; Zhang, L.; Li, H. Impact of cover crops in vineyard on the aroma compounds of Vitis vinifera L. cv Cabernet Sauvignon wine. Food Chem. 2011, 127, 516–522. [Google Scholar] [CrossRef]
- Diago, M.P.; Vilanova, M.; Blanco, J.A.; Tardaguila, J. Effects of mechanical thinning on fruit and wine composition and sensory attributes of Grenache and Tempranillo varieties (Vitis vinifera L.). Aust. J. Grape Wine Res. 2010, 16, 314–326. [Google Scholar] [CrossRef]
- Zamboni, M.; Bavaresco, L.; Komjanc, R. Influence of bud number on growth, yield, grape and wine quality of ‘Pinot Gris’, ‘Pinot Noir’ and ‘Sauvignon’ (Vitis vinifera L.). Acta Hortic. 1996, 427, 411–420. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Z. Volatile compounds of young wines from Cabernet Sauvignon, Cabernet Gernischet and Chardonnay Varieties grown in the Loess plateau region of China. Molecules 2010, 15, 9184–9196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, J.M.; Faria, M.; Sá, F.; Barros, F.; Araújo, I.M. C6-alcohols as varietal markers for assessment of wine origin. Anal. Chim. Acta 2006, 563, 300–309. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).