Evaluation of Trichogramma cacaeciae (Hymenoptera: Trichogrammatidae) and Blattisocius mali (Mesostigmata: Blattisociidae) in the Post-Harvest Biological Control of the Potato Tuber Moth (Lepidoptera: Gelechiidae): Use of Sigmoid Functions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Evaluation of the Effectiveness of T. cacaeciae and B. mali Used in PTM Control under Storage Conditions
2.2. Mathematical Model: Logistic Functions
3. Results
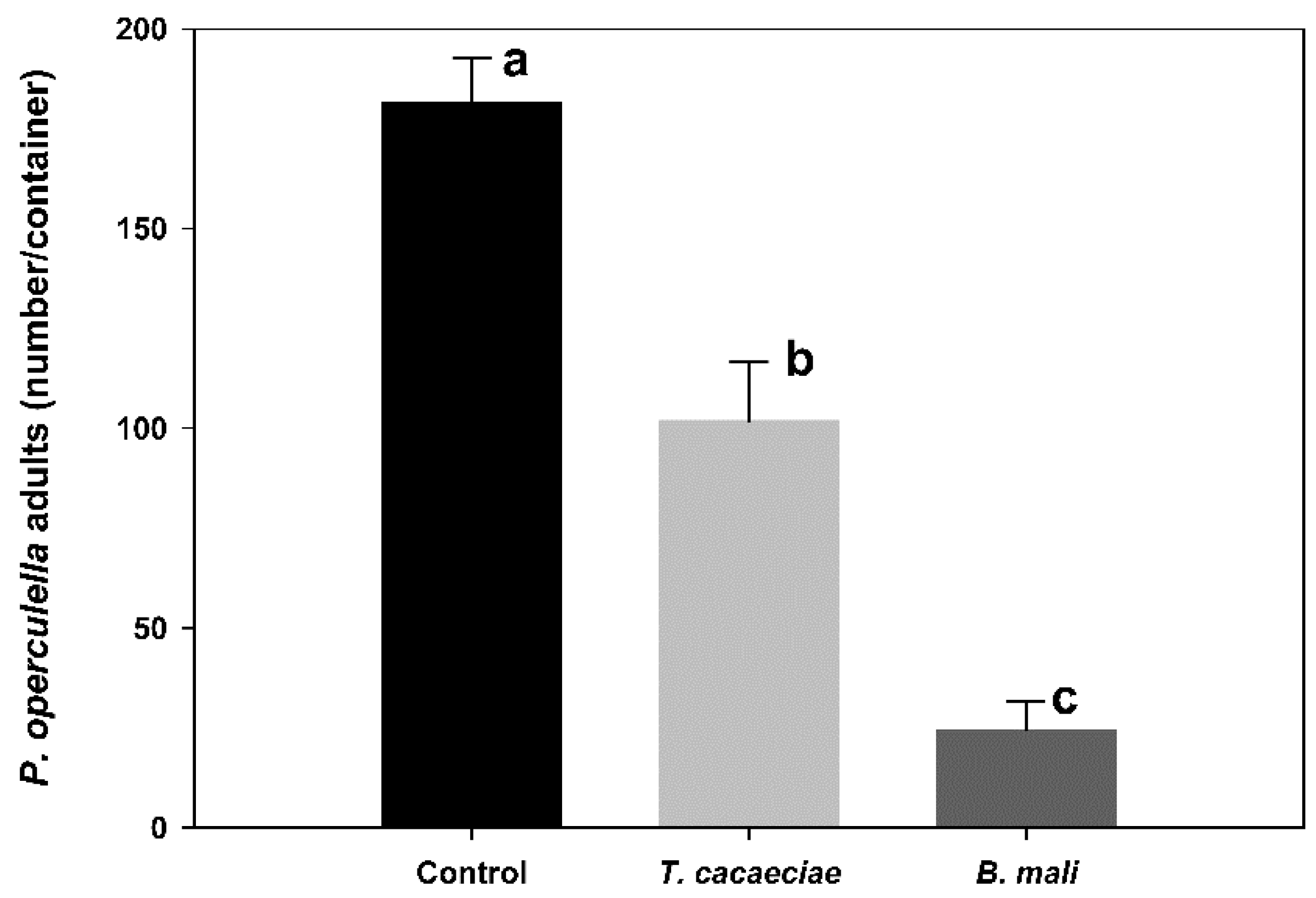
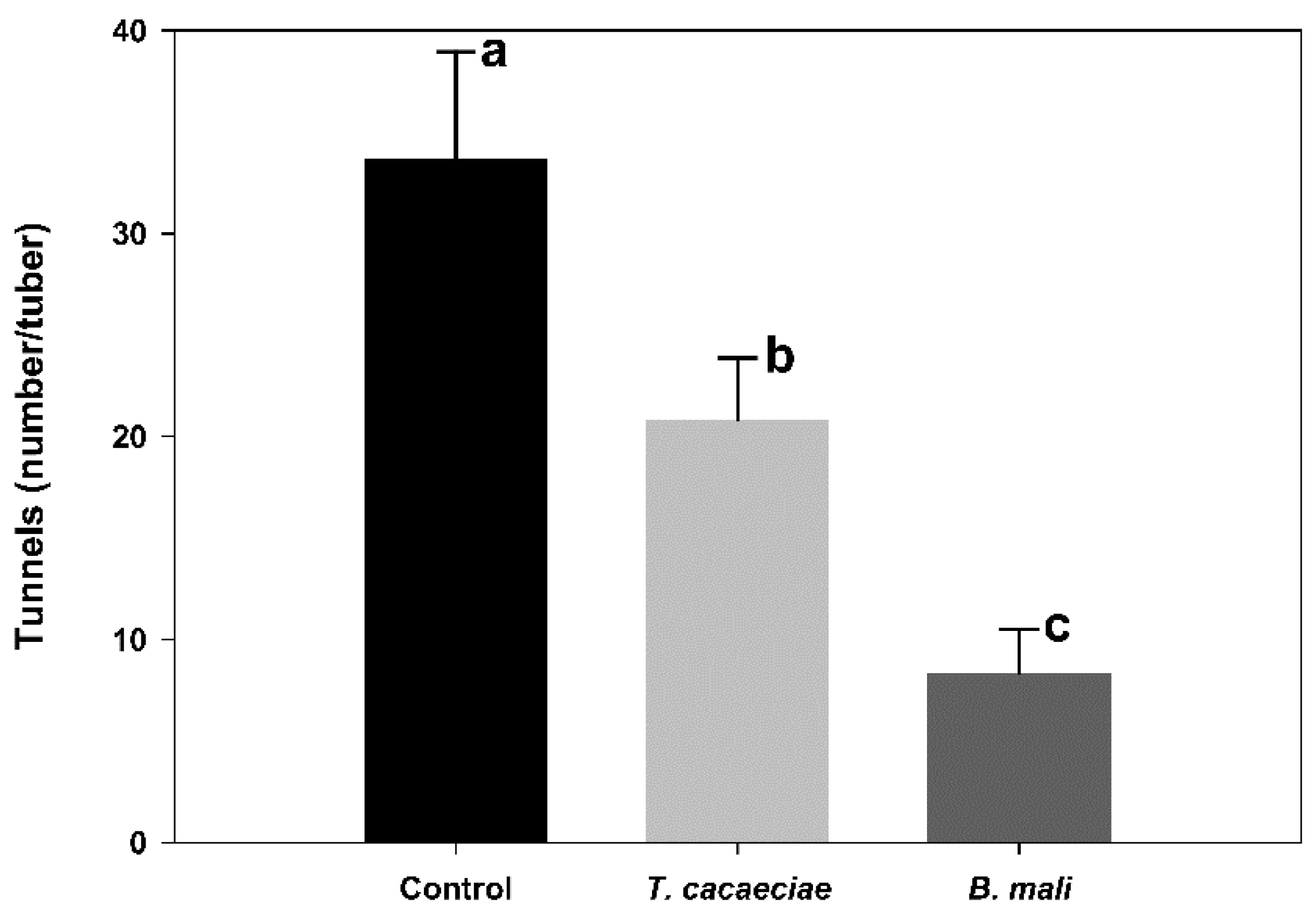
3.1. Effectiveness of T. cacaeciae and B. mali Used in PTM Control under Storage Conditions
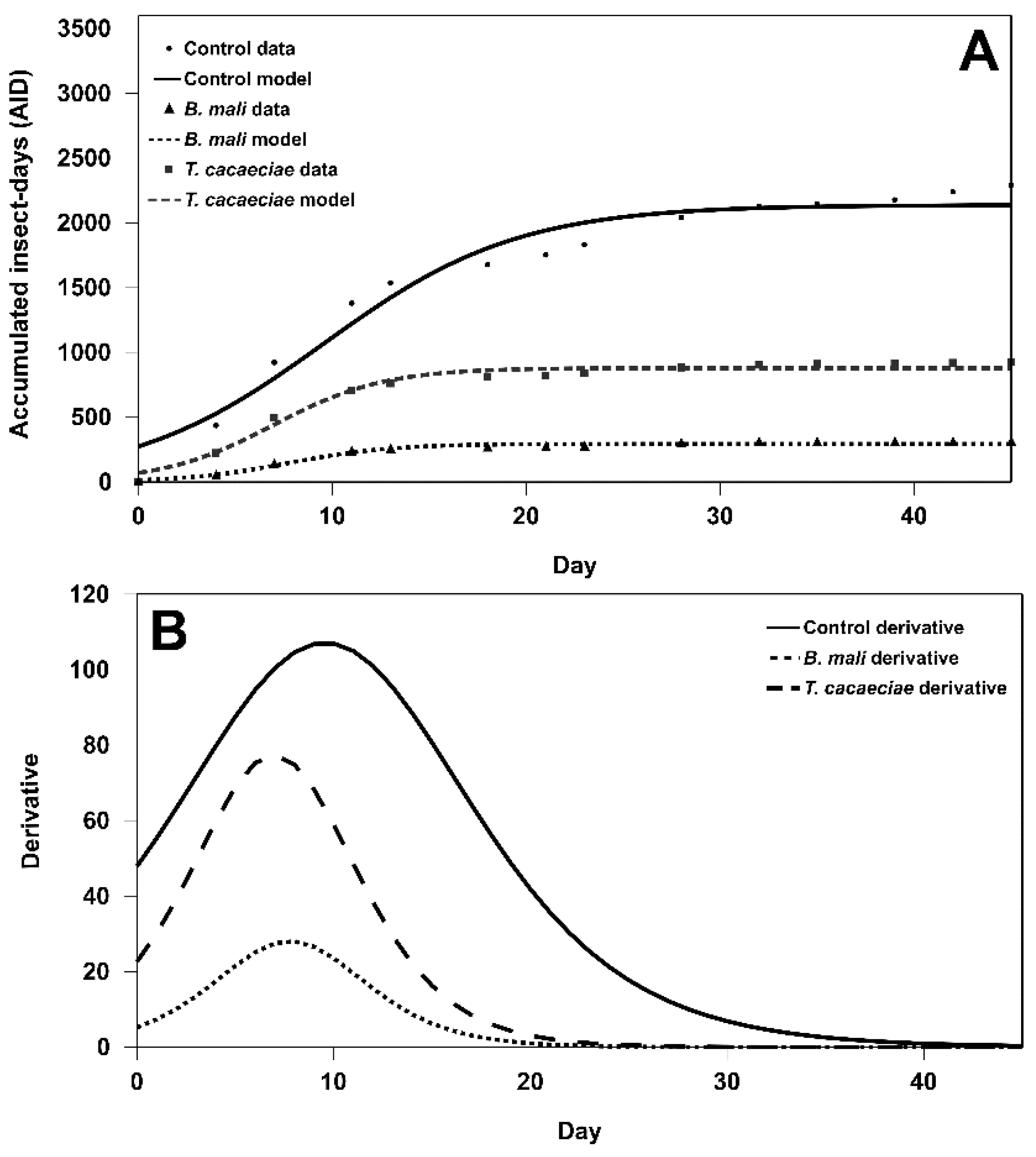
3.2. Effectiveness Evaluation Using the Mathematical Model
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kroschel, J.; Sporleder, M.; Carhuapoma, P. Potato tuber moth, Phthorimaea operculella. In Pest Distribution and Risk Atlas for Africa; Kroschel, J., Mujica, N., Carhuapoma, P., Sporleder, M., Eds.; International Potato Center (CIP): Lima, Peru, 2016; pp. 7–23. [Google Scholar]
- Gao, Y. Potato tuberworm: Impact and methods for control-mini review. CABI Rev. 2018, 13, 1–3. [Google Scholar] [CrossRef]
- Hagstrum, D.W.; Subramanyam, B. Fundamentals of Stored-Product Entomology; AACC International: St. Paul, MN, USA, 2006; p. 323. [Google Scholar]
- Rondon, S.I. The potato tuberworm: A literature review of its biology, ecology, and control. Am. J. Pot. Res. 2010, 87, 149–166. [Google Scholar] [CrossRef] [Green Version]
- Golizadeh, A.; Zalucki, M.P. Estimating temperature-dependent developmental rates of potato tuberworm, Phthorimaea operculella (Lep.: Gelechiidae). Insect Sci. 2012, 19, 609–620. [Google Scholar] [CrossRef]
- Hagstrum, D.W.; Klejdysz, T.; Subramanyam, B.; Nawrot, J. Atlas of Stored-Product Insects and Mites; AACC International: St. Paul, MN, USA, 2013; p. 589. [Google Scholar]
- Kroschel, J.; Schaub, B. Biology and ecology of potato tuber moths as major pests of potato. In Insect Pests of Potato: Global Perspectives on Biology and Management; Giordanengo, P., Vicent, C., Alyokhin, A., Eds.; Elsevier: Oxford, UK, 2013; pp. 165–192. [Google Scholar]
- Aryal, S.; Jung, C. IPM tactics of potato tuber moth, Phthorimaea operculella (Lep.: Gelechiidae): Literature study. Korean J. Soil Zool. 2015, 19, 42–51. [Google Scholar]
- Lacey, L.A.; Headrick, H.L.; Horton, D.R.; Schreiber, A. Effect of a granulovirus on mortality and dispersal of potato tuber worm (Lep.: Gelechiidae) in refrigerated storage warehouse conditions. Biocontrol Sci. Techn. 2010, 20, 437–447. [Google Scholar] [CrossRef]
- Kroschel, J.; Mujica, N.; Okonya, J.; Alyokhin, A. Insect pest affecting potatoes in tropical, subtropical, and temperate regions. In The Potato Crop; Campos, H., Ortiz, O., Eds.; Springer: Geneva, Switzerland, 2020; pp. 251–306. [Google Scholar]
- Hanafi, A. Integrated pest management of potato tuber moth in field and storage. Potato Res. 1999, 42, 373–380. [Google Scholar] [CrossRef]
- Schaub, B.; Kroschel, J. Developing a biocontrol strategy to protect stored potato tubers from infestation with potato tuber moth species in the Andean region. J. Appl. Entomol. 2017, 142, 78–88. [Google Scholar] [CrossRef] [Green Version]
- Sporleder, M.; Kroschel, J.; Gutierrez, M.R.; Lagnaoui, A. A temperature-based simulation model for the potato tuberworm, Phthorimaea operculella Zeller (Lep.: Gelechiidae). Environ. Entomol. 2004, 33, 477–486. [Google Scholar] [CrossRef]
- Fuglie, K.O.; Khatana, V.S.; Ilangantileke, S.G.; Scott, G.J.; Singh, J.; Kumar, D. Economics of potato storage in northern India. Q. J. Int. Agric. 2000, 39, 131–148. [Google Scholar]
- Gottschalk, K.; Ezekiel, R. Storage. In Handbook of Potato Production, Improvement, and Postharvest Management; Gopal, J., Kurana, S.M.P., Eds.; Food Products Press: New York, NY, USA, 2006; pp. 489–522. [Google Scholar]
- Gavara, J.; Piedra-Buena, A.; Hernandez-Suarez, E.; Gamez, M.; Cabello, T.; Gallego, J.R. Potential for the Postharvest Biological Control of Phthorimaea operculella (Lep.: Gelechiidae) by Blattisocius tarsalis (Mesostigmata, Blattisociidae). Agronomy 2021, 11, 288. [Google Scholar] [CrossRef]
- Saour, G. Efficacy assessment of some Trichogramma species (Hym.: Trichogrammatidae) in controlling the potato tuber moth Phthorimaea operculella Zell. (Lepidoptera: Gelechiidae). J. Pest Sci. 2004, 77, 229–234. [Google Scholar] [CrossRef]
- Mandour, N.S.; Sarhan, A.A.; Atwa, D.H. The integration between Trichogramma evanescens West. (Hym.: Trichogrammatidae) and selected bioinsecticides for controlling the potato tuber moth Phthorimaea operculella (Zell.) (Lep.: Gelechiidae) of stored potatoes. J. Plant Prot. Res. 2012, 52, 40–46. [Google Scholar] [CrossRef]
- Gallego, J.R.; Mellado-Lopez, L.; Cabello, T. Selección de una especie de Trichogramma (Hym., Trichogrammatidae) para el control biológico de la polilla de la patata Phthorimaea operculella (Lep., Gelechiidae) mediante el estudio del comportamiento de parasitación del huésped. ITEA Inf. Tec. Econ. Ag. 2020, 116, 2–18. [Google Scholar] [CrossRef]
- Gallego, J.R.; Caicedo, O.; Gamez, M.; Hernandez, J.; Cabello, T. Selection of predatory mites for the biological control of potato tuber moth in stored potatoes. Insects 2020, 11, 196. [Google Scholar] [CrossRef] [Green Version]
- Gallego, J.R.; Gamez, M.; Cabello, T. Potential of the Blattisocius mali mite (Acari: Blattisociidae) as a biological control agent of potato tubermoth (Lep.: Gelechiidae) in stored potatoes. Potato Res. 2020, 63, 241–251. [Google Scholar] [CrossRef]
- Mills, N. Techniques to evaluate the efficacy of natural enemies. In Methods in Ecological and Agricultural Entomology; Dent, D.R., Walton, M.P., Eds.; CAB International: Wallingford, UK, 1997; pp. 271–291. [Google Scholar]
- Van Driesche, R.G. Meaning of “percent parasitism” in studies of insect parasitoid. Environ. Entomol. 1983, 12, 1611–1622. [Google Scholar] [CrossRef]
- Latham, D.R.; Mills, N. Quantifying insect predation: A comparison of three methods for estimating daily per capita consumption of two aphidophagous predator. Environ. Entomol. 2009, 38, 1117–1125. [Google Scholar] [CrossRef] [Green Version]
- van Lenteren, J.C.; Bueno, V.H.P.; Burgio, G.; Lanzani, A.; Montes, F.C.; Silva, D.B.; Jong, P.W.; Hemerik, L. Pest kill rate as aggregate evaluation criterion to rank biological control agents: A case study with neotropical predators of Tuta absoluta on tomato. Bull. Entomol. Res. 2019, 109, 812–820. [Google Scholar] [CrossRef] [Green Version]
- Abbott, W.S. A method of computing effectiveness of an insecticide. J. Econ. Entomol. 1925, 18, 265–267. [Google Scholar] [CrossRef]
- Robertson, J.L.; Preisler, H.K. Pesticide Bioassays with Arthropods; CRC Press: Boca Raton, FL, USA, 1992; p. 127. [Google Scholar]
- Matthews, G.A. Techniques to evaluate insecticide efficacy. In Methods in Ecological and Agricultural Entomology; Dent, D.R., Walton, M.P., Eds.; CAB International: Wallingford, UK, 1997; pp. 243–269. [Google Scholar]
- Navon, A.; Ascher, K.R.S. Bioassays of Entomopathogenic Microbes and Nematodes; CABI Publishing: Walingford, UK, 2000; p. 324. [Google Scholar]
- Carey, J.R. Applied Demography for Biologists; Oxford University Press: New York, NY, USA, 1993; p. 206. [Google Scholar]
- Fleming, R.; Retnakaran, A. Evaluating single treatment data using Abbott’s formula with reference to insecticides. J. Econ. Entomol. 1985, 78, 1179–1181. [Google Scholar] [CrossRef]
- Rosenheim, J.A.; Hoy, M.A. Confidence intervals for the Abbott’s formula correction of bioassay data for control response. J. Econ. Entomol. 1989, 82, 331–335. [Google Scholar] [CrossRef] [Green Version]
- Berryman, A.A. The theoretical foundations of biological control. In Theoretical Approaches to Biological Control; Hawkins, B.A., Cornell, H.V., Eds.; Cambridge University Press: Cambridge, UK, 2004; pp. 3–21. [Google Scholar]
- Arthurs, S.; Lacey, L.A.; Pruneda, J.N.; Rondon, S.I. Semi-field evaluation of a granulovirus and Bacillus thuringiensis ssp. kurstaki for season-long control of the potato tuber moth, Phthorimaea operculella. Entomol Exp. Appl. 2008, 129, 276–285. [Google Scholar] [CrossRef]
- Ruppel, R.F. Cumulative insect-days as an index of crop protection. J. Econ. Entomol. 1983, 76, 375–377. [Google Scholar] [CrossRef]
- Cabello, T.; Gallego, J.R.; Fernandez, F.J.; Gamez, M.; Vila, E.; Pino, M.; Hernandez-Suarez, E. Biological control strategies for the South American tomato moth Tuta absoluta (Lep.: Gelechiidae) on greenhouse tomatoes. J. Econ. Entomol. 2012, 105, 2085–2096. [Google Scholar] [CrossRef]
- Carreño, R.; Andujar, A.; Cabello, T. Evaluación de la eficacia de pesticidas agrícolas. In Proceedings of the Actas de la 5ª Conferencia Española de Biometria, Región Española de la International Biometric Society, Valencia, Spain, 21–23 June 1995. [Google Scholar]
- Gurr, G.M.; Barlow, N.D.; Memmott, J.; Wratten, S.D.; Greathead, D.J. A History of methodological, theoretical and empirical approaches to biological control. In Biological Control: Measures of Success; Gurr, G., Wratten, S., Eds.; Springer: Dordrecht, The Netherlands, 2000; pp. 3–37. [Google Scholar]
- Barlow, N.D. Models in biological control: A field guide. In Theoretical Approaches to Biological Control; Hawkins, B.A., Cornell, H.V., Eds.; Cambridge University Press: Cambridge, UK, 2004; pp. 43–68. [Google Scholar]
- Sanchez, J.A.; Lacasa, A. Impact of the zoophytophagous plant bug Nesidiocoris tenuis (Het.: Miridae) on tomato yield. J. Econ. Entomol. 2008, 101, 1864–1870. [Google Scholar] [CrossRef]
- Hoy, M.A. Almonds (California). In Spider Mites: Their Biology, Natural Enemies and Control; Helle, W., Sabelis, M.W., Eds.; Elsevier: Amsterdam, The Netherlands, 1985; Volume 1, pp. 299–310. [Google Scholar]
- Carreño, R.; Gamez, M.; Barranco, P.; Belda, J.; Cabello, T. Modelos Analíticos Aplicados a la Dinámica de Población de Especies Plagas. In Actas de la VI Conferencia Española de Biometría; Caridad-Ocerin, J.M., Ed.; Región Española de la International Biometric Society, Universidad de Córdoba: Córdoba, Spain, 21–24 September 1997. [Google Scholar]
- Cabello, T.; Carreño, R. Modelos logísticos aplicados a la fenología de noctuidos plagas en el sur de España (Lep.: Noctuidae). Bol. San. Veg. Plagas 2002, 28, 319–336. [Google Scholar]
- Solano-Rojas, Y.; Gamez, M.; Lopez, I.; Garay, J.; Varga, Z.; Cabello, T. Conservation strategy for palm groves: Optimal chemical control model for red palmweevil, Rhynchophorus ferrugineus. Agronomy 2021, 11, 920. [Google Scholar] [CrossRef]
- Berger, R.D. Comparison of the Gompertz and logistic equations to describe plant disease progress. Phytopathology 1981, 71, 716–719. [Google Scholar] [CrossRef]
- Amorin, L.; Bergamin, A.; Hau, B. Analysis of progress curves of surgarcane smut on different cultivars using functions of doble sigmoid pattern. Phytopathology 1993, 83, 933–936. [Google Scholar] [CrossRef]
- Hau, B.; Amorim, L.; Bergamin, A. Mathematical function to describe disease progress curves of double sigmoid pattern. Phytopothology 1993, 83, 928–932. [Google Scholar] [CrossRef]
- Jeger, M.J. Analysis of disease progress as a basis for evaluating disease management practices. Annu. Rev. Phytopathol. 2004, 42, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Birch, L.C. The intrinsic rate of natural increase of an insect population. J. Anim. Ecol. 1948, 17, 15–26. [Google Scholar] [CrossRef]
- Tommasini, M.G.; van Lenteren, J.C.; Burgio, G. Biological traits and predation capacity of four Orius species on two prey species. Bull. Insect. 2004, 57, 79–93. [Google Scholar]
- Van Lenteren, J.C. Ecology: Cool Science, But Does It Help? Wageningen University: Wageningen, UK, 2010; p. 44. [Google Scholar]
- Van Lenteren, J.C.; Lanzoni, A.; Hemerik, L.; Bueno, V.H.P.; Cuervo, J.G.B.; Biondi, A.; Burgio, G.; Calvo, F.J.; de Jong, P.W.; López, S.N.; et al. The pest kill rate of thirteen natural enemies as aggregate evaluation criterion of their biological control potential of Tuta absoluta. Sci. Rep. 2021, 11, 10756. [Google Scholar] [CrossRef] [PubMed]
- Bellows, T.S.; Van Driesche, R.G. Life table construction and analysis for evaluating biological control agents. In Handbook of Biological Control; Bellows, T.S., Fisher, T.W., Eds.; Academic Press: San Diego, CA, USA, 1999; pp. 199–223. [Google Scholar]
- Southwood, T.R.E.; Henderson, P.A. Ecological Methods, 3rd ed.; Blackwell Science: Hoboken, NJ, USA, 2000; p. 575. [Google Scholar]
- Andujar, A.; Barranco, P.; Belda, J.E.; Cabello, T.; Carreño, R. Análisis de eficacia de productos fitosanitarios. Phytoma 1997, 92, 32–40. [Google Scholar]
- Vila, E.; Cabello, T. Biosystems engineering applied to greenhouse pest control. In Biosystems Engineering: Biofactories for Food Production in the XXI Century; Torres, I., Guevara, R., Eds.; Springer: Cham, Switzerland, 2014; pp. 99–128. [Google Scholar]
| Treatment | Function | Adjustment Parameters | Statistical Parameter | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| X01 | r1 | K1 | X02 | r2 | K2 | X03 | r3 | K3 | |||
| Control | Single | 6.80 ± 2.21 | 0.20 ± 0.03 | 2137.22 ± 65.94 | - | - | - | - | - | 190.51 * | |
| Double | 14.78 ± 5.94 | 0.39 ± 0.07 | 1704.57 ± 88.47 | 9.32 × 1019 | 1.96 ± 16.12 | 464.54 ± 95.44 | - | - | 206.54 | ||
| Triple | 19.68 ± 6.60 | 0.49 ± 0.07 | 1510.97 ± 152.1 | 325.41 ± 1366.9 | 0.25 ± 0.2 | 660.06 ± 462.81 | 25,458,201 ± 1.39 × 1011 | 0.24 ± 3.42 | 44,543.93 ± 2.49 × 1011 | 245.65 | |
| B. mali | Single | 19.41 ± 8.34 | 0.38 ± 0.05 | 294.20 ± 5.55 | - | - | - | - | - | - | 136.41 * |
| Double | 36.09 ± 8.63 | 0.52 ± 0.04 | 265.24 ± 4.55 | 5.2 × 1023 ± 3.7 × 1026 | 2.32 ± 31.54 | 39.92 ± 5.05 | - | - | - | 152.29 | |
| Triple | 8.04 ± 1901.71 | 0.37 ± 15.67 | 298.6 ± 14,188.8 | 1.6 × 1013± 4.0 × 1018 | 2.74 ± 22,096.5 | −6.45 ± 14,198.23 | −3.8 × 1011 | −2.06 | −8.1 × 1010 | 190.75 | |
| T. cacaeciae | Single | 11.60 ± 3.86 | 0.35 ± 0.05 | 879.37 ± 15.11 | - | - | - | - | - | - | 165.42 * |
| Double | 17.67 ± 4.90 | 0.47 ± 0.05 | 801.93 ± 19.79 | 1.6 × 1020± 6.2 × 1022 | 1.99 ± 16.63 | 104.38 ± 22.05 | - | - | - | 181.24 | |
| Triple | 16.60 ± 5.17 | 0.45 ± 0.05 | 812.82 ± 17.72 | 8.67 × 1024 ± 1.8 × 1029 | 2.08 ± 774.62 | 97.08 ± 49.02 | 754,962 ± 3.52 × 108 | 0.33 ± 11.39 | 2.60 ± 82.00 | 220.24 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solano-Rojas, Y.; Gallego, J.R.; Gamez, M.; Garay, J.; Hernandez, J.; Cabello, T. Evaluation of Trichogramma cacaeciae (Hymenoptera: Trichogrammatidae) and Blattisocius mali (Mesostigmata: Blattisociidae) in the Post-Harvest Biological Control of the Potato Tuber Moth (Lepidoptera: Gelechiidae): Use of Sigmoid Functions. Agriculture 2022, 12, 519. https://doi.org/10.3390/agriculture12040519
Solano-Rojas Y, Gallego JR, Gamez M, Garay J, Hernandez J, Cabello T. Evaluation of Trichogramma cacaeciae (Hymenoptera: Trichogrammatidae) and Blattisocius mali (Mesostigmata: Blattisociidae) in the Post-Harvest Biological Control of the Potato Tuber Moth (Lepidoptera: Gelechiidae): Use of Sigmoid Functions. Agriculture. 2022; 12(4):519. https://doi.org/10.3390/agriculture12040519
Chicago/Turabian StyleSolano-Rojas, Yohan, Juan R. Gallego, Manuel Gamez, Jozsef Garay, Joaquin Hernandez, and Tomas Cabello. 2022. "Evaluation of Trichogramma cacaeciae (Hymenoptera: Trichogrammatidae) and Blattisocius mali (Mesostigmata: Blattisociidae) in the Post-Harvest Biological Control of the Potato Tuber Moth (Lepidoptera: Gelechiidae): Use of Sigmoid Functions" Agriculture 12, no. 4: 519. https://doi.org/10.3390/agriculture12040519
APA StyleSolano-Rojas, Y., Gallego, J. R., Gamez, M., Garay, J., Hernandez, J., & Cabello, T. (2022). Evaluation of Trichogramma cacaeciae (Hymenoptera: Trichogrammatidae) and Blattisocius mali (Mesostigmata: Blattisociidae) in the Post-Harvest Biological Control of the Potato Tuber Moth (Lepidoptera: Gelechiidae): Use of Sigmoid Functions. Agriculture, 12(4), 519. https://doi.org/10.3390/agriculture12040519








