Spatiotemporal Distribution Patterns of Pest Species (Lepidoptera: Noctuidae) Affected by Meteorological Factors in an Agroecosystem
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Collection and Preservation
2.3. Genitalia Extraction and Identification
2.4. Relative Abundance (RA) Percentage
2.5. Spatial and Temporal Analysis
2.6. Statistical Analysis
2.6.1. Pooled OLS
2.6.2. Fixed Effect Model
Fixed Effect Hypothesis Testing
3. Results
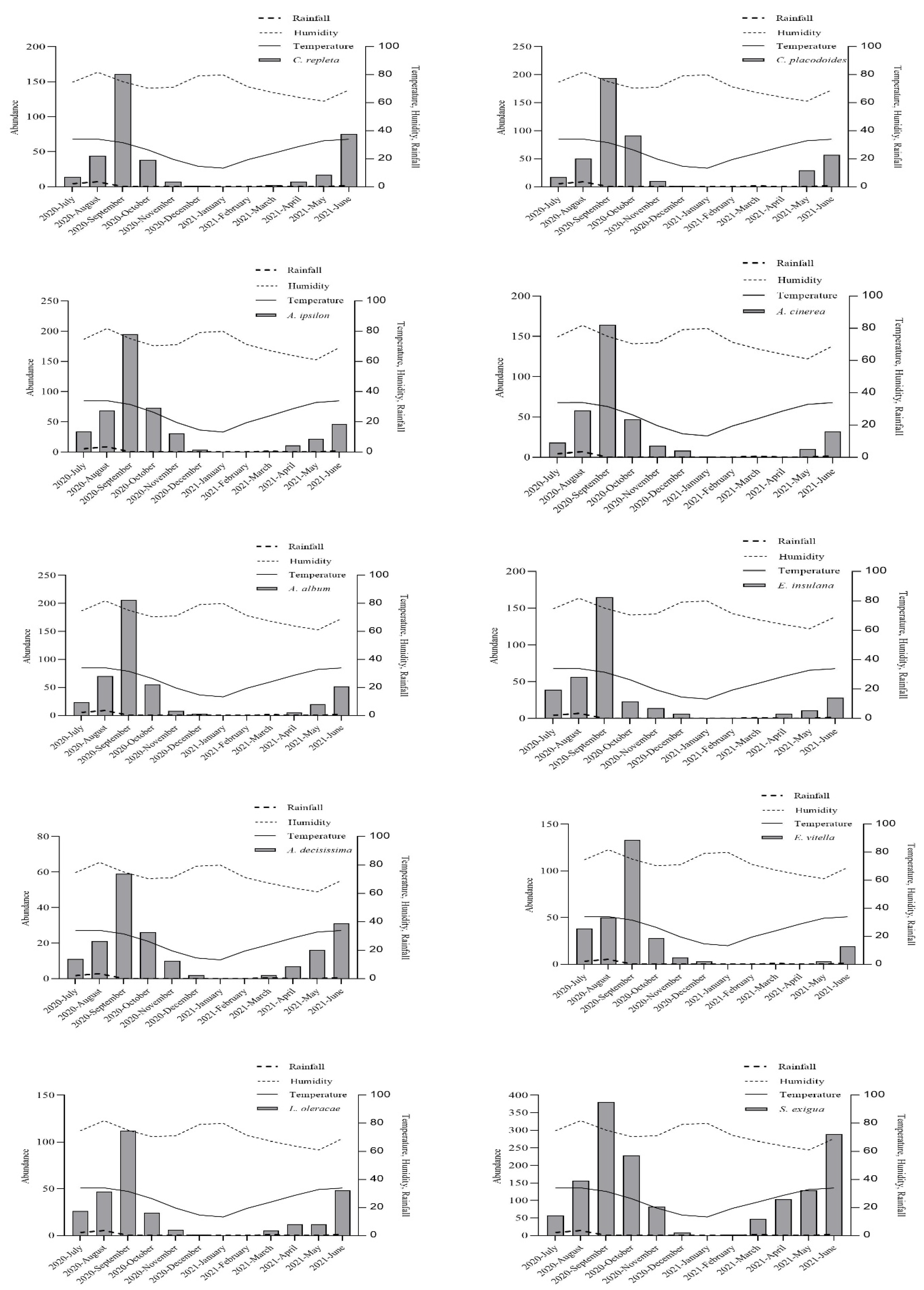
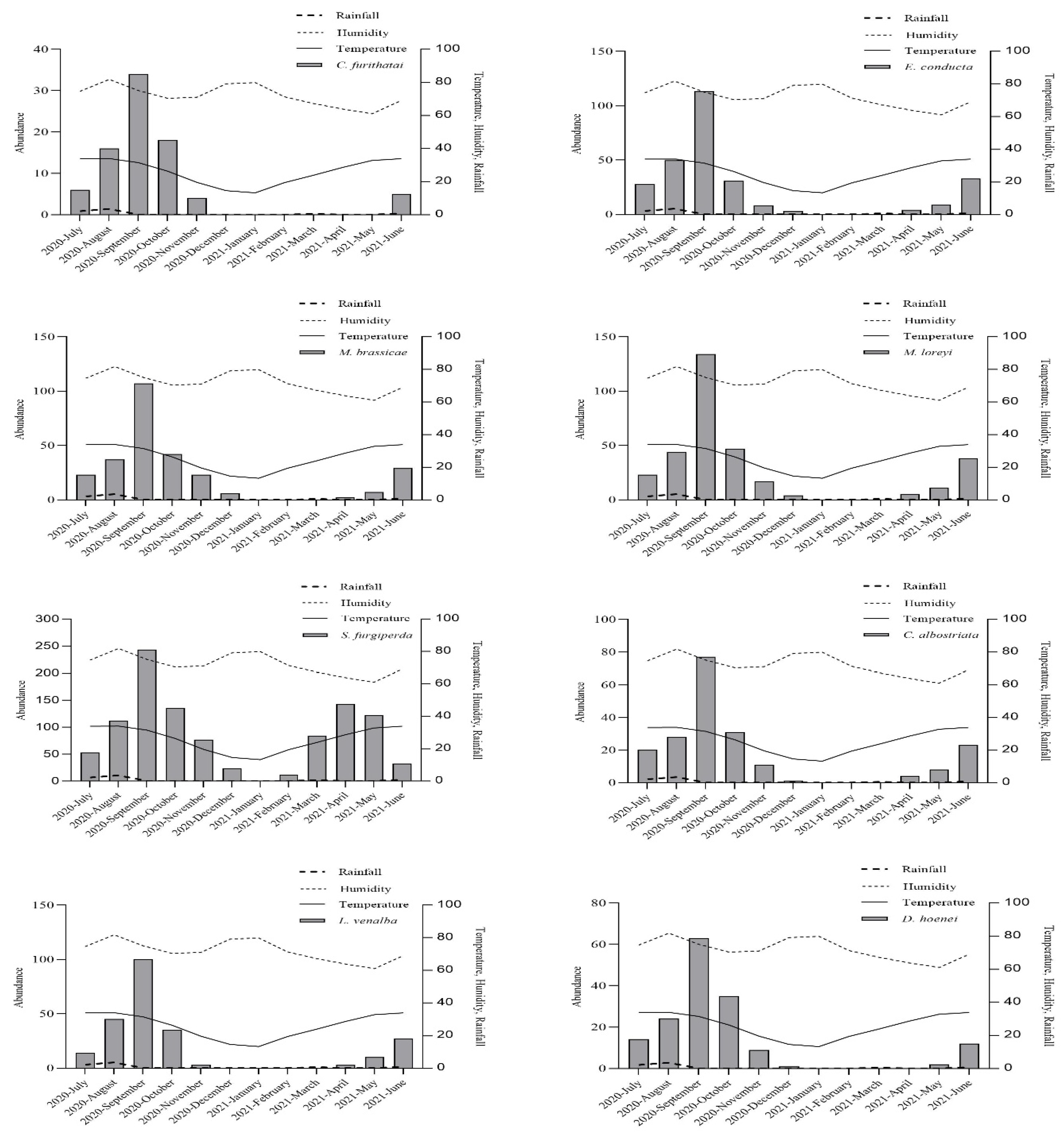
3.1. Abundance of Noctuid Moths
3.2. Effect of Meteorological Factors on Species Abundance
3.3. Temporal Distribution
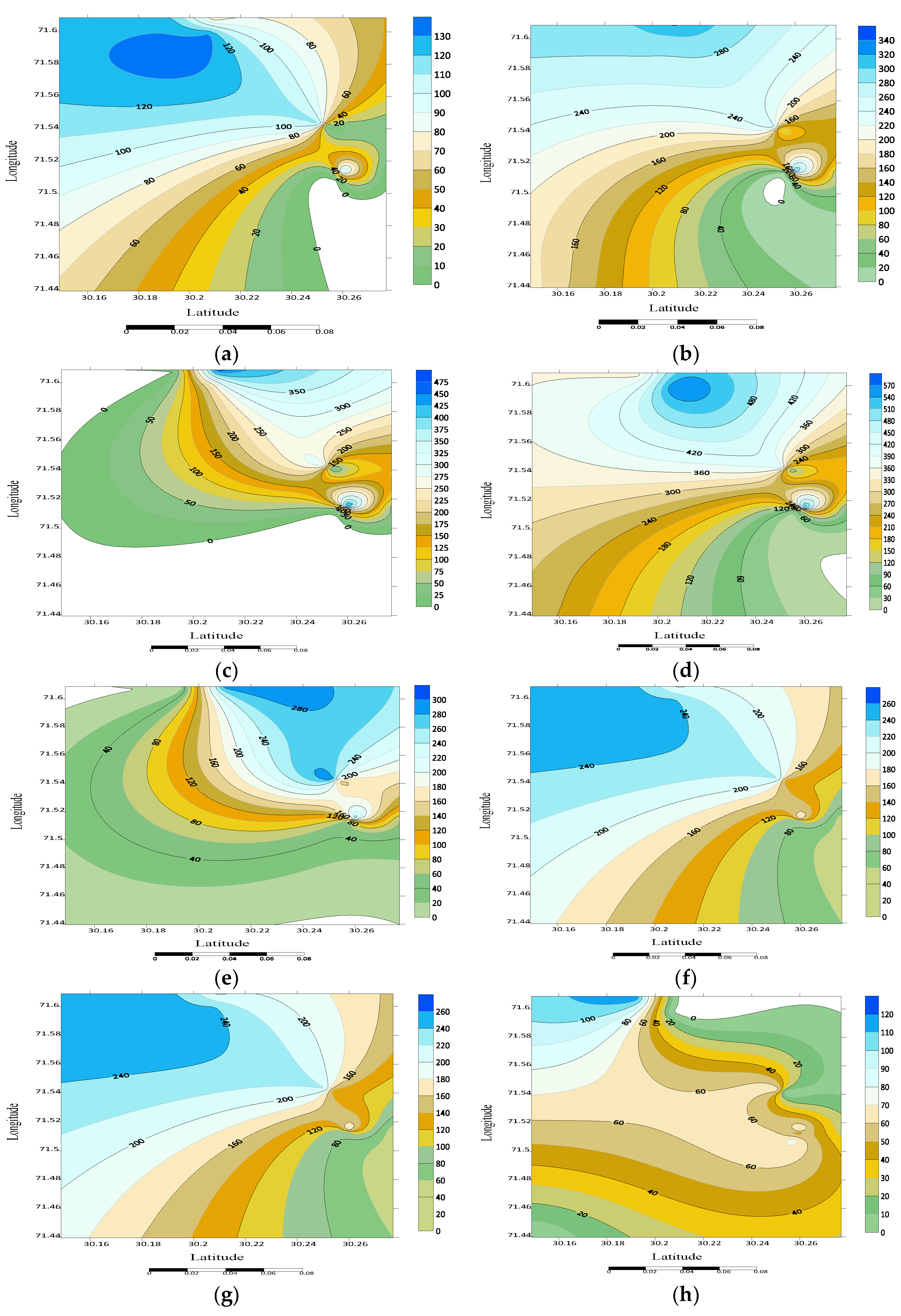
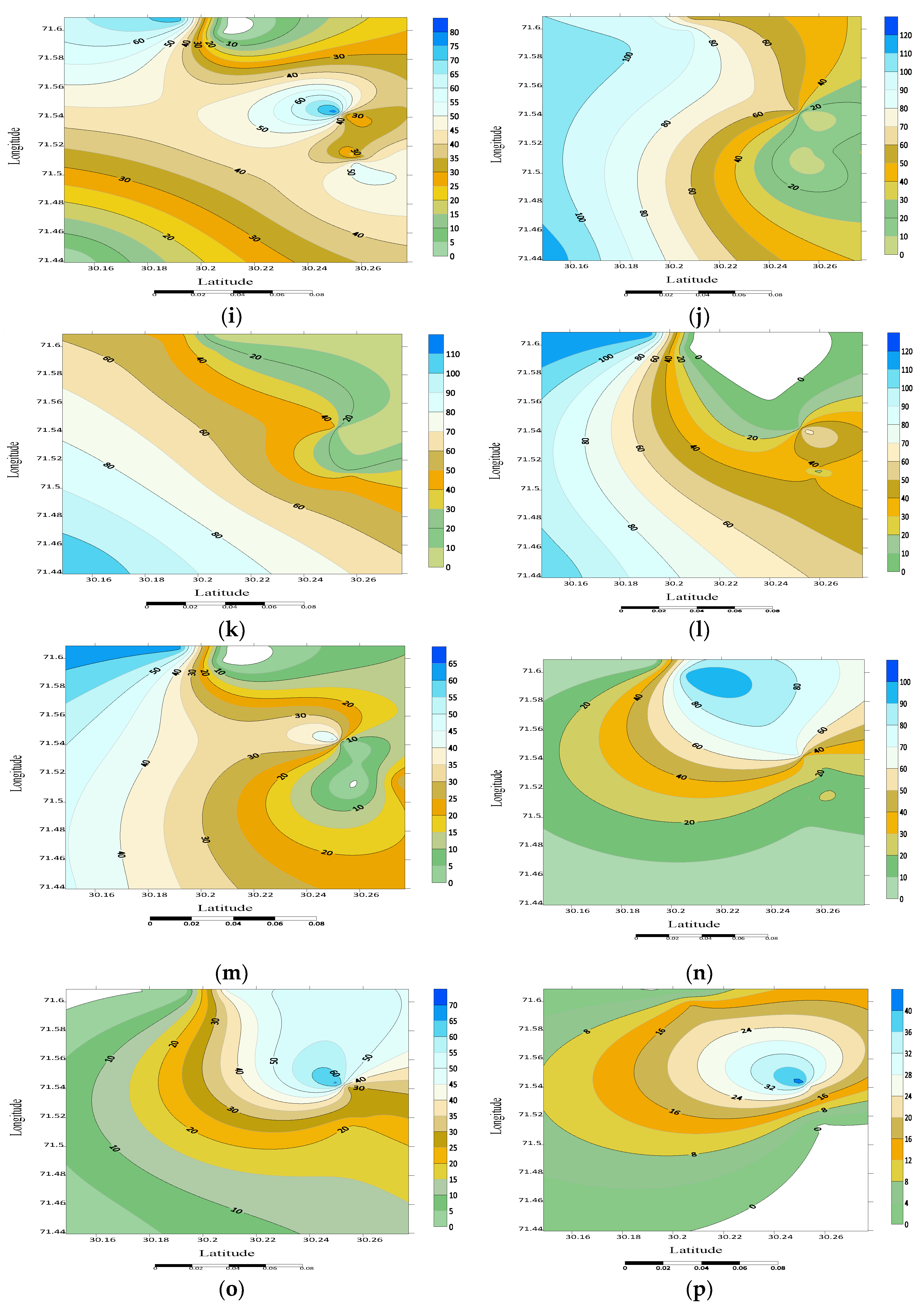
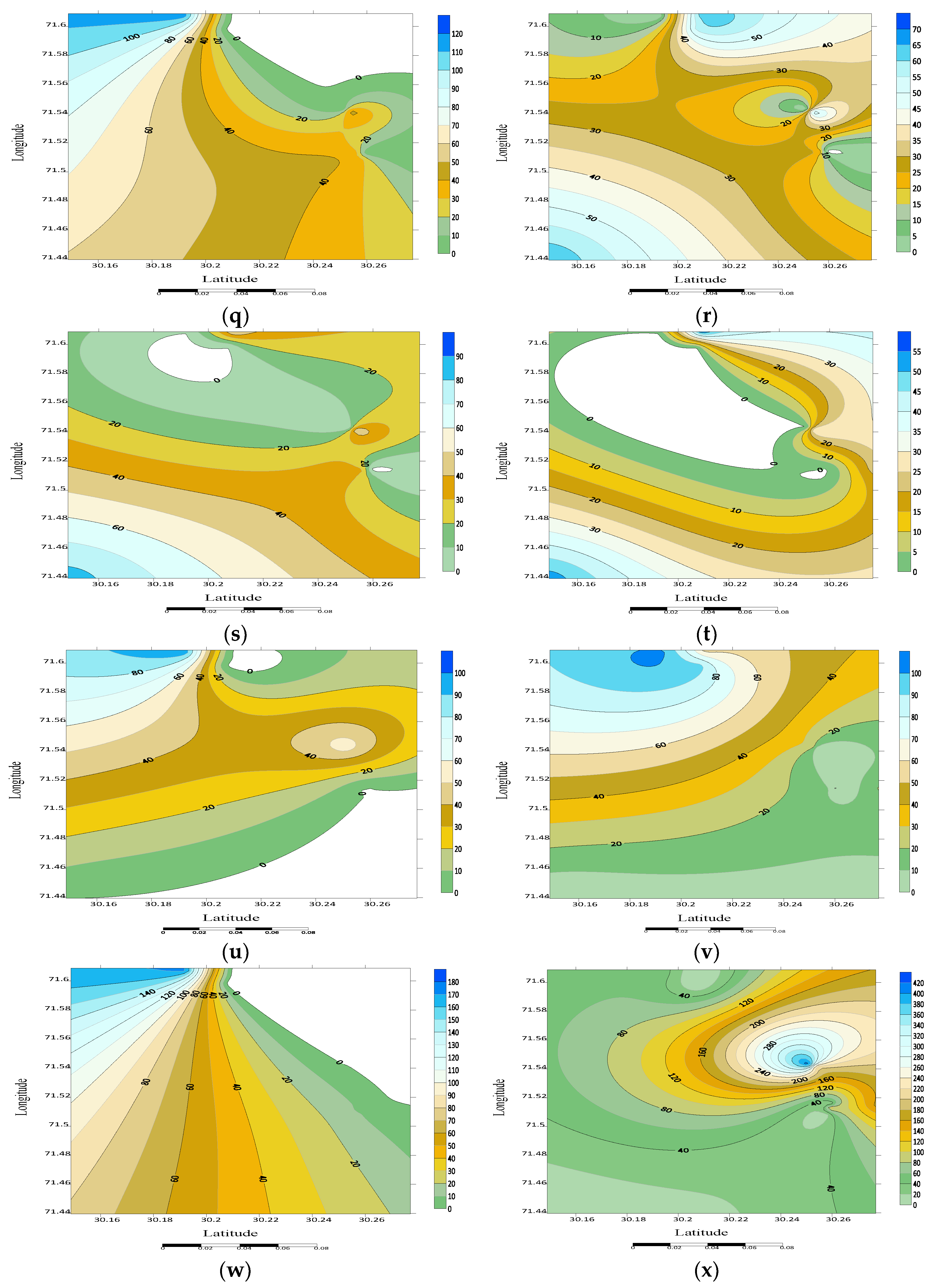
3.4. Spatial Distribution
3.5. Noctuid Moth Abundance from Different Habitats
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Resh, V.H.; Cardé, R.T. (Eds.) Encyclopedia of Insects; Academic Press: Cambridge, MA, USA, 2009; pp. 501–502. [Google Scholar]
- Sivasankaran, K.; Ignacimuthu, S.; Paulraj, M.G.; Prabakaran, S. A checklist of Noctuidae (Insecta: Lepidoptera: Noctuoidea) of India. Rec. Zool. Surv. India 2011, 111, 79–101. [Google Scholar] [CrossRef]
- Maruthadurai, R.; Ramesh, R. Occurrence, damage pattern and biology of fall armyworm, Spodoptera frugiperda (JE smith) (Lepidoptera: Noctuidae) on fodder crops and green amaranth in Goa, India. Phytoparasitica 2020, 48, 15–23. [Google Scholar] [CrossRef]
- Stacke, R.F.; Arnemann, J.A.; Rogers, J.; Stacke, R.S.; Strahl, T.T.; Perini, C.R.; Dossin, M.F.; Pozebon, H.; De-Arruda-Cavallin, L.; Guedes, J.V. Damage assessment of Helicoverpa armigera (Lepidoptera: Noctuidae) in soybean reproductive stages. Crop Protect. 2018, 112, 10–17. [Google Scholar] [CrossRef]
- Yang, Xi.; Song, Yi.; Sun, Xi.; Shen, Xi.; Wu, Qi.; Zhang, Ha.; Zhang, Da.; Zhao, Sh.; Liang, Ge.; Wu, Ko. Population occurrence of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), in the winter season of China. J. Integr. Agric. 2021, 20, 772–782. [Google Scholar] [CrossRef]
- Luttrell, R.G.; Wan, L.; Knighten, K. Variation in susceptibility of noctuid (Lepidoptera) larvae attacking cotton and soybean to purified endotoxin proteins and commercial formulations of Bacillus thuringiensis. J. Econ. Entomol. 1999, 92, 21–32. [Google Scholar] [CrossRef]
- Kalleshwaraswamy, C.; Asokan, R.; Mahadevaswamy, H.; Sharanabasappa, C. First record of invasive fall armyworm, Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) on rice (Oryza sativa) from India. J. Entomol. Zool. 2019, 7, 332–337. [Google Scholar]
- Paula-Moraes, S.V.; Silva, F.D.A.; Specht, A.; Rapisarda, C.; Cocuzza, G.E.M. Options and challenges for pest control in intensive cropping systems in tropical regions. J. Integr. Pest Manag. Tropical. 2017, 1, 18–32. [Google Scholar]
- Allstadt, A.J.; Liebhold, A.M.; Johnson, D.M.; Davis, R.E.; Haynes, K.J. Temporal variation in the synchrony of weather and its consequences for spatiotemporal population dynamics. Ecology 2015, 96, 2935–2946. [Google Scholar] [CrossRef] [Green Version]
- Colinet, H.; Sinclair, B.J.; Vernon, P.; Renault, D. Insects in fluctuating thermal environments. Annu. Rev. Entomol. 2015, 60, 123–140. [Google Scholar] [CrossRef] [Green Version]
- Robinet, C.; Roques, A. Direct impacts of recent climate warming on insect populations. Integer. Zool. 2010, 5, 132–142. [Google Scholar] [CrossRef]
- Lu, Z.Z.; Baker, G. Spatial and temporal dynamics of Helicoverpa armigera (Lepidoptera, Noctuidae) in contrasting agricultural landscapes in northwestern China. Int. J. Pest Manag. 2013, 59, 25–34. [Google Scholar] [CrossRef]
- Bianchi, F.J.; Booij, C.; Tscharntke, T. Sustainable pest regulation in agricultural landscapes: A review on landscape composition, biodiversity and natural pest control. Proc. Biol. Sci. 2006, 273, 1715–1727. [Google Scholar] [CrossRef]
- Vinatier, F.; Tixier, P.; Duyck, P.F.; Lescourret, F. Factors and mechanisms explaining spatial heterogeneity: A review of methods for insect populations. Methods Ecol. Evol. 2011, 2, 11–22. [Google Scholar] [CrossRef]
- Da-Silva, R.S.; Kumar, L.; Shabani, F.; Da Silva, E.M.; da Silva Galdino, T.V.; Picanço, M.C. Spatio-temporal dynamic climate model for Neoleucinodes elegantalis using CLIMEX. Int. J. Biometeorol. 2017, 61, 785–795. [Google Scholar] [CrossRef]
- Milonas, P.; Gogou, C.; Papadopoulou, A.; Fountas, S.; Liakos, V.; Papadopoulos, N.T. Spatio-temporal distribution of Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae) and Pectinophora gossypiella (Saunders) (Lepidoptera: Gelechiidae) in a cotton production area. Neo. Entomol. 2016, 45, 240–251. [Google Scholar] [CrossRef]
- Hernandez-Mendoza, J.L.; López-Barbosa, E.C.; Garza-González, E.; Mayek-Perez, N. Spatial distribution of Spodoptera frugiperda (Lepidoptera: Noctuidae) in maize landraces grown in Colima, México. Int. J. Trop. Insect Sci. 2008, 28, 126–129. [Google Scholar] [CrossRef]
- Merkl, M.E.; Pfrilllmcr, T.R. Light·trap investigations at Stoncville. J. Econ. Entomol. 1995, 48, 740–743. [Google Scholar] [CrossRef]
- Sajjad, M.; Sarwar, Z.M.; Binyameen, M.; Qasim, M.; Bashir, M.H. A New Species of Genus Agrotis Ochsenheimer (Lepidoptera: Noctuidae) in Pakistan. J. Anim. Plant Sci. 2020, 30, 685–692. [Google Scholar]
- Khan, M.R.; Rafi, M.A.; Nazir, N.; Khan, M.R.; Khan, I.A.; Hayat, A.; Ghaffar, A.; Rahim, J.; Perveen, F. Biodiversity of butterflies from poonch division of Azad Kashmir, Pakistan. J. Agric. Technol. 2014, 10, 885–898. [Google Scholar]
- Fonseca-Medrano, M.; Specht, A.; Silva, F.A.M.; Otanásio, P.N.; Malaquias, J.V. The population dynamics of three polyphagous owlet moths (Lepidoptera: Noctuidae) and the influence of meteorological factors and ENSO on them. Rev. Bras. Entomol. 2020, 63, 308–315. [Google Scholar] [CrossRef]
- Santos, S.R.D.; Specht, A.; Carneiro, E.; Casagrande, M.M. The influence of agricultural occupation and climate on the spatial distribution of Plusiinae (Lepidoptera: Noctuidae) on a latitudinal gradient in Brazil. Rev. Bras. Entomol. 2021, 65. [Google Scholar] [CrossRef]
- Jian-Yu, L.; Yan-Ting, C.; Meng-Zhu, S.; Jian-Wei, L.; Rui-Bin, X.; Gabor, P.; Shen, M. Spatio-temporal distribution patterns of Plutella xylostella (Lepidoptera: Plutellidae) in a fine-scale agricultural landscape based on geostatistical analysis. Sci. Rep. 2021, 11, 13622. [Google Scholar]
- Xiong, L.G.; Wu, Q.J.; Wang, S.L.; Xu, B.Y.; Zhu, G.R.; Zhang, Y.J. Biological characteristic of overwintering in the diamondback moth, Plutella xylostella. Plant Prot. 2010, 36, 90–93. [Google Scholar]
- Frank, K.D.; Rich, C.; Longcore, T. Effects of artificial night lighting on moths. In Ecological Consequences of Artificial Night Lighting; Island Press: Washington, DC, USA, 2006; pp. 305–344. [Google Scholar]
- Zhao, Z.H.; Hui, C.; He, D.H.; Ge, F. Effects of position within wheat field and adjacent habitats on the density and diversity of cereal aphids and their natural enemies. Biocontrol 2013, 58, 765–776. [Google Scholar] [CrossRef]
- Sciarretta, A.; Trematerra, P. Geostatistical tools for the study of insect spatial distribution: Practical implications in the integrated management of orchard and vineyard pests. Plant Prot. Sci. 2014, 50, 97–110. [Google Scholar] [CrossRef] [Green Version]
- Furlong, M.J.; Wright, D.J.; Dosdall, L.M. Diamondback moth ecology and management: Problems, progress, and prospects. Annu. Rev. Entomol. 2013, 58, 517–541. [Google Scholar] [CrossRef]
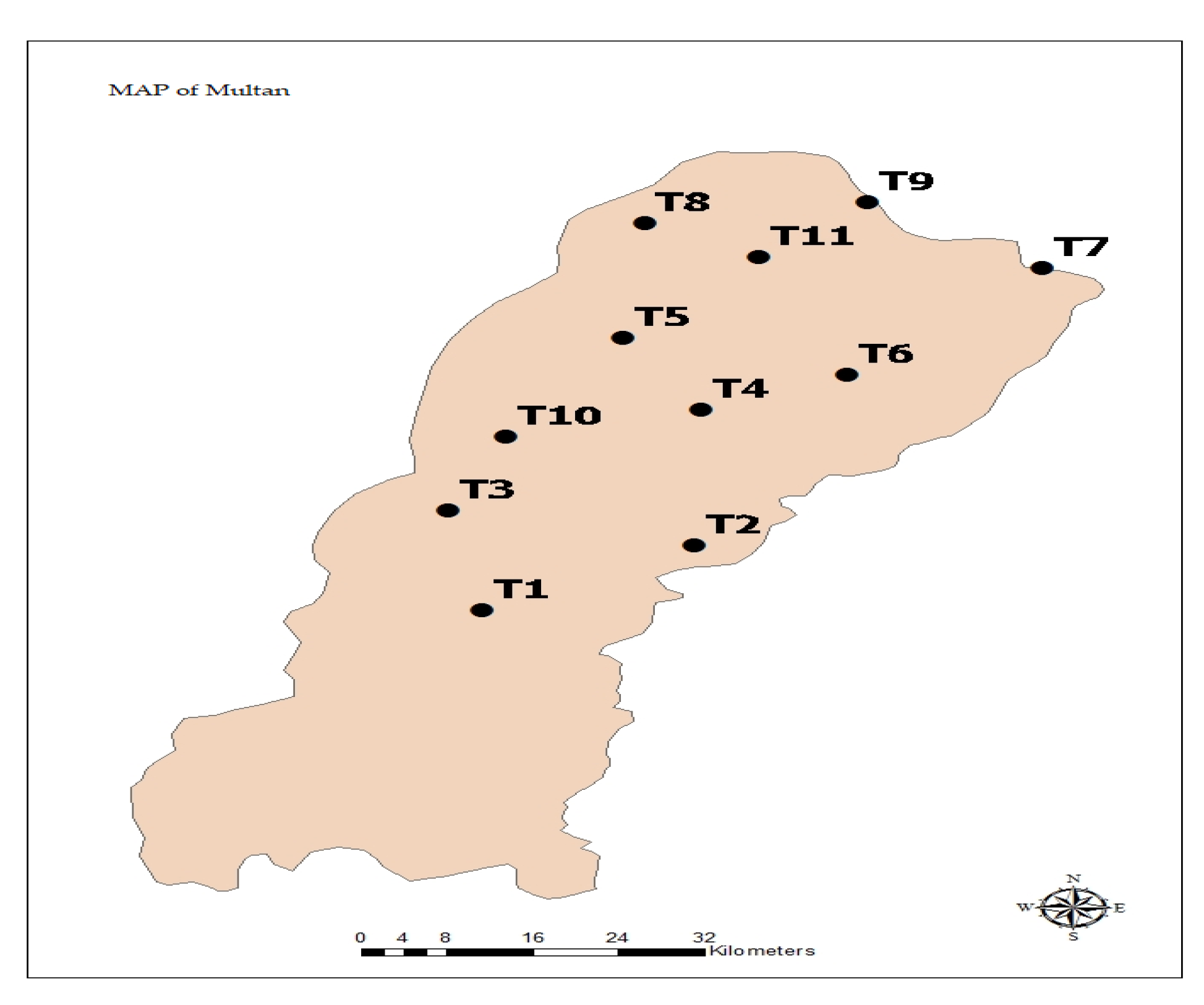


| Traps | Coordinates | Habitat | Location |
|---|---|---|---|
| T1 | 29.7591 N; 71.3203 E | Cotton, Rice, Maize, Tomato | Crop area/Near urban |
| T2 | 29.8255 N; 71.5593 E | Maize, Rice, Mung bean | Crop area/Near urban |
| T3 | 29.9017 N; 71.2915 E | Forest | Forest area/Near urban |
| T4 | 30.0468 N; 71.5044 E | Forest | Forest area/Near urban |
| T5 | 30.1491 N; 71.4387 E | Forest | Forest area/Near urban |
| T6 | 30.0960 N; 71.6275 E | Rice, Maize, Sugarcane | Crop area/Near urban |
| T7 | 30.2497 N; 71.7916 E | Maize, Cotton, Cabbage, Tomato, Okra | Crop area/Away urban |
| T8 | 30.3138 N; 71.4565 E | Cotton, Cabbage, Okra, Grasses | Crop area/Away urban |
| T9 | 30.3440 N; 71.6446 E | Cotton, Maize | Crop area/Away urban |
| T10 | 30.0080 N; 71.3404 E | Cotton, Maize, tomato, Sorghum | Crop area/Away urban |
| T11 | 30.2646 N; 71.5526 E | Maize, Cotton, Cabbage, Okra | Crop area/Away urban |
| Rank | Species Name | Abundance | Relative Abundance (%) | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | T11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | S. litura | 3171 | 18.6% | 595 | 187 | 43 | 72 | 80 | 84 | 401 | 262 | 522 | 574 | 351 |
| 2 | H. Tripoli | 2074 | 12.2% | 325 | 212 | 17 | 26 | 69 | 74 | 253 | 188 | 322 | 289 | 299 |
| 3 | H. jahangiri | 1813 | 10.6% | 484 | 219 | 19 | 59 | 42 | 0 | 292 | 0 | 464 | 234 | 0 |
| 4 | H. armigera | 1724 | 10.1% | 187 | 98 | 81 | 87 | 48 | 127 | 221 | 189 | 197 | 246 | 243 |
| 5 | S. exigua | 1480 | 8.7% | 267 | 93 | 59 | 66 | 60 | 151 | 297 | 0 | 286 | 201 | 0 |
| 6 | S. furgiperda | 1034 | 6.1% | 24 | 121 | 11 | 28 | 164 | 212 | 414 | 0 | 0 | 0 | 60 |
| 7 | H. stigmosa | 721 | 4.2% | 96 | 104 | 0 | 15 | 0 | 0 | 112 | 68 | 72 | 132 | 122 |
| 8 | A. ipsilon | 486 | 2.8% | 17 | 18 | 0 | 4 | 31 | 0 | 61 | 118 | 53 | 93 | 91 |
| 9 | C. placodoides | 449 | 2.6% | 77 | 39 | 74 | 49 | 25 | 0 | 67 | 0 | 0 | 0 | 118 |
| 10 | A. album | 442 | 2.6% | 42 | 11 | 41 | 25 | 32 | 65 | 0 | 109 | 0 | 0 | 117 |
| 11 | C. replete | 366 | 2.1% | 37 | 28 | 52 | 21 | 48 | 27 | 80 | 0 | 0 | 0 | 73 |
| 12 | A. cinerea | 352 | 2.1% | 15 | 19 | 43 | 15 | 29 | 0 | 47 | 110 | 0 | 28 | 46 |
| 13 | E. insulana | 348 | 2% | 21 | 24 | 12 | 9 | 15 | 22 | 73 | 0 | 74 | 98 | 0 |
| 14 | M. loreyi | 323 | 1.9% | 0 | 0 | 10 | 15 | 21 | 0 | 26 | 0 | 56 | 93 | 102 |
| 15 | L. oleracea | 293 | 1.7% | 8 | 0 | 34 | 31 | 0 | 45 | 0 | 59 | 0 | 0 | 116 |
| 16 | E. vitella | 281 | 1.6% | 24 | 19 | 18 | 19 | 24 | 29 | 67 | 0 | 47 | 34 | 0 |
| 17 | E. conducta | 279 | 1.6% | 0 | 0 | 16 | 24 | 0 | 55 | 0 | 65 | 63 | 56 | 0 |
| 18 | M. brassicae | 276 | 1.6% | 5 | 0 | 11 | 9 | 0 | 0 | 0 | 78 | 0 | 0 | 173 |
| 19 | H. platigera | 240 | 1.4% | 24 | 11 | 16 | 10 | 0 | 12 | 80 | 0 | 0 | 0 | 87 |
| 20 | L. venalba | 237 | 1.4% | 0 | 0 | 22 | 32 | 0 | 48 | 0 | 85 | 50 | 0 | 0 |
| 21 | C. albostriata | 203 | 1.2% | 0 | 0 | 0 | 0 | 0 | 50 | 56 | 0 | 0 | 0 | 97 |
| 22 | A. decisissima | 185 | 1.1% | 0 | 0 | 0 | 0 | 30 | 0 | 47 | 46 | 0 | 0 | 62 |
| 23 | D. hoenei | 160 | 0.9% | 0 | 0 | 0 | 0 | 22 | 30 | 0 | 53 | 55 | 0 | 0 |
| 24 | C. furthatai | 83 | 0.5% | 0 | 0 | 0 | 0 | 0 | 25 | 42 | 0 | 0 | 16 | 0 |
| Total Individuals | N = 17020 | ΣN = 100% | N1 = 2248 | N2 = 1203 | N3 = 579 | N4 = 616 | N5 = 740 | N6 = 1056 | N7 = 2636 | N8 = 1430 | N9 = 2261 | N10 = 2094 | N11 = 2157 | |
| Species | Temperature (°C) | Humidity (%) | Rainfall (mm) | SE | Pooled OLS (F-Test) Value | |||
|---|---|---|---|---|---|---|---|---|
| Coefficient | p Value | Coefficient | p Value | Coefficient | p Value | |||
| H. stigmosa | 1.08 * | <0.0001 | 0.90 * | <0.0001 | −6.14 * | <0.0001 | 6.39 | 9.28× 1014 |
| H. trifoli | 3.11 * | <0.0001 | 2.40 * | <0.0001 | −16.68 * | <0.0001 | 12.12 | 1.40 × 1023 |
| H. jahangiri | 2.43 * | <0.0001 | 1.70 * | <0.0001 | −13.18 * | <0.0001 | 14.27 | 4.67 × 1021 |
| S. litura | 4.37 * | <0.0001 | 3.12 * | <0.0001 | −22.38 * | <0.0001 | 17.23 | 3.51× 1026 |
| S. exigua | 2.13 * | <0.0001 | 1.47 * | <0.0001 | −11.21 * | <0.0001 | 9.83 | 3.78× 1022 |
| H. armigera | 2.83 * | <0.0001 | 2.17 * | <0.0001 | −14.69 * | <0.0001 | 8.53 | 6.22× 1029 |
| H. platigera | 0.48 * | <0.0001 | 0.46 * | <0.0001 | −2.78 * | <0.0001 | 3.50 | 1.14× 1012 |
| C. placodoides | 1.02 * | <0.0001 | 0.96 * | <0.0001 | −6.24 * | <0.0001 | 5.74 | 3.31× 1013 |
| C. repleta | 0.85 * | <0.0001 | 0.78 * | <0.0001 | −4.92 * | <0.0001 | 3.86 | 1.55 × 1017 |
| A. ipsilon | 0.95 * | <0.0001 | 0.92 * | <0.0001 | −5.40 * | <0.0001 | 5.31 | 1.39 × 1014 |
| A. cinerea | 0.78 * | <0.0001 | 0.82 * | <0.0001 | −4.57 * | <0.0001 | 3.98 | 1.77 × 1016 |
| A. album | 1.03 * | <0.0001 | 1.01 * | <0.0001 | −5.83 * | <0.0001 | 5.46 | 3.97 × 1015 |
| A. decisissima | 0.32 * | <0.0001 | 0.26 * | <0.0001 | −1.73 * | <0.0001 | 2.70 | 2.72 × 1011 |
| E. insulana | 0.77 * | <0.0001 | 0.79 * | <0.0001 | −4.20 * | <0.0001 | 4.09 | 2.15 × 1016 |
| E. vitelli | 0.64 * | <0.0001 | 0.67 * | <0.0001 | −3.33 * | <0.0001 | 3.07 | 1.56 × 1016 |
| C. furithatai | 0.17 * | <0.0001 | 0.18 * | <0.0001 | 0.90 * | 0.0018 | 1.93 | 3.38 × 1007 |
| L. oleracea | 0.58 * | <0.0001 | 0.52 * | <0.0001 | −2.84 * | <0.0001 | 4.49 | 7.14 × 1011 |
| E. conducta | 0.57 * | <0.0001 | 0.56 * | <0.0001 | −2.86 * | <0.0001 | 3.70 | 1.48 × 1012 |
| L. venalba | 0.51 * | <0.0001 | 0.50 * | <0.0001 | −2.67 * | <0.0001 | 3.72 | 3.35 × 1011 |
| D. hoenei | 0.32 * | <0.0001 | 0.32 * | <0.0001 | −1.79 * | <0.0001 | 2.97 | 2.05 × 1008 |
| C. albostriata | 0.39 * | <0.0001 | 0.37 * | <0.0001 | −2.12 * | 0.0002 | 3.77 | 2.33 × 1010 |
| M. loreyi | 0.67 * | <0.0001 | 0.66 * | <0.0001 | −3.83 * | <0.0001 | 4.16 | 1.47 × 1014 |
| M. brassicae | 0.52 * | <0.0001 | 0.53 * | <0.0001 | −2.99 * | 0.0003 | 5.54 | 2.62 × 1011 |
| S. furgiperda | 0.84 * | <0.0001 | 0.51 * | 0.0036 | −3.91 * | 0.0007 | 7.64 | 9.35 × 1025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, Z.; Sarwar, Z.M.; Akbar, A.; Alhag, S.K.; Ahmed, N.; Alam, P.; Almadiy, A.A.; Zouidi, F.; Jawalkar, N.B. Spatiotemporal Distribution Patterns of Pest Species (Lepidoptera: Noctuidae) Affected by Meteorological Factors in an Agroecosystem. Agriculture 2022, 12, 2003. https://doi.org/10.3390/agriculture12122003
Hussain Z, Sarwar ZM, Akbar A, Alhag SK, Ahmed N, Alam P, Almadiy AA, Zouidi F, Jawalkar NB. Spatiotemporal Distribution Patterns of Pest Species (Lepidoptera: Noctuidae) Affected by Meteorological Factors in an Agroecosystem. Agriculture. 2022; 12(12):2003. https://doi.org/10.3390/agriculture12122003
Chicago/Turabian StyleHussain, Zafar, Zahid Mahmood Sarwar, Atif Akbar, Sadeq K. Alhag, Nazeer Ahmed, Pravej Alam, Abdulrahman A. Almadiy, Ferjeni Zouidi, and Nilesh Baburao Jawalkar. 2022. "Spatiotemporal Distribution Patterns of Pest Species (Lepidoptera: Noctuidae) Affected by Meteorological Factors in an Agroecosystem" Agriculture 12, no. 12: 2003. https://doi.org/10.3390/agriculture12122003
APA StyleHussain, Z., Sarwar, Z. M., Akbar, A., Alhag, S. K., Ahmed, N., Alam, P., Almadiy, A. A., Zouidi, F., & Jawalkar, N. B. (2022). Spatiotemporal Distribution Patterns of Pest Species (Lepidoptera: Noctuidae) Affected by Meteorological Factors in an Agroecosystem. Agriculture, 12(12), 2003. https://doi.org/10.3390/agriculture12122003







