Effects of Kaolin and Shading Net on the Ecophysiology and Berry Composition of Sauvignon Blanc Grapevines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting and Pilot Design
2.2. Leaf Gas Exchange, Predawn and Midday Leaf Water Potential, Leaf-Specific Hydraulic Conductance, Leaf Chlorophyll a Fluorescence, and Content
2.3. Berry Temperature and Composition
2.4. Statistical Analysis
3. Results
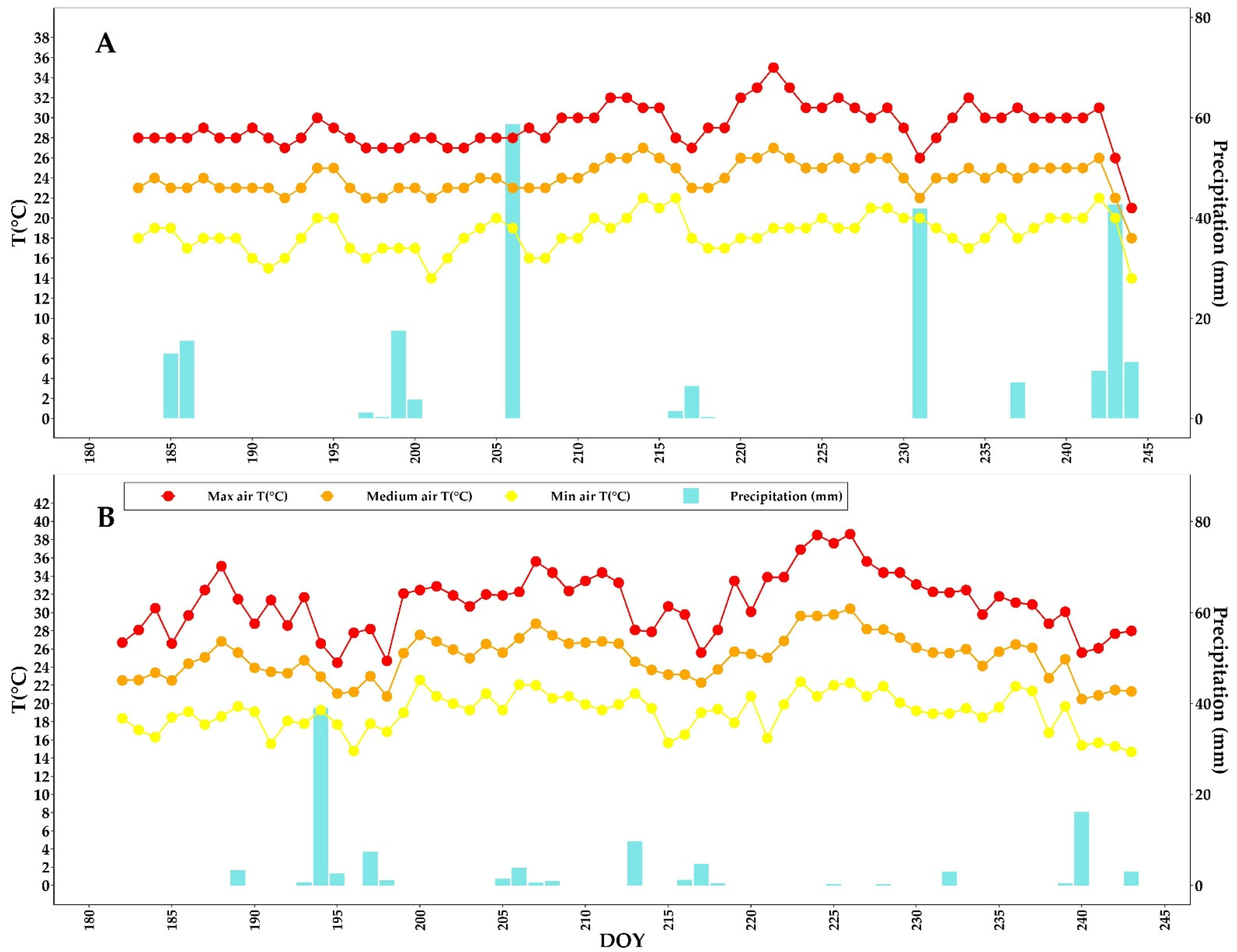
3.1. Meteorological Conditions
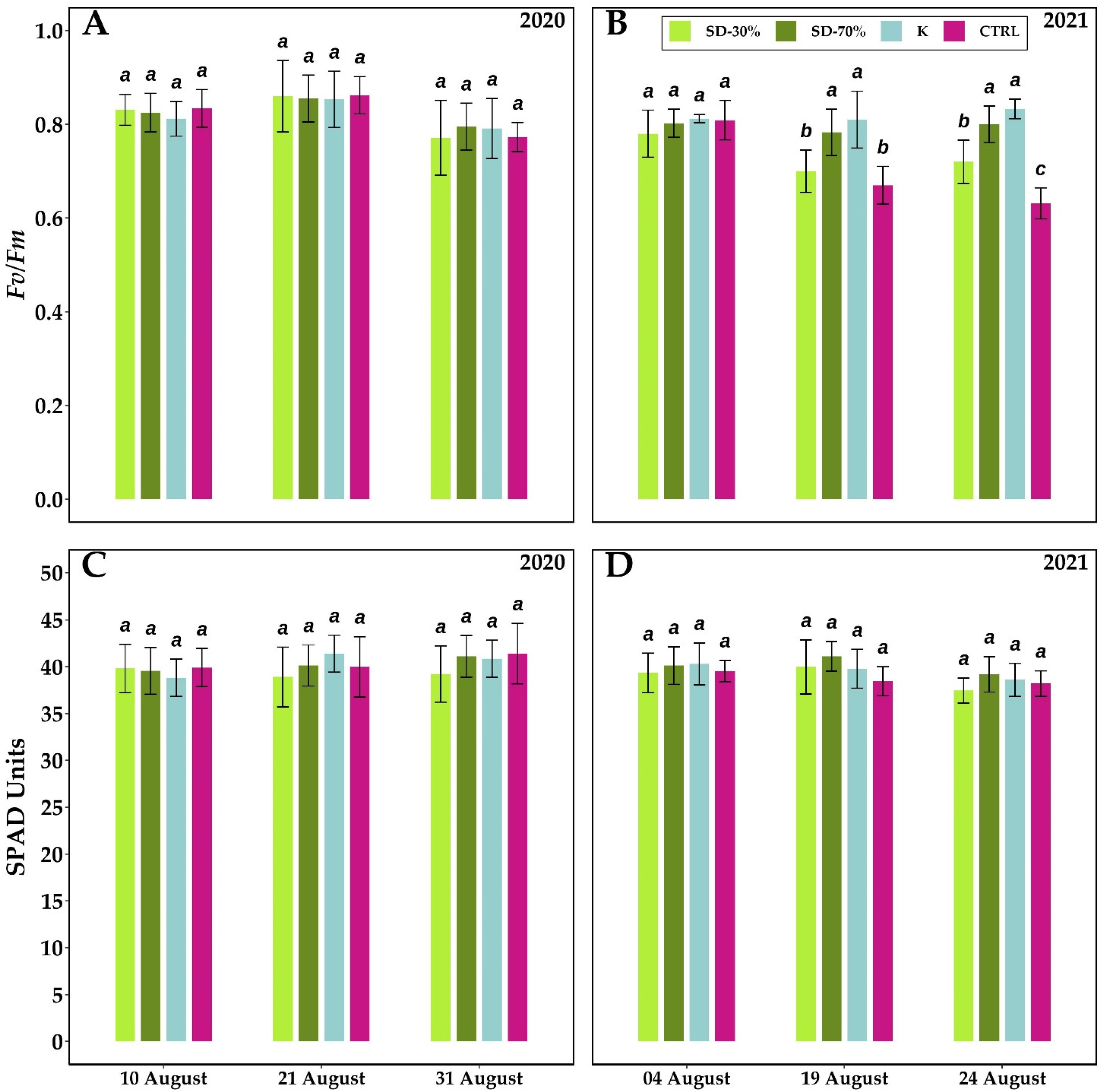
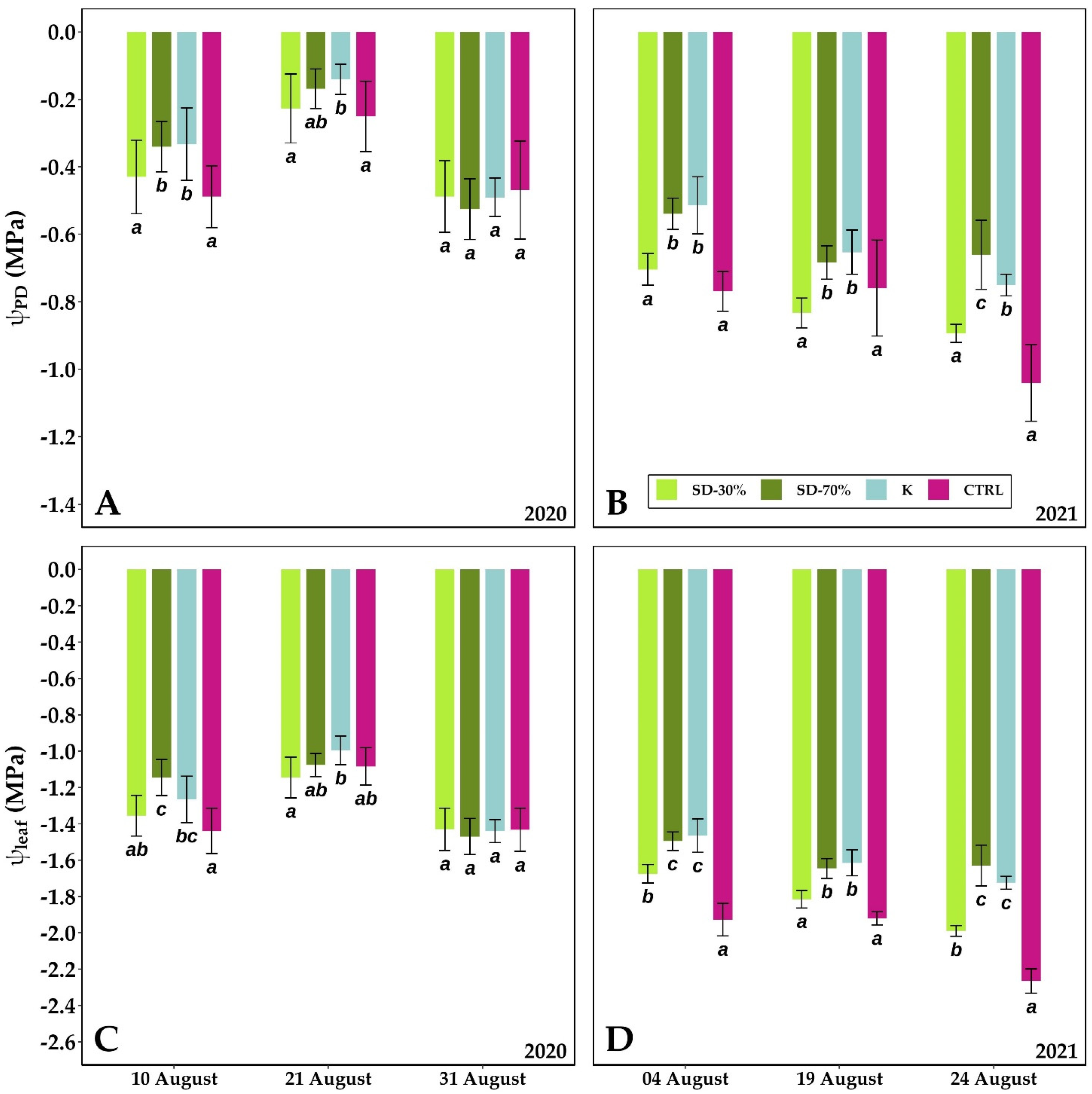
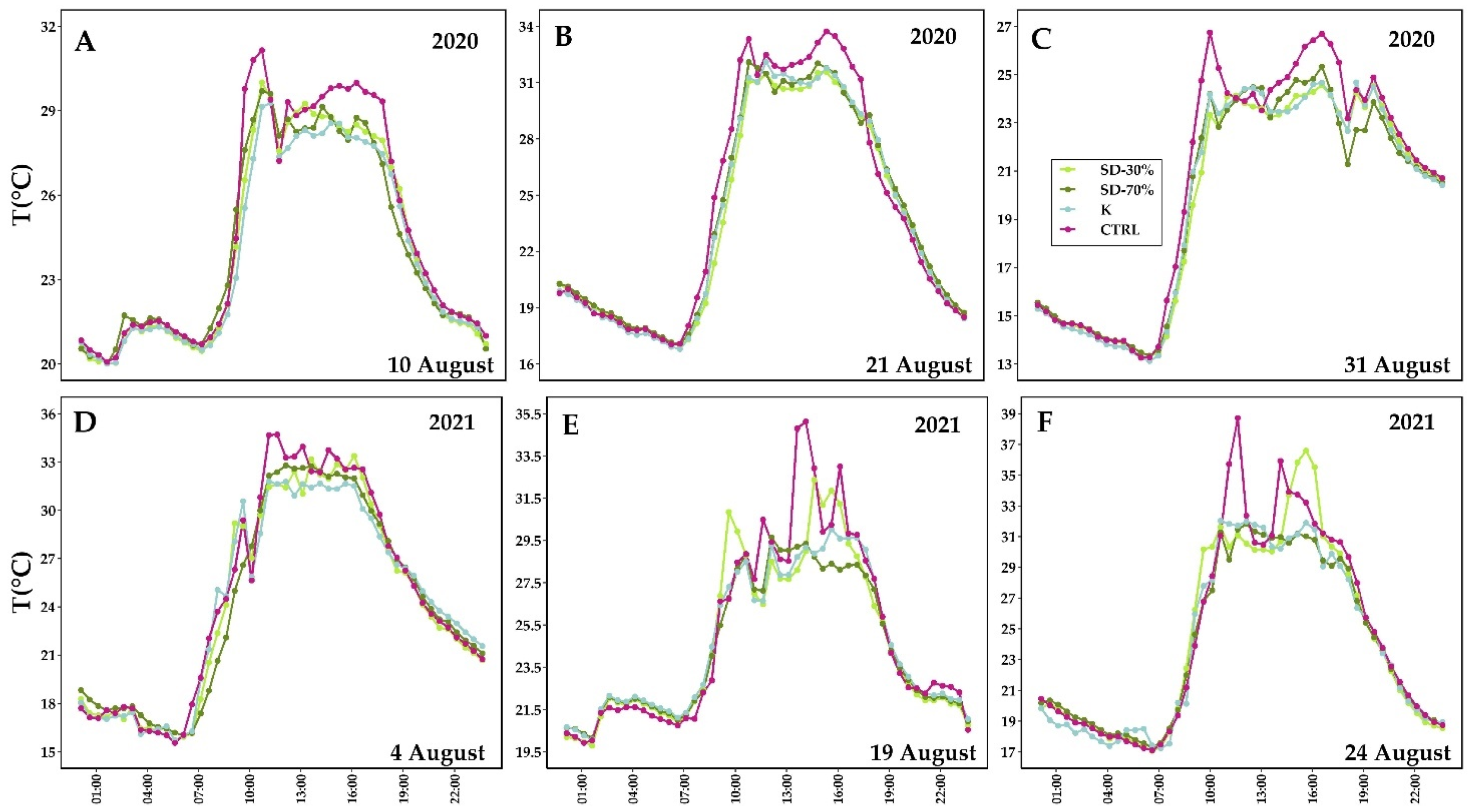
3.2. Leaf Gas Exchange, Predawn and Leaf Water Potential, Leaf-Specific Hydraulic Conductance, Leaf Chlorophyll a Fluorescence and Content
3.3. Berry Temperature and Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Thornton, P.K.; Ericksen, P.J.; Herrero, M.; Challinor, A.J. Climate variability and vulnerability to climate change: A review. Glob. Chang. Biol. 2014, 20, 3313–3328. [Google Scholar] [CrossRef]
- Mitchell, J.F.; Lowe, J.; Wood, R.A.; Vellinga, M. Extreme events due to human-induced climate change. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2006, 364, 2117–2133. [Google Scholar] [CrossRef]
- Meehl, G.A.; Covey, C.; Delworth, T.; Latif, M.; McAvaney, B.; Mitchell, J.F.; Sttouffer, R.J.; Taylor, K.E. The WCRP CMIP3 multimodel dataset: A new era in climate change research. Bull. Am. Meteorol. Soc. 2007, 88, 1383–1394. [Google Scholar] [CrossRef] [Green Version]
- Dong, Z.; Pan, Z.; An, P.; Wang, L.; Zhang, J.; He, D.; Han, H.; Pan, X. A novel method for quantitatively evaluating agricultural vulnerability to climate change. Ecol. Indic. 2015, 48, 49–54. [Google Scholar] [CrossRef]
- Pokhrel, Y.; Felfelani, F.; Satoh, Y.; Boulange, J.; Burek, P.; Gädeke, A.; Wada, Y. Global terrestrial water storage and drought severity under climate change. Nat. Clim. Chang. 2021, 11, 226–233. [Google Scholar] [CrossRef]
- Irimia, L.M.; Patriche, C.V.; Renan, L.; Herve, Q.; Cyril, T.; Sfîcă, L. Projections of climate suitability for wine production for the Cotnari wine region (Romania). Environ. Dev. Sustain. 2019, 1, 5–18. [Google Scholar]
- Ammoniaci, M.; Kartsiotis, S.P.; Perria, R.; Storchi, P. State of the art of monitoring technologies and data processing for precision viticulture. Agriculture 2021, 11, 201. [Google Scholar] [CrossRef]
- Jones, G.V. The climate component of terroir. Elem. An. Int. Mag. Mineral. Geochem. Petrol. 2018, 14, 167–172. [Google Scholar] [CrossRef]
- Priori, S.; Pellegrini, S.; Perria, R.; Puccioni, S.; Storchi, P.; Valboa, G.; Costantini, E.A. Scale effect of terroir under three contrasting vintages in the Chianti Classico area (Tuscany, Italy). Geoderma 2019, 334, 99–112. [Google Scholar] [CrossRef]
- Bindi, M.; Miglietta, F.; Gozzini, B.; Orlandini, S.; Seghi, L. A simple model for simulation of growth and development in grapevine (Vitis vinifera L.). 1. Model description. VITIS-J. Grapevine Res. 2015, 36, 67. [Google Scholar]
- Reshef, N.; Agam, N.; Fait, A. Grape berry acclimation to excessive solar irradiance leads to repartitioning between major flavonoid groups. J. Agric. Food Chem. 2018, 66, 3624–3636. [Google Scholar] [CrossRef] [PubMed]
- Del-Castillo-Alonso, M.Á.; Monforte, L.; Tomás-Las-Heras, R.; Ranieri, A.; Castagna, A.; Martínez-Abaigar, J.; Núñez-Olivera, E. Secondary metabolites and related genes in Vitis vinifera L. cv. Tempranillo grapes as influenced by ultraviolet radiation and berry development. Physiol. Plant. 2021, 173, 709–724. [Google Scholar] [CrossRef] [PubMed]
- De Orduna, R.M. Climate change associated effects on grape and wine quality and production. Int. Food Res. J. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Ju, Y.L.; Yue, X.F.; Zhao, X.F.; Zhao, H.; Fang, Y.L. Physiological, micro-morphological and metabolomic analysis of grapevine (Vitis vinifera L.) leaf of plants under water stress. Plant Physiol. Biochem. 2018, 130, 501–510. [Google Scholar] [CrossRef]
- Erasmus, D.J.; van der Merwe, G.K.; van Vuuren, H.J. Genome-wide expression analyses: Metabolic adaptation of Saccharomyces cerevisiae to high sugar stress. FEMS Yeast Res. 2003, 3, 375–399. [Google Scholar] [CrossRef] [Green Version]
- Coulter, A.D.; Henschke, P.A.; Simos, C.A.; Pretorius, I.S. When the heat is on, yeast fermentation runs out of puff. Aust. NZ Wine Ind. J. 2008, 23, 29–33. [Google Scholar]
- Palliotti, A.; Tombesi, S.; Silvestroni, O.; Lanari, V.; Gatti, M.; Poni, S. Changes in vineyard establishment and canopy management urged by earlier climate-related grape ripening: A review. Sci. Hortic. 2014, 178, 43–54. [Google Scholar] [CrossRef]
- Jones, G.V. Climate, grapes and wine: Structure and suitability in a changing climate. In Proceedings of the International Symposium on the XXVIII International Horticultural Congress on Science and Horticulture for People (IHC2010), Lisbon, Portugal, 22–27 August 2010; pp. 19–28. [Google Scholar]
- Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Santos, J.A. An overview of climate change impacts on European viticulture. Food Energy Secur. 2012, 1, 94–110. [Google Scholar] [CrossRef]
- Hall, A.; Jones, G.V. Effect of potential atmospheric warming on temperature-based indices describing Australian winegrape growing conditions. Aust. J. Grape Wine Res. 2009, 15, 97–119. [Google Scholar] [CrossRef]
- Droulia, F.; Charalampopoulos, I. Future Climate Change Impacts on European Viticulture: A Review on Recent Scientific Advances. Atmosphere 2021, 12, 495. [Google Scholar] [CrossRef]
- Lobit, P.; Genard, M.; Soing, P.; Habib, R. Modelling malic acid accumulation in fruits: Relationships with organic acids, potassium, and temperature. J. Exp. Bot. 2006, 57, 1471–1483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, A.; Eiras-Dias, J.; Castellarin, S.D.; Gerós, H. Berry phenolics of grapevine under challenging environments. Int. J. Mol. Sci. 2013, 14, 18711–18739. [Google Scholar] [CrossRef] [Green Version]
- Koch, A.; Doyle, C.L.; Matthews, M.A.; Williams, L.E.; Ebeler, S.E. 2-Methoxy-3-isobutylpyrazine in grape berries and its dependence on genotype. Phytochemistry 2010, 71, 2190–2198. [Google Scholar] [CrossRef]
- Deloire, A.J. Grapevine berry ripening and wine aroma. Wynboer 2012, 269, 108. [Google Scholar]
- Šuklje, K.; Česnik, H.B.; Janeš, L.; Kmecl, V.; Vanzo, A.; Deloire, A.; Sivillotti, P.; Lisjak, K. The effect of leaf area to yield ratio on secondary metabolites in grapes and wines of Vitis vinifera L. cv. Sauvignon blanc. OENO One 2013, 47, 83–97. [Google Scholar] [CrossRef]
- Borgogno-Mondino, E.; de Palma, L.; Novello, V. Investigating Sentinel 2 Multispectral Imagery Efficiency in Describing Spectral Response of Vineyards Covered with Plastic Sheets. Agronomy 2020, 10, 1909. [Google Scholar] [CrossRef]
- Bergqvist, J.; Dokoozlian, N.; Ebisuda, N. Sunlight exposure and temperature effects on berry growth and composition of Cabernet Sauvignon and Grenache in the Central San Joaquin Valley of California. Am. J. Enol. Vitic. 2021, 52, 1–7. [Google Scholar]
- Van Leeuwen, C.; Destrac-Irvine, A. Modified grape composition under climate change conditions requires adaptations in the vineyard. Oeno One 2017, 51, 147–154. [Google Scholar] [CrossRef]
- Carlomagno, A.; Novello, V.; Ferrandino, A.; Genre, A.; Lovisolo, C.; Hunter, J.J. Pre-harvest berry shrinkage in cv ‘Shiraz’ (Vitis vinifera L.): Understanding sap flow by means of tracing. Sci. Hortic. 2018, 233, 394–406. [Google Scholar] [CrossRef]
- Lobos, G.A.; Acevedo-Opazo, C.; Guajardo-Moreno, A.; Valdés-Gómez, H.; Taylor, J.A.; Laurie, V.F. Effects of kaolin-based particle film and fruit zone netting on Cabernet Sauvignon grapevine physiology and fruit quality. OENO One 2015, 49, 137–144. [Google Scholar] [CrossRef]
- Georgieva, K.; Tsonev, T.; Velikova, V.; Yordanov, I. Photosynthetic activity during high temperature treatment of pea plants. J. Plant Physiol. 2000, 157, 169–176. [Google Scholar] [CrossRef]
- Lei, Y.; Xie, S.; Guan, X.; Song, C.; Zhang, Z.; Meng, J. Methoxypyrazines biosynthesis and metabolism in grape: A review. Food Chem. 2018, 245, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Mattii, G.B. Effect of Agronomic Techniques on Aroma Composition of White Grapevines: A Review. Agronomy 2021, 11, 2027. [Google Scholar] [CrossRef]
- Scafidi, P.; Pisciotta, A.; Patti, D.; Tamborra, P.; Di Lorenzo, R.; Barbagallo, M.G. Effect of artificial shading on the tannin accumulation and aromatic composition of the Grillo cultivar (Vitis vinifera L.). BMC Plant Biol. 2013, 13, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cataldo, E.; Salvi, L.; Paoli, F.; Fucile, M.; Mattii, G.B. Effects of Defoliation at Fruit Set on Vine Physiology and Berry Composition in Cabernet Sauvignon Grapevines. Plants 2021, 10, 1183. [Google Scholar] [CrossRef] [PubMed]
- Chorti, E.; Guidoni, S.; Ferrandino, A.; Novello, V. Effect of different cluster sunlight exposure levels on ripening and anthocyanin accumulation in Nebbiolo grapes. Am. J. Enol. Vitic. 2010, 61, 23–30. [Google Scholar]
- Jeong, S.T.; Goto-Yamamoto, N.; Kobayashi, S.; Esaka, M.J.P.S. Effects of plant hormones and shading on the accumulation of anthocyanins and the expression of anthocyanin biosynthetic genes in grape berry skins. Plant Sci. 2004, 167, 247–252. [Google Scholar] [CrossRef]
- Gutiérrez-Gamboa, G.; Zheng, W.; de Toda, F.M. Current viticultural techniques to mitigate the effects of global warming on grape and wine quality: A comprehensive review. Int. Food Res. J. 2020, 139, 109946. [Google Scholar] [CrossRef]
- Tzortzakis, N.; Chrysargyris, A.; Aziz, A. Adaptive response of a native Mediterranean grapevine cultivar upon short-term exposure to drought and heat stress in the context of climate change. Agronomy 2020, 10, 249. [Google Scholar] [CrossRef] [Green Version]
- Dinis, L.T.; Bernardo, S.; Matos, C.; Malheiro, A.; Flores, R.; Alves, S.; Costa, C.; Rocha, S.; Correia, C.; Luzio, A.; et al. Overview of Kaolin Outcomes from vine to wine: Cerceal white variety case study. Agronomy 2020, 10, 1422. [Google Scholar] [CrossRef]
- Singh, R.K.; Afonso, J.; Nogueira, M.; Oliveira, A.A.; Cosme, F.; Falco, V. Silicates of Potassium and Aluminium (Kaolin); Comparative Foliar Mitigation Treatments and Biochemical Insight on Grape Berry Quality in Vitis vinifera L. (cv. Touriga National and Touriga Franca). Biology 2020, 9, 58. [Google Scholar] [CrossRef] [Green Version]
- Bernardo, S.; Dinis, L.T.; Machado, N.; Barros, A.; Pitarch-Bielsa, M.; Malheiro, A.C.; Gómez-Cadenas, A.; Moutinho-Pereira, J. Uncovering the effects of kaolin on balancing berry phytohormones and quality attributes of Vitis vinifera grown in warm-temperate climate regions. J. Sci. Food Agric. 2021, 102, 782–793. [Google Scholar] [CrossRef]
- Ćosić, M.; Stričević, R.; Djurović, N.; Lipovac, A.; Bogdan, I.; Pavlović, M. Effects of irrigation regime and application of kaolin on canopy temperatures of sweet pepper and tomato. Sci. Hortic. 2018, 238, 23–31. [Google Scholar] [CrossRef]
- AbdAllah, A. Impacts of Kaolin and Pinoline foliar application on growth, yield and water use efficiency of tomato (Solanum lycopersicum L.) grown under water deficit: A comparative study. J. Saudi Soc. Agric. 2019, 18, 256–268. [Google Scholar] [CrossRef]
- Marko, V.; Blommers, L.H.M.; Bogya, S.; Helsen, H. Kaolin particle films suppress many apple pests, disrupt natural enemies and promote woolly apple aphid. J. Appl. Entomol. 2008, 132, 26–35. [Google Scholar] [CrossRef]
- Salerno, G.; Rebora, M.; Kovalev, A.; Gorb, E.; Gorb, S. Kaolin nano-powder effect on insect attachment ability. J. Pest. Sci. 2020, 93, 315–327. [Google Scholar] [CrossRef]
- Tubajika, K.M.; Civerolo, E.L.; Puterka, G.J.; Hashim, J.M.; Luvisi, D.A. The effects of kaolin, harpin, and imidacloprid on development of Pierce’s disease in grape. Crop Prot. 2007, 26, 92–99. [Google Scholar] [CrossRef]
- Glenn, D.M. The mechanisms of plant stress mitigation by kaolin-based particle films and applications in horticultural and agricultural crops. HortScience 2012, 47, 710–711. [Google Scholar] [CrossRef] [Green Version]
- Glenn, M.D.; Wuensche, J.; McIvor, I.; Nissen, R.; George, A. Ultraviolet radiation effects on fruit surface respiration and chlorophyll fluorescence. J. Hortic. Sci. Biotechnol. 2008, 83, 43–50. [Google Scholar] [CrossRef]
- Glenn, D.M. Particle film mechanisms of action that reduce the effect of environmental stress in ‘Empire’ apple. J. Am. Soc. Hortic. Sci. 2009, 134, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.C.; Wei, W.; Wang, Y.; Duan, C.Q.; Chen, W.; Wang, J. Effects of sunlight exclusion on leaf gas exchange, berry composition, and wine flavour profile of Cabernet-Sauvignon from the foot of the north side of Mount Tianshan and a semi-arid continental climate. OENO One 2021, 55, 267–283. [Google Scholar] [CrossRef]
- Naulleau, A.; Gary, C.; Prévot, L.; Hossard, L. Evaluating strategies for adaptation to climate change in grapevine production–A systematic review. Front. Plant Sci. 2021, 11, 2154. [Google Scholar] [CrossRef] [PubMed]
- Novello, V.; De Palma, L. Viticultural strategy to reduce alcohol levels in wine. In Alcohol Level Reduction in Wine (pp. 3–8). Vigne et Vin Publications Internationales; Oenoviti International Network: Bordeaux, France, 2013. [Google Scholar]
- Villalobos-Soublett, E.; Gutiérrez-Gamboa, G.; Balbontín, C.; Zurita-Silva, A.; Ibacache, A.; Verdugo-Vásquez, N. Effect of Shading Nets on Yield, Leaf Biomass and Petiole Nutrients of a Muscat of Alexandria Vineyard Growing under Hyper-Arid Conditions. Horticulturae 2021, 7, 445. [Google Scholar] [CrossRef]
- Zha, Q.; Wu, J.; Xi, X.; He, Y.; Yin, X.; Jiang, A. Effects of Colored Shade Nets on Grapes and Leaves of Shine Muscat Grown under Greenhouse Conditions. Am. J. Enol. Vitic. 2022, 73, 39–47. [Google Scholar] [CrossRef]
- Zhang, Z.; Qiao, D.; He, L.; Pan, Q.; Wang, S. Effects of vine top shading on the accumulation of C6/C9 compounds in’Cabernet Sauvignon’(Vitis vinifera L.) grape berries in northwestern China. J. Sci. Food Agric. 2021, 102, 1862–1871. [Google Scholar] [CrossRef] [PubMed]
- Bureau, S.M.; Baumes, R.L.; Razungles, A.J. Effects of vine or bunch shading on the glycosylated flavor precursors in grapes of Vitis vinifera L. cv. Syrah. J. Agric. Food Chem. 2000, 48, 1290–1297. [Google Scholar] [CrossRef]
- Shirazi, M.A.; Boersma, L. A unifying quantitative analysis of soil texture. Soil Sci. Soc. Am. J. 1984, 48, 142–147. [Google Scholar] [CrossRef]
- Ghiglieno, I.; Mattivi, F.; Cola, G.; Trionfini, D.; Perenzoni, D.; Simonetto, A.; Giloli, G.; Valenti, L. The effects of leaf removal and artificial shading on the composition of Chardonnay and Pinot noir grapes. OENO One 2020, 54, 761–777. [Google Scholar] [CrossRef]
- Eichhorn, K.W.; Lorenz, D.H. Phänologische Entwick-lungs- stadien der Rebe. Nachr. Des. Dtsch. Pflanz. Schutzdienstes Braunschw. 1977, 29, 119–120. [Google Scholar]
- Lorenz, D.; Eichhorn, K.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Growth Stages of the Grapevine: Phenological growth stages of the grapevine (Vitis vinifera L. ssp. vinifera)-Codes and descriptions according to the extended BBCH scale. Aust. J. Grape Wine Res. 1995, 1, 100–103. [Google Scholar] [CrossRef]
- Scholander, P.F.; Hammel, H.T.; Bradstreet, E.D.; Hemmingsen, E.A. Sap pressure in vascular plants. Science 1965, 148, 339–346. [Google Scholar] [CrossRef]
- Sperry, J.S.; Pockman, W.T. Limitation of transpiration by hydraulic conductance and xylem cavitation in Betula occidentalis. Plant Cell Environ. 1993, 16, 279–287. [Google Scholar] [CrossRef]
- Markwell, J.; Osterman, J.C.; Mitchell, J.L. Calibration of the Minolta SPAD-502 leaf chlorophyll meter. Photosynth. Res. 1995, 46, 467–472. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Cola, G.; Failla, O.; Mariani, L. BerryTone—A simulation model for the daily course of grape berry temperature. Agric. For. Meteorol. 2009, 149, 1215–1228. [Google Scholar] [CrossRef]
- Ramful, D.; Tarnus, E.; Aruoma, O.I.; Bourdon, E.; Bahorun, T. Polyphenol composition, vitamin C content and antioxidant capacity of Mauritian citrus fruit pulps. Food Res. Int. 2011, 44, 2088–2099. [Google Scholar] [CrossRef]
- Larcher, R.; Tonidandel, L.; Villegas, T.R.; Nardin, T.; Fedrizzi, B.; Nicolini, G. Pre-fermentation addition of grape tannin increases the varietal thiols content in wine. Food Chem. 2015, 166, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Hanusz, Z.; Tarasinska, J.; Zielinski, W. Shapiro-Wilk test with known mean. REVSTAT-Stat. J. 2016, 14, 89–100. [Google Scholar]
- Gastwirth, J.L.; Gel, Y.R.; Miao, W. The impact of Levene’s test of equality of variances on statistical theory and practice. Stat. Sci. 2009, 24, 343–360. [Google Scholar] [CrossRef] [Green Version]
- Aickin, M.; Gensler, H. Adjusting for multiple testing when reporting research results: The Bonferroni vs Holm methods. Am. J. Public Health 1996, 86, 726–728. [Google Scholar] [CrossRef] [Green Version]
- Marín, D.; Armengol, J.; Carbonell-Bejerano, P.; Escalona, J.M.; Gramaje, D.; Hernández-Montes, E.; Intrigliolo, D.S.; Martínez-Zapater, J.M.; Medrano, H.; Mirás-Avalos, J.M.; et al. Challenges of viticulture adaptation to global change: Tackling the issue from the roots. Aust. J. Grape Wine Res. 2021, 27, 8–25. [Google Scholar] [CrossRef]
- Kariyapperuma, N.; Collins, E. Family logics and environmental sustainability: A study of the New Zealand wine industry. Bus. Strategy Environ. 2021, 30, 3626–3650. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, Y.; Tang, K. Aroma of Icewine: A Review on How Environmental, Viticultural, and Oenological Factors Affect the Aroma of Icewine. J. Agric. Food Chem. 2021, 69, 6943–6957. [Google Scholar] [CrossRef]
- da Silva, P.S.O.; Junior, L.F.G.O.; Gonzaga, M.I.S.; Sena, E.D.O.A.; dos Santos Maciel, L.B.; Fiaes, M.P.; Carnelossi, M.A.G. Effects of calcium particle films and natural shading on ecophysiological parameters of conilon coffee. Sci. Hortic. 2019, 245, 171–177. [Google Scholar] [CrossRef]
- Mathur, S.; Jain, L.; Jajoo, A. Photosynthetic efficiency in sun and shade plants. Photosynthetica 2018, 56, 354–365. [Google Scholar] [CrossRef]
- González, C.V.; Prieto, J.A.; Mazza, C.; Jeréz, D.N.; Biruk, L.N.; Jofre, M.F.; Giordano, C.V. Grapevine morphological shade acclimation is mediated by light quality whereas hydraulic shade acclimation is mediated by light intensity. Plant Sci. 2021, 307, 110893. [Google Scholar] [CrossRef] [PubMed]
- Brito, C.; Dinis, L.T.; Moutinho-Pereira, J.; Correia, C. Kaolin, an emerging tool to alleviate the effects of abiotic stresses on crop performance. Sci. Hortic. 2019, 250, 310–316. [Google Scholar] [CrossRef]
- Dinis, L.T.; Malheiro, A.C.; Luzio, A.; Fraga, H.; Ferreira, H.; Gonçalves, I.; Pinto, G.; Correia, C.M.; Moutinho-Pereira, J. Improvement of grapevine physiology and yield under summer stress by kaolin-foliar application: Water relations, photosynthesis and oxidative damage. Photosynthetica 2018, 56, 641–651. [Google Scholar] [CrossRef]
- Conde, A.; Pimentel, D.; Neves, A.; Dinis, L.T.; Bernardo, S.; Correia, C.M.; Geròs, H.; Moutinho-Pereira, J. Kaolin foliar application has a stimulatory effect on phenylpropanoid and flavonoid pathways in grape berries. Front. Plant Sci. 2016, 7, 1150. [Google Scholar] [CrossRef] [Green Version]
- Conde, A.; Neves, A.; Breia, R.; Pimentel, D.; Dinis, L.T.; Bernardo, S.; Correia, C.M.; Cunha, A.; Geros, H.; Moutinho-Pereira, J. Kaolin particle film application stimulates photoassimilate synthesis and modifies the primary metabolome of grape leaves. J. Plant Physiol. 2018, 223, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Paciello, P.; Mencarelli, F.; Palliotti, A.; Ceccantoni, B.; Thibon, C.; Darriet, P.; Pasquini, M.; Bellincontro, A. Nebulized water cooling of the canopy affects leaf temperature, berry composition and wine quality of Sauvignon blanc. J. Sci. Food Agric. 2017, 97, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Poni, S.; Gatti, M.; Palliotti, A.; Dai, Z.; Duchêne, E.; Truong, T.T.; Ferrara, G.; Matarrese, A.M.S.; Gallotta, A.; Bellincontro, A.; et al. Grapevine quality: A multiple choice issue. Sci. Hortic. 2018, 234, 445–462. [Google Scholar] [CrossRef] [Green Version]
- Frioni, T.; Saracino, S.; Squeri, C.; Tombesi, S.; Palliotti, A.; Sabbatini, P.; Mugnanini, E.; Poni, S. Understanding kaolin effects on grapevine leaf and whole-canopy physiology during water stress and re-watering. J. Plant Physiol. 2019, 242, 153020. [Google Scholar] [CrossRef] [PubMed]
- Yanykin, D.; Sundyreva, M.; Khorobrykh, A.; Semenova, G.; Savchenko, T. Functional characterization of the corticular photosynthetic apparatus in grapevine. Biochim. Et Biophys. Acta (BBA)-Bioenerg. 2020, 1861, 148260. [Google Scholar] [CrossRef]
- Levin, A.D. Re-evaluating pressure chamber methods of water status determination in field-grown grapevine (Vitis spp.). Agric. Water Manag. 2019, 221, 422–429. [Google Scholar] [CrossRef]
- Tuccio, L.; Piccolo, E.L.; Battelli, R.; Matteoli, S.; Massai, R.; Scalabrelli, G.; Remorini, D. Physiological indicators to assess water status in potted grapevine (Vitis vinifera L.). Sci. Hortic. 2019, 255, 8–13. [Google Scholar] [CrossRef]
- Pou, A.; Medrano, H.; Tomàs, M.; Martorell, S.; Ribas-Carbó, M.; Flexas, J. Anisohydric behaviour in grapevines results in better performance under moderate water stress and recovery than isohydric behaviour. Plant Soil 2012, 359, 335–349. [Google Scholar] [CrossRef]
- Suter, B.; Triolo, R.; Pernet, D.; Dai, Z.; Van Leeuwen, C. Modeling stem water potential by separating the effects of soil water availability and climatic conditions on water status in grapevine (Vitis vinifera L.). Front. Plant Sci. 2019, 10, 1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, G.; Boini, A.; Manfrini, L.; Torres-Ruiz, J.M.; Pierpaoli, E.; Zibordi, M.; Losciale, P.; Morandi, B.; Corelli-Grappadelli, L. Effect of shading and water stress on light interception, physiology and yield of apple trees. Agric. Water Manag. 2018, 210, 140–148. [Google Scholar] [CrossRef]
- Szabó, A.; Tamás, J.; Nagy, A. The influence of hail net on the water balance and leaf pigment content of apple orchards. Sci. Hortic. 2021, 283, 110112. [Google Scholar] [CrossRef]
- Dinis, L.T.; Bernardo, S.; Luzio, A.; Pinto, G.; Meijón, M.; Pintó-Marijuan, M.; Cotado, A.; Correira, C.; Moutinho-Pereira, J. Kaolin modulates ABA and IAA dynamics and physiology of grapevine under Mediterranean summer stress. J. Plant Physiol. 2018, 220, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Tosin, R.; Pôças, I.; Cunha, M. Spectral and thermal data as a proxy for leaf protective energy dissipation under kaolin application in grapevine cultivars. Open Agric. 2019, 4, 294–304. [Google Scholar] [CrossRef]
- Frioni, T.; Tombesi, S.; Sabbatini, P.; Squeri, C.; Lavado Rodas, N.; Palliotti, A.; Poni, S. Kaolin reduces ABA biosynthesis through the inhibition of neoxanthin synthesis in grapevines under water deficit. Int. J. Mol. Sci. 2020, 21, 4950. [Google Scholar] [CrossRef] [PubMed]
- Dinis, L.T.; Ferreira, H.; Pinto, G.; Bernardo, S.; Correia, C.M.; Moutinho-Pereira, J. Kaolin-based, foliar reflective film protects photosystem II structure and function in grapevine leaves exposed to heat and high solar radiation. Photosynthetica 2016, 54, 47–55. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.T.; Ferreira, H.; Rocha, L.; Pavia, I.; Moutinho-Pereira, J.; Correia, C.M. Kaolin particle film modulates morphological, physiological and biochemical olive tree responses to drought and rewatering. Plant Physiol. Biochem. 2018, 133, 29–39. [Google Scholar] [CrossRef]
- Li, T.; Dai, J.; Zhang, Y.; Kong, X.; Li, C.; Dong, H. Topical shading substantially inhibits vegetative branching by altering leaf photosynthesis and hormone contents of cotton plants. Field Crops Res. 2019, 238, 18–26. [Google Scholar] [CrossRef]
- Milenković, L.; Mastilović, J.; Kevrešan, Ž.; Bajić, A.; Gledić, A.; Stanojević, L.; Cvetković, D.; Šunić, L.; Ilić, Z.S. Effect of shading and grafting on yield and quality of tomato. J. Sci. Food Agric. 2020, 100, 623–633. [Google Scholar] [CrossRef]
- Kishore, K.; Rupa, T.R.; Samant, D. Influence of shade intensity on growth, biomass allocation, yield and quality of pineapple in mango-based intercropping system. Sci. Hortic. 2021, 278, 109868. [Google Scholar] [CrossRef]
- Martinez-Luscher, J.; Chen, C.C.L.; Brillante, L.; Kurtural, S.K. Partial solar radiation exclusion with color shade nets reduces the degradation of organic acids and flavonoids of grape berry (Vitis vinifera L.). J. Agric. Food Chem. 2017, 65, 10693–10702. [Google Scholar] [CrossRef]
- Sadras, V.O.; Petrie, P.R.; Moran, M.A. Effects of elevated temperature in grapevine. II juice pH, titratable acidity and wine sensory attributes. Aust. J. Grape Wine Res. 2013, 19, 107–115. [Google Scholar] [CrossRef]
- Ranjitha, K.; Shivashankar, S.; Prakash, G.S.; Sampathkumar, P.; Roy, T.K.; Suresh, E.R. Effect of vineyard shading on the composition, sensory quality and volatile flavours of Vitis vinifera L. cv. Pinot Noir wines from mild tropics. J. Appl. Hortic. 2015, 17, 3–6. [Google Scholar] [CrossRef]
- Pillet, J.; Egert, A.; Pieri, P.; Lecourieux, F.; Kappel, C.; Charon, J.; Gomès, E.; Keller, F.; Delrot, S.; Lecourieux, D. VvGOLS1 and VvHsfA2 are involved in the heat stress responses in grapevine berries. Plant Cell Physiol. 2012, 53, 1776–1792. [Google Scholar] [CrossRef]
- Lecourieux, F.; Kappel, C.; Lecourieux, D.; Serrano, A.; Torres, E.; Arce-Johnson, P.; Delrot, S. An update on sugar transport and signalling in grapevine. J. Exp. Bot. 2014, 65, 821–832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez-Gamboa, G.; Pardo, C.; Moreno-Simunovic, Y. Effects on Berry Shrinkage in Vitis vinifera. L cv. ‘Merlot’ From Changes in Canopy/Root Ratio: A Preliminary Approach. S. Afr. J. Enol. Vitic. 2019, 40, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Conde, A.; Soares, F.; Breia, R.; Gerós, H. Postharvest dehydration induces variable changes in the primary metabolism of grape berries. Int. Food Res. J. 2018, 105, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Triolo, R.; Roby, J.P.; Pisciotta, A.; Di Lorenzo, R.; van Leeuwen, C. Impact of vine water status on berry mass and berry tissue development of Cabernet franc (Vitis vinifera L.), assessed at berry level. J. Sci. Food Agric. 2019, 99, 5711–5719. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lüscher, J.; Chen, C.C.L.; Brillante, L.; Kurtural, S.K. Mitigating heat wave and exposure damage to “cabernet sauvignon” wine grape with partial shading under two irrigation amounts. Front. Plant Sci. 2020, 1760. [Google Scholar] [CrossRef]
- Ferrari, V.; Disegna, E.; Dellacassa, E.; Coniberti, A. Influence of timing and intensity of fruit zone leaf removal and kaolin applications on bunch rot control and quality improvement of Sauvignon blanc grapes, and wines, in a temperate humid climate. Sci. Hortic. 2017, 223, 62–71. [Google Scholar] [CrossRef]
- Wenter, A.; Zanotelli, D.; Montagnani, L.; Tagliavini, M.; Andreotti, C. Effect of different timings and intensities of water stress on yield and berry composition of grapevine (cv. Sauvignon blanc) in a mountain environment. Sci. Hortic. 2018, 236, 137–145. [Google Scholar] [CrossRef]
- Pons, A.; Allamy, L.; Schüttler, A.; Rauhut, D.; Thibon, C.; Darriet, P. What is the expected impact of climate change on wine aroma compounds and their precursors in grape? OENO One 2017, 51, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Drappier, V.; Hilbert, G.; Guillaumie, S.; Dai, Z.; Geny, L.; Delrot, S.; Darriet, P.; Thibon, C.; Pieri, P. The effects of a moderate grape temperature increase on berry secondary metabolites. OENO One 2019, 53, 321–333. [Google Scholar] [CrossRef]
- Coniberti, A.; Ferrari, V.; Dellacassa, E.; Boido, E.; Carrau, F.; Gepp, V.; Disegna, E. Kaolin over sun-exposed fruit affects berry temperature, must composition and wine sensory attributes of Sauvignon blanc. Eur. J. Agron. 2013, 50, 75–81. [Google Scholar] [CrossRef]
- Rienth, M.; Vigneron, N.; Darriet, P.; Sweetman, C.; Burbidge, C.; Bonghi, C.; Walker, R.P.; Famiani, F.; Castellarin, S.D. Grape Berry Secondary Metabolites and Their Modulation by Abiotic Factors in a Climate Change Scenario—A Review. Front. Plant Sci. 2021, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.A.; Schilmiller, A.L. Oxylipin metabolism in response to stress. Curr. Opin. Plant Biol. 2002, 5, 230–236. [Google Scholar] [CrossRef]
- Kobayashi, H.; Takase, H.; Suzuki, Y.; Tanzawa, F.; Takata, R.; Fujita, K.; Kohno, M.; Mochizuki, M.; Suzuki, S.; Konno, T. Environmental stress enhances biosynthesis of flavor precursors, S-3-(hexan-1-ol)-glutathione and S-3-(hexan-1-ol)-L-cysteine, in grapevine through glutathione S-transferase activation. J. Exp. Bot. 2011, 62, 1325–1336. [Google Scholar] [CrossRef] [PubMed]
- Dunlevy, J.D.; Soole, K.L.; Perkins, M.V.; Nicholson, E.L.; Maffei, S.M.; Boss, P.K. Determining the methoxypyrazine biosynthesis variables affected by light exposure and crop level in Cabernet Sauvignon. Am. J. Enol. Vitic. 2013, 64, 450–458. [Google Scholar] [CrossRef]




| Pn (µmol CO2 m−2 s−1) | gs (mmol H2O m−2 s−1) | |||||||
| Stage | SD30% | SD70% | K | CTRL | SD30% | SD70% | K | CTRL |
| 10 August 2020 | 4.21 ± 1.24 c | 3.36 ± 1.34 c | 6.91 ± 3.16 b | 8.29 ± 2.40 a | 129.10 ± 30.44 c | 120.24 ± 12.82 c | 167.50 ± 27.63 a | 142.40 ± 24.10 b |
| 21 August 2020 | 3.42 ± 1.03 b | 2.21 ± 0.37 c | 10.97 ± 4.10 a | 10.40 ± 1.31 a | 145.65 ± 27.87 ab | 139.35 ± 33.54 b | 154.21 ± 27.31 a | 156.00 ± 32.11 a |
| 31 August 2020 | 3.61 ± 1.95 c | 1.52 ± 0.29 d | 8.40 ± 1.31 b | 9.76 ± 2.11 a | 148.00 ± 13.12 a | 140.4 ± 21.22 a | 148.40 ± 31.31 a | 152.8 ± 35.51 a |
| 4 August 2021 | 2.44 ± 1.10 bc | 1.66 ± 0.34 c | 4.78 ± 1.27 a | 2.98 ± 0.98 b | 100.50 ± 22.10 b | 75.11 ± 5.23 c | 131.40 ± 37.67 a | 125.70 ± 31.07 a |
| 19 August 2021 | 3.25 ± 1.01 b | 1.71 ± 0.20 c | 5.31 ± 1.55 a | 4.05 ± 2.14 b | 42.23 ± 15.01 a | 44.62 ± 9.72 a | 59.34 ± 21.00 a | 48.75 ± 26.27 a |
| 24 August 2021 | 2.95 ± 1.60 b | 1.51 ± 1.57 c | 4.93 ± 1.94 a | 4.35 ± 2.14 a | 41.30 ± 8.37 b | 49.55 ± 2.13 b | 63.50 ± 15.78 a | 62.00 ± 55.18 a |
| eWUE (µmol CO2/mmol H2O) | T leaf (°C) | |||||||
| Stage | SD30% | SD70% | K | CTRL | SD30% | SD70% | K | CTRL |
| 10 August 2020 | 1.67 ± 1.38 b | 1.28 ± 1.46 b | 3.33 ± 0.75 a | 3.10 ± 1.12 a | 32.17 ± 0.84 ab | 31.88 ± 2.68 b | 32.27 ± 0.86 a | 32.48 ± 2.58 a |
| 21 August 2020 | 2.81 ± 0.88 c | 2.05 ± 0.42 c | 4.02 ± 0.66 b | 5.80 ± 0.32 a | 31.71 ± 1.37 a | 31.11 ± 1.13 a | 31.89 ± 0.95 a | 31.99 ± 0.62 a |
| 31 August 2020 | 2.00 ± 1.37 a | 2.33 ± 1.12 a | 2.13 ± 1.67 a | 2.14 ± 1.05 a | 20.94 ± 0.68 a | 20.27 ± 1.92 a | 20.96 ± 0.96 a | 20.93 ± 1.41 a |
| 4 August 2021 | 1.03 ± 0.77 ab | 0.84 ± 0.91 b | 1.59 ± 1.52 a | 1.87 ± 0.82 a | 34.32 ± 0.60 b | 32.95 ± 1.01 d | 33.61 ± 0.68 c | 35.89 ± 0.66 a |
| 19 August 2021 | 2.39 ± 0.32 ab | 1.35 ± 0.52 c | 3.22 ± 1.30 a | 2.09 ± 1.00 b | 36.16 ± 0.48 b | 35.88 ± 1.31 bc | 35.32 ± 0.37 c | 38.38 ± 0.81 a |
| 24 August 2021 | 2.64 ± 0.51 a | 0.70 ± 1.35 b | 2.86 ± 1.10 a | 2.09 ± 1.56 a | 36.93 ± 1.06 b | 34.52 ± 2.65 c | 35.06 ± 1.59 c | 39.20 ± 1.13 a |
| Kl (mmol MPa−1 m−2 s−1) | E (mmol H2O m−2 s−1) | |||||||
| Stage | SD30% | SD70% | K | CTRL | SD30% | SD70% | K | CTRL |
| 10 August 2020 | 5.24 ± 1.20 b | 3.91 ± 2.28 c | 6.28 ± 2.92 a | 7.06 ± 2.74 a | 3.63 ± 0.74 b | 2.74 ± 2.07 c | 4.25 ± 2.01 ab | 4.48 ± 0.14 a |
| 21 August 2020 | 4.55 ± 1.33 b | 3.85 ± 0.46 b | 3.94 ± 1.89 b | 5.43 ± 1.00 a | 3.28 ± 0.85 ab | 3.15 ± 1.04 b | 3.22 ± 1.16 ab | 4.18 ± 1.08 a |
| 31 August 2020 | 5.94 ± 0.96 a | 5.25 ± 1.36 a | 6.00 ± 2.09 a | 5.90 ± 1.70 a | 4.34 ± 0.60 ab | 3.91 ± 1.07 b | 5.28 ± 0.99 a | 4.94 ± 1.10 a |
| 4 August 2021 | 3.31 ± 1.73 b | 2.14 ± 0.73 c | 5.31 ± 1.39 a | 1.60 ± 1.13 d | 2.97 ± 1.15 ab | 1.96 ± 0.52 c | 3.74 ± 1.12 a | 2.36 ± 0.88 bc |
| 19 August 2021 | 1.53 ± 1.14 b | 1.00 ± 0.78 b | 2.07 ± 0.88 a | 0.82 ± 1.12 b | 1.61 ± 0.34 ab | 0.93 ± 1.03 b | 1.91 ± 0.61 a | 1.66 ± 1.25 ab |
| 24 August 2021 | 1.32 ± 0.97 b | 2.63 ± 1.72 a | 2.30 ± 1.24 a | 1.08 ± 0.84 b | 1.41 ± 0.69 a | 1.77 ± 0.69 a | 2.15 ± 0.83 a | 1.51 ± 0.73 a |
| Sugar Content (°Brix) | TA (g L−1 Tartaric Acid) | |||||||
| Stage | SD30% | SD70% | K | CTRL | SD30% | SD70% | K | CTRL |
| 10 August 2020 | 12.88 ± 0.35 a | 12.85 ± 0.15 a | 12.35 ± 0.12 a | 13.31 ± 0.25 a | 13.35 ± 0.22 a | 13.54 ± 0.68 a | 13.23 ± 0.28 a | 11.17 ± 0.73 b |
| 21 August 2020 | 15.95 ± 0.70 ab | 15.00 ± 0.47 b | 15.80 ± 0.45 ab | 16.00 ± 0.55 a | 9.55 ± 0.23 b | 10.00 ± 0.43 ab | 10.30 ± 0.26 a | 9.05 ± 0.12 b |
| 31 August 2020 | 18.58 ± 0.42 b | 18.25 ± 0.15 b | 18.02 ± 0.37 b | 19.82 ± 0.09 a | 7.22 ± 0.50 b | 8.41 ± 0.53 a | 8.15 ± 0.40 ab | 7.20 ± 0.33 b |
| 4 August 2021 | 14.95 ± 0.15 a | 13.54 ± 0.32 b | 13.88 ± 0.30 b | 15.18 ± 0.50 a | 12.11 ± 0.47 ab | 12.64 ± 0.46 a | 12.60 ± 0.41 a | 11.30 ± 0.33 b |
| 19 August 2021 | 18.40 ± 0.32 a | 17.21 ± 0.22 b | 17.36 ± 0.25 b | 18.72 ± 0.25 a | 6.48 ± 0.20 b | 8.00 ± 0.15 a | 7.90 ± 0.10 a | 6.44 ± 0.35 b |
| 24 August 2021 | 21.72 ± 0.22 a | 19.00 ± 0.33 c | 20.40 ± 0.41 b | 22.46 ± 0.20 a | 4.22 ± 0.02 b | 5.91 ± 0.07 a | 6.16 ± 0.08 a | 4.04 ± 0.04 b |
| pH | Berry Weight (g) | |||||||
| Stage | SD30% | SD70% | K | CTRL | SD30% | SD70% | K | CTRL |
| 10 August 2020 | 2.94 ± 0.05 a | 3.01 ± 0.08 a | 2.95 ± 0.07 a | 3.00 ± 0.07 a | 1.34 ± 0.13 a | 1.27 ± 0.11 a | 1.31 ± 0.13 a | 1.15 ± 0.15 a |
| 21 August 2020 | 3.30 ± 0.06 a | 3.21 ± 0.10 a | 3.18 ± 0.11 a | 3.20 ± 0.03 a | 1.54 ± 0.27 a | 1.51 ± 0.17 a | 1.49 ± 0.14 a | 1.51 ± 0.29 a |
| 31 August 2020 | 3.28 ± 0.04 a | 3.25 ± 0.11 a | 3.22 ± 0.06 a | 3.55 ± 0.07 a | 1.77 ± 0.07 a | 1.71 ± 0.80 a | 1.73 ± 0.16 a | 1.69 ± 0.13 a |
| 4 August 2021 | 3.01 ± 0.08 a | 3.09 ± 0.07 a | 3.08 ± 0.11 a | 3.26 ± 0.11 a | 0.95 ± 0.21 b | 1.11 ± 0.18 ab | 1.28 ± 0.12 a | 0.88 ± 0.09 b |
| 19 August 2021 | 3.21 ± 0.07 a | 3.16 ± 0.05 a | 3.30 ± 0.04 a | 3.33 ± 0.12 a | 1.27 ± 0.15 b | 1.54 ± 0.12 a | 1.49 ± 0.08 a | 1.33 ± 0.08 b |
| 24 August 2021 | 3.24 ± 0.10 b | 3.28 ± 0.07 b | 3.46 ± 0.05 ab | 4.02 ± 0.09 a | 1.30 ± 0.07 b | 1.51 ± 0.11 a | 1.44 ± 0.08 a | 1.27 ± 0.12 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cataldo, E.; Fucile, M.; Mattii, G.B. Effects of Kaolin and Shading Net on the Ecophysiology and Berry Composition of Sauvignon Blanc Grapevines. Agriculture 2022, 12, 491. https://doi.org/10.3390/agriculture12040491
Cataldo E, Fucile M, Mattii GB. Effects of Kaolin and Shading Net on the Ecophysiology and Berry Composition of Sauvignon Blanc Grapevines. Agriculture. 2022; 12(4):491. https://doi.org/10.3390/agriculture12040491
Chicago/Turabian StyleCataldo, Eleonora, Maddalena Fucile, and Giovan Battista Mattii. 2022. "Effects of Kaolin and Shading Net on the Ecophysiology and Berry Composition of Sauvignon Blanc Grapevines" Agriculture 12, no. 4: 491. https://doi.org/10.3390/agriculture12040491
APA StyleCataldo, E., Fucile, M., & Mattii, G. B. (2022). Effects of Kaolin and Shading Net on the Ecophysiology and Berry Composition of Sauvignon Blanc Grapevines. Agriculture, 12(4), 491. https://doi.org/10.3390/agriculture12040491








