Abstract
The use of nitrogen fertilizers in agriculture is currently under high pressure to reduce its environmental impact and improve its currently low efficiency. Nitrification inhibitors and/or intercrops emerged in recent decades as useful tools to combat these problems. The objective of the experiment is to study the effect of these techniques on the yield, the nitrogen use efficiency (NUE) and N leaching in a maize–wheat rotation. Six treatments were studied, combining the use of ammonium nitrate sulfate (ASN) alone or with a nitrification inhibitor (DMPSA or 3,4-dimethylpyrazole succinic acid) and the use or absence of vetch (Vicia sativa L.) as an intercrop. The results showed that fertilized treatments did not show significant differences in crop development, but the use of DMPSA delayed the nitrate (NO3−) availability and reduced N leaching losses (average N leaching reductions around 25% after maize harvest). On the other hand, the use of vetch as an intercrop helped to reduce the negative effects of N deficiency and, at the same time, increased the concentration of N in the soil during the following crop harvest (4.5 kg N ha−1 on average after wheat harvest) and reduced losses due to leaching (average N leaching reductions around 14% after the maize–wheat season). The combination of both techniques (DMPSA and vetch intercrop) at the same time presented a synergic effect and greatly improved the environmental impact of the irrigated maize–wheat system.
1. Introduction
Nitrogen (N) is the most important macronutrient for plants’ nutrition and is usually regarded as one of the most limiting factors for plant growth. Therefore, it is no surprise that N-based fertilizers are the most widely demanded and applied in agriculture []. This N is applied largely in the form of ammoniacal fertilizers manufactured in energy-intensive industrial processes. Rising energy costs translate into higher N fertilizer prices, bringing increasing pressure on grain producers to maximize fertilizer N efficiency []. The problem arises from the generally low efficiency of N-based fertilizers, given that only about 42–47% of the nitrogen added to croplands globally is harvested as crop product [,]. Most of the lost N becomes pollutants and a threat to human and ecosystem health on local and global scales. In agricultural and horticultural systems, mineral N is mainly prone to losses through ammonia volatilization, leaching (i.e., removal in drainage water) and denitrification (i.e., transformations into gaseous forms) [].
Nitrate (NO3−) is usually the more abundant form of mineral N in the agricultural soils, but it is prone to leach and to suffer denitrification processes, both of them deriving large N losses []. One method of limiting nitrification processes and maintaining N in the soil as ammonium (NH4+) is to add a nitrification inhibitor (as DMPSA; 3,4-dimethylpyrazole succinic acid) with the fertilizer. Nitrification inhibitors have been reported to increase NUE and crop yield [,] and, under some conditions, reduce N leaching []. In addition, the assimilation of NO3− requires the energy equivalent of 20 ATP mol−1, whereas NH4+ assimilation requires only 5 ATP mol−1 []. These energy savings could lead to greater dry matter production for plants supplied solely with NH4+, although the process of passive diffusion from the soil to the roots is more difficult for NH4+ than for NO3−, mostly because NH4+ presents higher retention to the soil particles (where most of the charges are negative) and also because NH4+ tends to be easily oxidized to NO3− in the aerated soils.
Intercropping is an ancient practice, which has regained interest and importance as a mean to address some of the major problems associated with modern farming, including moderate yield, pest and pathogen accumulation, soil degradation and environmental deterioration [,,]. Between its many advantages, intercropping has shown its capability to reduce N leaching [,,,] and increase nitrogen use efficiency (NUE) [,] in many previous experiments in different agrosystems. In this regard, vetch (Vicia sativa L.) is a very well adapted to Mediterranean conditions forage crop, usually used as an intercrop []. Its use is highly recommended, not only due to its high yield and protein content, but also because of its many and well-known effects of improving soil moisture, nutrient content, microbial community [] and the overall crop sustainability [].
Maize is one of the most important crops in the world, standing as the second most produced crop, behind sugar cane and followed by wheat. In 2019, 1148 Mt of maize and 766 Mt of wheat were produced, which respectively represents 38.5% and 25.7% of the world’s total cereal production. In the 2020–2029 period, global cereal production is projected to expand by 375 Mt, to reach 3054 Mt in 2029, mainly driven by higher yields []. In the same line, wheat and maize are two of the most important crops in Spain; therefore, increasing the economic and environmental benefits in these systems is key to increase their medium- and long-term sustainability.
The positive effects of the use of nitrification inhibitors or intercrops have been, to a large extent, studied separately, but there is little literature that reflects the effects that may result from the interaction between both techniques. Additionally, there is little information about the effect of nitrification inhibitors on nitrate leaching, as they have mostly focused on denitrification gaseous losses. Therefore, the objective of the present experiment was to compare the nitrate leaching from a nitrogen fertilizer with or without a nitrification inhibitor (DMPSA) in a maize/wheat rotation, with or without a legume intercrop in between maize lines. We expected to reduce N leaching without decreasing crop yield with both nitrification inhibitors and intercrops, and, at the same time, we expected to better assess the interaction between both techniques in a typical grain rotation.
2. Materials and Methods
2.1. Field Experiment
The field experiment was conducted from April 2018 to July 2019 at ‘La Canaleja’ Field Station from the National Institute of Agricultural and Food Research and Technology (INIA). It was located in Alcalá de Henares, (Madrid province), Spain (40°32′ N, 3°20′ W, 600 m.a.s.l.). The climate is Mediterranean semi-arid with an annual rainfall of 389 mm (186–547 mm) that mainly occurs in autumn and spring and is almost negligible in summer, with a mean annual temperature of 13.5 °C (Figure 1). The soil was classified as a Typic Calcixerept by the soil taxonomy classification (Soil Survey Staff 2014) and as a Calcic Cambisol by the Food and Agriculture Organization (FAO) classification (IUSS Working Group WRB 2007). The principal soil initial properties can be found in Table 1.
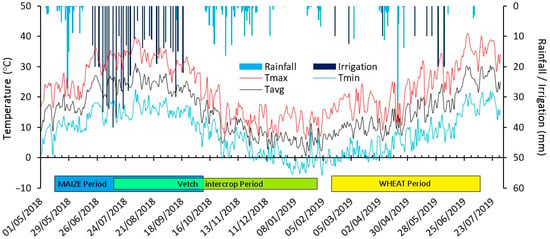
Figure 1.
Weather conditions during the experiment (daily maximum, minimum and average temperature and daily rainfall and irrigation) during maize–intercrop–wheat periods. Arrows indicate the N fertilizer application dates.

Table 1.
Soil chemical properties at the beginning of the experiment.
The experiment was included in a maize/wheat crop rotation (Figure 1) and consisted of maize (Zea mays L. cv P1574) sown on May 14 and harvested on October 8 followed by spring wheat (Triticum aestivum L. cv Badiel) sown on February 14 and harvested on July 10. The vetch (Vicia salitva L cv Aitana) intercrop was broadcasted on July 12, the same day as the second maize fertilizer dressing, followed by a cultivator pass in order to bury the fertilizer, the vetch seeds and the emerged weeds. On January 31 the vetch was chopped and left over the soil before the wheat was sown. The same soil management was applied to all plots since 2008, and the same crop rotation (maize wheat) was used since 2016 in order to homogenize the field.
The maize (plant density of 80,000 plants ha−1) and wheat (sowing rate of 230 kg ha−1) were grown inside an irrigated field with a 16 × 16 m2 permanent sprinkle system. Maize and vetch density were chosen as the recommended values in the region (in the higher part of the range for vetch because the sowing method and time are not the more appropriate and the termination date is sooner). The wheat sowing rate was higher than the recommended values for traditional wheat because this variety presents a very short cycle with a very low tillering rate. The P and K fertilization consisted of 70 kg P2O5 ha−1 and 120 kg K2O ha−1, before maize sowing, following the traditional dose in the region, in order to avoid phosphorus (P) or potassium (K) deficit. Soil management consisted of no tillage, sowing maize and wheat with a direct seeder. The vetch intercrop was broadcasted (160 kg ha−1) over the maize crop, without incorporation. All crop residues were left over the surface after being chopped.
The experiment was carried out with 6 different treatments, combining 3 fertilization treatments and the use or not of an intercrop: (1) ammonium sulfate nitrate alone (ASN-C), (2) ammonium sulfate nitrate with vetch intercrop (ASN-IC), (3) ammonium sulfate nitrate + nitrification inhibitor alone (DMPSA-C), (4) ammonium sulfate nitrate + nitrification inhibitor with vetch intercrop (DMPSA-IC), (5) non-fertilized control alone (0 N-C) and (6) non-fertilized control with vetch intercrop (0 N-IC). Each treatment had 4 replications. All treatments and replications were randomly distributed in 20 8 × 16 m2 plots. The maize fertilizer rate was 175 kg N ha−1, which was applied as ASN and split in two dressings applied on June 18 and July 12. The fertilizer rate was based on previous N response trials conducted in the same research fields [,], and it was adjusted according to the initial Nmin content in the soil. The spring wheat crop was sown over the previous maize treatments and the previously fertilized treatments received again 75 kg ha−1 of ASN on May 3, which was later and lower than the recommended rate, in order to better appreciate the residual effect of both DMPSA and vetch treatments in yield and grain quality.
After each N fertilizer application, a 20 mm irrigation event was applied in order to incorporate the fertilizer and avoid direct volatilization. The irrigation schedule and doses were estimated from the daily values of crop evapotranspiration (ETc) and rainfall. This was calculated as ETc = Kc × ET0, where ET0 is the reference evapotranspiration calculated by the FAO Penman–Monteith model [] using daily local data, and Kc was daily obtained by the Martinez–Cob method []. The total precipitation during the maize crop was 87 mm, the ET0 was 651 mm, the ETc was 556 mm and the water was irrigated around 700 mm. Total water input was ~20–30% larger than the ETc to ensure a leaching fraction and avoid an increase in soil salinity. The spring wheat was irrigated in order to compensate the difference between the potential evapotranspiration and the actual precipitation. The total precipitation during the wheat crop was 93 mm, the ETc was 290 mm and the water was irrigated around 125 mm. As the applied water presented on average 12.3 mg N-NO3− L−1 along the cropping season, the total amount of nitrate applied with the irrigation was 86.0 and 17.5 kg N ha−1 during the maize and wheat periods, respectively.
2.2. Crop Analysis: Yield, Grain Quality, N Content and Nutritional Status
For the maize crop, the harvest index (grain/(grain + rest of aboveground biomass)) was calculated before mechanical harvest. A 5 m stripe next to the central row was harvested by hand and separated into plant components (grain vs. rest of aboveground biomass), and a subsample of each component was oven-dried (65 °C, 48 h) and weighed. These subsamples were used to determine total N concentration by the Dumas combustion method (LECO FP-428 analyzer, St. Joseph, MI, USA). At harvest, two 10 m stripe of the central rows from each plot were harvested by an experimental combiner, and the maize yield was recorded. A grain subsample was oven-dried (65 °C, 48 h), weighed and ground. For each plot, the N content of each crop component was calculated by multiplying its dry biomass by its N concentration and adding up both to obtain the total crop above ground N content.
For the vetch intercrop, four samples per plot of plants (inside a 0.25 m2 square) were hand-cut at the termination date. Samples were oven-dried (65 °C, 48 h) and weighed. A subsample was ground, and the total N concentration was determined by the Dumas combustion method (LECO FP-428 analyzer, St. Joseph, MI, USA). For each plot, the vetch aboveground N content was calculated by multiplying its dry biomass by its N concentration.
Regarding wheat, one 1.5 × 10 m stripe for the central rows of each plot was harvested by an experimental combiner, and the wheat yield was recorded. A grain subsample was oven-dried (65 °C, 48 h), weighed for humidity correction and ground for total N concentration determination by the same method used in maize.
The crop nutritional state in maize was evaluated with Dualex® Scientific (Force-A, Orsay, France) chlorophyll meter. It is a leaf clip sensor that measures chlorophyll content (Chl) as the difference between the light transmitted at the red and infrared wavelengths. The device also measures flavonols (Flav) and anthocyanins (Anth), both substances are highly correlated with crop stress and the nutritional balance index (NBI) [,]. Ten readings were carried out from June 12 (before first dressing) to October 3 (before harvest), including remarkable dates on July 11 (before the second dressing), August 6 (at flowering) or September 19 (at kernel black layer appearance). On each sampling date, 20 measurements were taken from the uppermost fully developed leaf (or in the ear leaf from flowering date) of 20 representative plants in the central rows of each plot, following the sampling method defined for maize by Gabriel et al. []. The representative value of each plot was obtained by the 20 measurement average.
Similar measurements were done during the wheat crop period on April 9, May 3 (before fertilization) and May 30 (at flowering). In this case, two nutritional indexes were measured: the NDVI (structural and growth index) and NDRE (nitrogen content index). Both of them were acquired using the RapidScan® CS-45 (Holland Scientific Inc., Lincoln, NE, USA), integrating measurements every 0.5 s in the central 12 m row per plot.
2.3. Soil Mineral N Content (Nmin)
The soil nitrate and ammonium content were determined at four dates: before the maize crop sowing (May 11), at flowering (August 6), after the maize harvest (October 11) and after the wheat harvest (July 14). A combined soil sample from three soil cores was taken from each plot by 0.2 m intervals with an Eijkelkamp® helicoidal auger (Eijkelkamp Agrisearch Equipment, Geisbeek, The Netherlands). The samples were taken at a depth of up to 1 m. The samples were placed in a plastic bag and firmly closed immediately, transported and refrigerated (4–6 °C). Within the five consecutive days, a soil subsample of each box was extracted with 2 M KCl (~5 g of soil: 50 mL of KCl), centrifuged and decanted, and a subsample of the supernatant volume was stored in a freezer until later analysis (FIAstar™ 5000, FOSS Analytical AB, Höganäs, Sweden). The nitrate concentration was determined by the Griess–Ilosvay method [], and ammonium was measured using the method of Solorzano []. Soil Nmin was calculated for each layer and plot.
2.4. Nitrate Leaching
Nitrate leaching was measured based on the combined method proposed by Gabriel et al. [] estimating the water balance employing a hydrological model (WAVE []) and the analysis of the nitrate concentration of the free water at 1 m depth obtained with suction cups []. Six suction cups were installed per treatment, with a suction pressure maintained at close to 333 cm (the field capacity) during the entire experimental period. The cups were installed in the medium row at least 1.5 m from the nearest cup, the EnviroSCAN® capacitance probe, a soil sampling point or the plot edge. Samples of the soil solution at 1 m depth were taken every 15 days or after 20 mm of rain with a hand-operated vacuum pump connected to a capillary tube and then transferred to a storage bottle. Soil solutions were stored in a freezer for later analysis. The nitrate concentration in the soil solution was determined by spectrophotometry after reduction with a cadmium column [], and ammonium was measured using the method of Solorzano [].
The hydrological model was calibrated and validated based on continuous data (measurements every 6 h) obtained from 9 EnviroSCAN® capacitance probes (SENTEK Technologies, Stepney, Australia) with sensors placed at 10, 20, 30, 50, 70 and 90 cm depth. Once the model was able to correctly predict the water movement observed with the soil sensors, we extracted the daily drainage estimated by the model and multiplied by the nitrate (and ammonium) concentration measured at each treatment with the suction cups during the corresponding period.
2.5. Statistical Analysis
Statistical analysis was carried out using SPSS for Windows (v. 23.0). A Shapiro–Wilk test was carried out in order to assess data normality. The variables of maize green biomass and wheat grain yield were transformed by calculating their square to perform parametric tests. All multifactor analyses were performed using GLM or, in the case of non-normality of the data, the Kruskal–Wallis non-parametric test (only for grain yield and N uptake in maize variables). Post hoc multiple comparisons of means were carried out using Duncan’s (parametric) or Bonferroni’s (non-parametric) tests as appropriate. Only a p < 0.05 value was considered statistically significant.
3. Results
3.1. Maize Crop Analysis
Results obtained with clip sensor showed differences in maize nutritional status between fertilized and non-fertilized treatments, but there were not differences between both fertilizers, or with or without vetch as the intercrop (Figure 2). After the first N fertilizer dressing, the fertilized treatments increased the Chl and reduced both Flav and Anth indexes with respect to both non-fertilized controls. This effect also resulted in a higher NBI, as it responds to the relation between Chl and Flav. Looking at the application of DMPSA, there was no clear effect on the nutritional status. However, looking to the presence or not of vetch as an intercrop, and although there were no differences at p < 0.05, there was a tendency to increase Chl and to reduce Flav (no so clear with the Anth) in the treatments with intercrop with respect to their respective non-intercropped treatment. This tendency was also seen in the last weeks of the crop cycle regarding the NBI index.
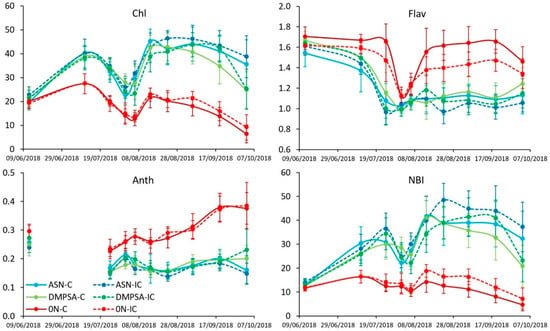
Figure 2.
Chlorophyll (Chl), flavonol (Flav), anthocyanins (Anth) and NBI indexes (all of them dimensionless) estimated with the Dualex® for the different treatments along the maize cycle. Bars represent the standard error. Treatments were combinations of ASN, ASN + DMPSA (DMPSA) or no N fertilization (0 N) and the intercropping use (IC) or absence (C).
At maize harvest, the biggest differences were found between the fertilized treatments (ASN and DMPSA) and the non-fertilized control (0 N), with the latter having the lowest results for all the measured variables (Figure 3). However, no differences were found in these variables between fertilized treatments (ASN or DMPSA, with or without the vetch intercrop). In this case, it could be observed just a small tendency to reduce the yield, height, N concentration and N content in the treatments with DMPSA with respect to the regular ASN, although these differences were not statically significant. On the other hand, the use of intercrops tended to reduce the grain N uptake in the fertilized treatments, showing some possible competence for N when N was available. This effect can be related to the lower green biomass obtained in the IC treatments. Nevertheless, intercrops tended to increase N availability in the unfertilized treatment (0 N), resulting in an increased yield, green biomass and height.
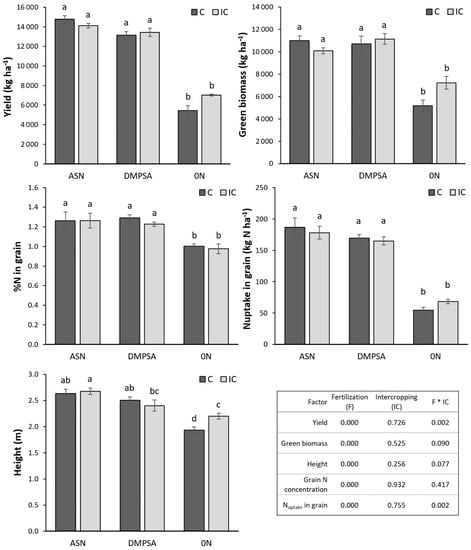
Figure 3.
Maize grain yield (dry matter), green biomass (dry matter) and height for the different treatments. Letters indicate statistical differences between treatments (p < 0.05). Treatments were combinations of ASN, ASN + DMPSA (DMPSA) or no N fertilization (0 N) and the intercropping use (IC) or absence (C). The table shows p-values for different factors in relation to its effect on the different measured variables.
3.2. Vetch Crop Analysis
At the termination date, the vetch biomass was 1937 kg ha−1 on average, without differences between fertilization treatments. The N concentration in the aerial biomass was 3.75% and the total N uptake 72.6 kg N ha−1.
3.3. Wheat Crop Analysis
Along the wheat period, there were differences in wheat growth and nutritional status between fertilization treatments, but they were not so evident between intercropping treatments. The wheat grown after the maize fertilized with regular ASN presented the largest growth (NDVI) and the largest N content (NDRE) before wheat fertilization. On the other hand, the wheat grown after the unfertilized maize presented the lowest NDVI and NDRE. The wheat grown after maize fertilized with DMPSA presented an intermediate response, but in this case, the combination of DMPSA with vetch intercrop tended to increase NDVI and NDRE, reaching values more similar to the regular ASN treatments before the fertilization. At wheat flowering, after the wheat fertilization, the differences between treatments were reduced, although the same patron was observed among fertilized treatments (Figure 4). At harvest, there were no differences in wheat grain yield among fertilized treatments, while unfertilized treatments showed a significant decrease in yield (Figure 5).
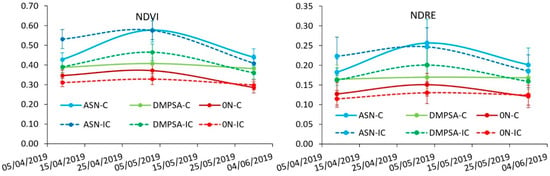
Figure 4.
NDVI and NDRE indexes estimated with the RapidScan® in the different treatments along the wheat cycle. Bars represent the standard error. Treatments were combinations of ASN, ASN + DMPSA or no N fertilization (0 N) and the intercropping use (IC) or absence (C).
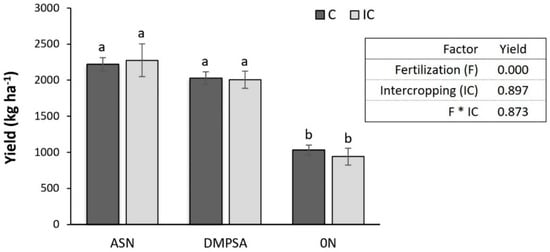
Figure 5.
Wheat grain yield (dry matter) different treatments. Bars represent the standard error. Letters indicate statistical differences between treatments (p < 0.05). Treatments were combinations of ASN, ASN + DMPSA or no N fertilization (0 N) and the intercropping use (IC) or absence (C). The table shows the p-value for different factors in relation to wheat grain yield.
3.4. Soil Inorganic N Content
Initial soil inorganic N content in the 0–60 cm layer was slightly high (110 kg N ha−1 and 153 kg N ha−1 at 0–100 cm depth), but the entire field was homogeneous and no differences were found between treatments. After maize harvest, the soil Nmin decreased by at least one-third in all the treatments, except in the ASN one without an intercrop (Figure 6). Non-fertilized treatments showed one of the most remarkable reductions in residual Nmin. However, this decrease was mostly due to the nitrate reduction, because ammonium content tended to increase with respect to the fertilized treatments. Focusing on the differences between fertilized treatments, it is possible to observe two important effects. First, the regular ASN without the intercrop treatment greatly increased the residual Nmin with respect to the other fertilized treatments (and mostly with respect to DMPSA with the intercrop). Secondly, DMPSA treatments decreased the residual Nmin content below 60 cm (reducing the leaching risk during the autumn and winter), but without a reduction in the superficial residual Nmin content (providing similar sowing conditions to the spring wheat).
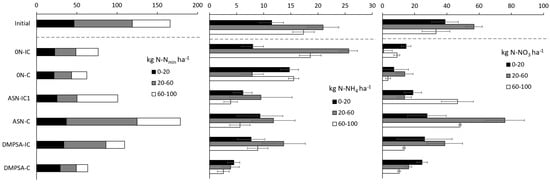
Figure 6.
Cumulative soil mineral N content (0–100 cm depth) at maize harvest. Treatments were combinations of ASN, ASN + DMPSA or no N fertilization (0 N) and the intercropping use (IC) or absence (C).
Analyzing the relationship between nitrate and ammonium content at each treatment and depth, three different patrons were observed, indicating the possible effect of the different fertilization strategies (Figure 6). In the non-fertilized soils, the nitrate content tends to be similar or smaller than the ammonium along the entire profile, while in the fertilized soils with regular ASN, the nitrate content was always larger than the ammonium, and this relationship increased with depth (from 3 at the surface, to 4 at medium depth and 10 at the deepest one). Finally, the fertilized soils with DMPSA presented an intermediate situation, with larger contents of nitrate with respect to the ammonium, but contents that were not so large and more constant or tending to decrease with depth (4.5 at the surface, 3.5 at medium depth and 2.75 at the deepest one). This difference in the proportion suggests an actual decrease in the nitrification rate; moreover, it also shows a smaller risk of nitrate leaching in the DMPSA treatments with respect to the regular ASN ones.
Regarding the wheat crop, after the wheat harvest, there was an increment in the upper layers Nmin in the fertilized treatments mostly produced by the late fertilization (Figure 7). Moreover, there was an intercropping effect, which also increased the amount of available Nmin in the upper layers with respect to the non-intercropped ones. Again, non-fertilized treatments decreased residual Nmin at maize harvest. However, this decrease was mostly due to a lower nitrate content, given that ammonium content tended to be similar for all treatments and depths. The nitrate reduction observed at maize harvest in the DMPSA treatments with respect to the regular ASN ones in the lower part of the profile was not observed. In this case, the nitrate leaching risk increased in the intercropped treatments because of the larger nitrate content.
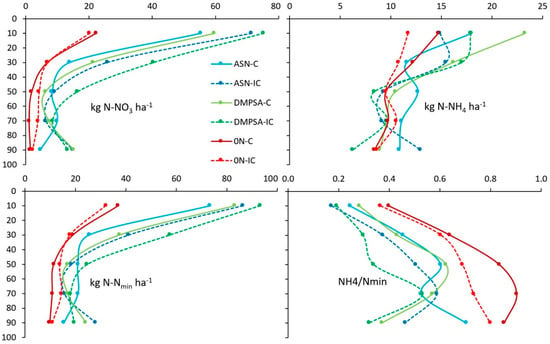
Figure 7.
Cumulative soil mineral N content (0–100 cm depth) at wheat harvest. Treatments were combinations of ASN, ASN + DMPSA or no N fertilization (0 N) and the intercropping use (IC) or absence (C).
Focusing on the nitrate–ammonium rate for each treatment and depth, three different patrons were also observed, indicating the possible effect of the different fertilization strategies, but in this case also combining with the intercropping effect. In the non-fertilized soils, the nitrate content was again similar or smaller than the ammonium along the entire profile, and the difference increased with the depth. In the fertilized soils, again, the amount of nitrate tended to be larger than the ammonium, although the proportion of ammonium also increased with depth (as in the unfertilized ones). However, the proportion of nitrate with respect to ammonium was higher in the intercropped treatments, whereas there was not difference between fertilized treatments.
Therefore, the difference observed after wheat harvest suggests that an actual decrease in the nitrification rate was avoided, meaning that the residual effect of the DMPSA faded away after one year and the main factor at this moment was the presence of a decomposing intercrop mulch. This intercropping effect could also be seen in the unfertilized treatment, although only in the lower layers.
3.5. Soil Water Balance and Nitrate Leaching
The ammonium concentration in the drainage water at 1 m depth along the entire maize–wheat cycle continued presenting very constant values without differences between treatments (around 0.39 mg N-NH4+ L−1 on average). However, the nitrate concentration presented differences not only between dates, but also between treatments, although always presenting values below the 15 mg N-NO3− L−1 threshold fixed for human consumption and even below the 12.3 mg N-NO3− L−1 original irrigation water concentration (Figure 8). Although there were few statistical differences due to the great variability between suction cups, there was a tendency for the nitrate concentration to increase during July. During August, the nitrate concentration in the drainage water decreased in all the treatments to values close to 0 mg N-NO3− L−1, but the reduction was slower in the ASN without intercrop treatment, suggesting a lower risk for nitrate contamination in the alternative cropping systems (including DMPSA or an intercrop). However, at the end of the maize cycle, a new increase in the nitrate concentration in the drainage water on the DMPSA treatment without the intercrop could be suggesting some uncoupling effect in the nitrification rate with respect to the crop demand, which can be ameliorated with vetch intercrops. During the wheat cycle, the larger concentrations were found in the DMPSA-C treatment, with an average concentration of 5.7 mg N-NO3− L−1 since the maize harvest. On the contrary, the minimum value among the fertilized treatments was usually the ASN-C, with an average concentration of 2.8 mg N-NO3− L−1 since the maize harvest. Between the previous treatments, we can find both intercropped treatments, DMPSA-IC and ASN-IC, with an average concentration of 3.2 and 3.9 mg N-NO3− L−1, respectively. The unfertilized plot usually presented very low values along the entire cycle, but the nitrate concentration tended to increase as the crops’ cycle advanced. In this case, there was no intercrop effect.
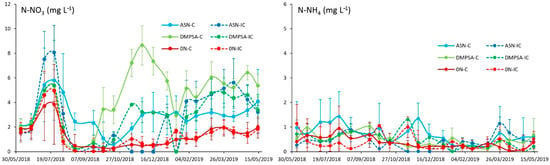
Figure 8.
Mineral N concentration in the drainage water at 1 m depth along the maize–wheat cycle. Treatments were combinations of ASN, ASN + DMPSA or no N fertilization (0 N) and the intercropping use (IC) or absence (C).
The water balance done with the WAVE model provided an estimated drainage during the maize period of 186 mm for the non-intercropped plots and 174 mm for the vetch intercropped plots. During this period, the intercropping had no effect on the N leached (as the vetch growth was still very limited), unlike fertilized treatments (Figure 9). In this case, the plots fertilized with regular ASN increased the N leached before the maize harvest to 11.8 kg N ha−1 (on average), with respect to the 7.2 kg N ha−1 estimated for the unfertilized treatments. Whereas the treatments fertilized with DMPSA only increased on average to 8.6 kg N ha−1 before the maize harvest. After the maize harvest, the vetch intercrop grew under a low precipitation period, which resulted generally in low water leaching (18 mL in the non-intercropped treatments and 3 mL in the intercropped treatments), most of which occurred between January 16 and February 14, corresponding with the wheat sowing. During the wheat period, the drainage was 37 mm for the non-intercropped plots and just 11 mm for the vetch intercropped plots. As happened previously during the intercropping period, the N leaching differences between the intercropped and non-intercropped treatments increased due to the combination of larger drainage and larger nitrate concentrations. Under these conditions, the final amount of N leached in the ASN-C treatment reached 13.5 kg N ha−1. Similar losses were observed for the treatments including just one alternative strategy (12.6 kg N ha−1 for the intercropped and 12.0 kg N ha−1 for the DMPSA). However, the DMPSA-IC (combining both strategies, DMPSA and intercrop) only lost 9.1 kg N ha−1, closer to the 7.8 kg N ha−1 lost in the unfertilized treatments.
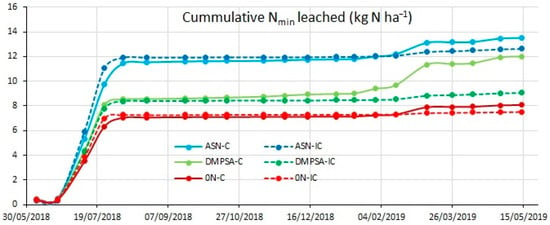
Figure 9.
Cumulative N leached with the drainage water at 1 m depth during the complete cycle. Treatments were combinations of ASN, ASN + DMPSA or no N fertilization (0 N) and the intercropping use (IC) or absence (C).
4. Discussion
Results obtained during the maize and wheat crop cycle showed little to no difference between the fertilized treatments, while unfertilized treatments generally showed poorer crop development. With respect to the pigments, lower chlorophyll and NBI values and higher flavonol and anthocyanin content were observed in the unfertilized treatments. However, as previously observed by other authors, vetch intercropped treatments showed a slightly increase in chlorophyll and NBI, while flavonol and anthocyanin content was mildly lower, although these differences were not statistically significant []. Some authors have pointed out that maize–legume (Crotalaria sp.) intercrops can show mixed results on the maize chlorophyll content depending on the legume sowing date []. On the other hand, the use of nitrification inhibitors has previously shown an increase in the chlorophyll content of maize plants compared to conventionally fertilized ones [].
Regarding maize biomass parameters, at harvest, the non-fertilized treatments showed the lowest results from all the studied treatments, as could be expected. The vetch intercrop effect over the different maize biomass parameters was faint, and it only had a slight impact ameliorating the detrimental effect of no fertilization (0 N) on yield, green biomass, height and N uptake. This ameliorating effect largely depends on the type of intercropping chosen, since the use of some types of legumes such as soybeans can lead to a reduction in green biomass and grain yield []. Anyway, the vetch growth until maize harvest was very limited by maize shadow, so small effects were expected, as Alonso-Ayuso et al. [] already observed. Among fertilized treatments, the plants treated with ASN + DMPSA showed a slight but not significant decrease in grain yield, plant height and N uptake, while green biomass and N concentration in grain was not affected. Similar results were obtained by Huérfano et al. [], who observed no significant differences in the grain yield, shoot yield and protein content of maize treated with ASN or ASN + DMPSA in a two year rotation of maize and ryegrass. Green biomass was higher in calcium ammonium nitrate (CAN) + DMPSA-fertilized maize compared with only CAN-fertilized plants in a two-year field experiment, while grain yield and aboveground N uptake were similar between both treatments []. Other wheat–maize rotations have also previously shown a slight but not significant decrease in maize and wheat grain yield and total N uptake in plants treated with nitrification inhibitors []. The effects observed in our experiment were probably caused by the fertilizer application schedule, which is optimal for ASN, but not for the DMPSA treatments. DMPSA, as observed, delayed the availability of NO3− for the maize, reducing in part the N uptake during the first stages. This delayed nitrification has been previously reported by other authors []. A better option could have been to adapt the DMPSA application to earlier stages, using all of it in the first side dressing, as we previously observed for other inhibitors []. The total amount of N available during the maize season was 370 kg N ha−1, resulting from 110 kg N ha−1 initially available in the soil, 175 kg N ha−1 applied with the fertilizer and 86 kg N ha−1 applied with the irrigation water. These application rates were in line with the optimal rates observed by other authors under similar conditions [,]. However, the nitrogen use efficiency of the nitrogen applied (considering N content in the grain versus N introduced with fertilization and irrigation water) moved from 63% to 71%. This recovery is in the upper range observed for maize by other authors such as Bundy and Andraski [] (ranging from 39% to 64%), Normand et al. [] (ranging from 64% to 66%) and Alonso-Ayuso et al. [] (55% on average), and it was also in the upper part of the range of the nitrogen use efficiency observed among 1240 farms in Europe [].
The use of nitrification inhibitors reduced more than half the residual Nmin in soil compared to the ASN alone fertilizer treatment, and this fact was even more relevant below the 60 cm depth, where the residual nitrate content is more prone to be leached. Other authors have also observed little to no difference in the N content of soil after the application of nitrification inhibitors to a maize crop. These results varied significantly depending on the amount of inhibitor applied and the phenology of the plant []. Nitrification inhibitors also showed a decrease in the nitrate liberation in a wheat–maize rotation in the first days after fertilizer application, while, on the contrary, the ammonium concentration increased due to the nitrification inhibitors’ effect []. The use of DMPSA in maize has also been related to general tendency to increase ammonium and decrease nitrate content in soil, even though this can be statically not significant and depend largely on other factors such as tillage management, phenology and fertilizer application rate, among others []. Guardia et al. [] observed that the amount of NH4+ in the first 10 cm of soil was greater in the maize fertilized with calcium ammonium nitrate (CAN) + DMPSA compared to the CAN alone fertilized plants, during the early crop cycle between the sow and the first dressing of a two-year field experiment.
As result, we obtained a large reduction of the NO3− leached in the DMPSA treatments, reducing it almost to the values observed for unfertilized treatments, during the maize period. On the other hand, the vetch had a lesser impact on nitrate leaching during this maize period. The increase observed in the nitrate concentration on the water leached during July was remarkable. This increase could be produced by the fertilization (June 18 and July 12), but this was not the only reason, because this tendency was also observed in the unfertilized treatments, although to a lesser extent (N mineralization estimated based on the Nmin balance in the 0 N-C treatment was around 80 kg N ha−1 over the entire maize–wheat season). It could also be produced by the high amount of nitrate observed in the irrigation water. Some other factors that could be producing this increase could be the increase of the mineralization rate (as July temperatures were higher) and soil humidity due to irrigation water, during a period when the maize was not developed enough to absorb all this NO3− []. Some kind of temporal immobilization of the N in the DMPSA treatments (probably as organic N) should be also considered because there was a decrease on the N in the maize, the N in the soil and the N leached. This effect needs further analysis with another experimental design.
At the end of the maize–wheat cycle, wheat showed similar yield results to the previous maize. Unfertilized controls showed a considerable decrease in grain yield, while fertilized treatments had no significant differences between their results, with a small effect of the DMPSA. Nitrification inhibitors have also previously shown no effect on grain or biomass yield in urea-fertilized wheat []. In another experiment, the effect of DMPSA was not so clear, and it depended on the fertilizer application rate and soil tillage management, even though the differences among fertilized treatments were not significant in any case []. In our experiment, wheat grain yield was also not affected by the previous vetch intercrop or crops grown over the previous intercrop systems, showing very similar results. This seems to indicate that the effects of the vetch intercrop on the grain yield have a greater weight during the development of the legume than after its harvest. In other studies, the effect of previous legume intercrops on a subsequent wheat rotation showed a beneficial effect on the grain yield similar to a 46 kg N ha−1 []. A similar experiment showed that legume intercropped with barley could lead to subsequent wheat productions similar to a 50 kg N ha−1, without comprising barley production and further increasing the whole crop rotation yield thanks to the legume production []. In both cases, the effect of using intercrops could also surpass the further application of conventional fertilizer, and this effect depended on the species chosen for intercropping and sowing density. Cowpea intercropped with maize has also shown the ability to increase the grain yield of the subsequent wheat grown in a maize–wheat rotation [].
With respect to the soil inorganic N, fertilized treatments showed a general increase in the nitrate and Nmin content of the soil in all the soil profile at the end of the maize–wheat cycle, with this effect being more pronounced in the soil’s upper layers. The use of vetch intercrops added to this effect, increasing the nitrate and Nmin content of the soil even more in the upper layers and the N-NO3−/N-NH4+ relation in the whole profile, which was again related to the N fixation ability of legumes. Although it was not measured in this study, previous studies in the region have shown that the vetch used as cover crop can fix between 32% and 68% of its total N uptake []. Intercropped treatments also showed a general decrease in the N-NH4+/Nmin relation, which was even lower than in the treatments without intercrops. Other experiments have also shown that the use of intercropped legumes with maize can improve N concentration in soil for later wheat crops, even though this effect was not statically significant []. Similar experiments based in barley–legume intercrops followed by wheat rotations showed an increase in nitrate content in the upper 60 cm of the soil profile after the intercrop harvest, even when compared to conventionally fertilized plots []. The differences between DMPSA and ASN alone fertilized treatments were small and not significant, which can mean that the effect of the DMPSA applied during the maize fertilization disappeared and was almost irrelevant in the subsequent wheat, in the line of the observed by others [,], but contrary to the observed for other inhibitors by other authors [].
Ammonium concentration in the drainage water after maize harvest and during the wheat cycle was mostly unaffected by the different treatments studied. On the other hand, nitrate concentration was surprisingly higher in the DMPSA-treated plots than in only ASN-treated plants. These data could confirm the uncoupling effect in the nitrification rate with respect to the crop demand produced when the DMPSA was applied in dressing fertilizations, and observed during the maize period. This uncoupling was partially solved including an intercrop in the maize crop, avoiding the fallow period between maize and wheat, recycling the nitrogen and reducing the nitrate leaching risk. Other authors already identified this catch crop effect of some legumes [,] under some conditions, even when they increase the total N availability due to N2 fixation. However, further solutions, such as earlier fertilizations, should be considered when DMPSA is used. In any case, the nitrate concentration found in the drainage waters was always below the 15 mg N-NO3− L−1 threshold fixed for human consumption. Previous experiments have shown that the use of nitrification inhibitors can reduce the amount of N-NO3− leached, while the N-NH4+ leached increases or remains the same, depending on the soil properties and the inhibitor formulation []. In the same line, other studies have shown a NO3− reduction in drainage water related to the use of nitrification inhibitor []. In both cases, the N source was urine. The formulation of the nitrification inhibitor also has to be minded, given that previous results have showed that DMPSA can be useful to reduce N2O emissions, while DMPSA+NBTP can not only reduce N2O emissions, but also NH3 losses [].
The WAVE model estimation for the amount of drainage water in non-intercropped treatments was larger than in the intercropped plots (37 mm and 11 mm, respectively). This ultimately leads to a reduction in the cumulative Nmin losses through leaching in the intercropped treatments, with the DMPSA-IC treatment showing approximately a 30% reduction in the amount of N leached and reaching results similar to the non-fertilized treatments. These results follows the same pattern as previous experiments, where maize intercropped with different species helped to ameliorate nitrate leaching [,,].
5. Conclusions
The results of this experiment indicate that the use of nitrification inhibitors in combination with intercropping techniques could be a very interesting way to reduce the environmental impact of the cropping systems without compromising economic sustainability. However, the fertilization schedule and rates should be readjusted in order to avoid deferred contamination.
In this experiment, the use of DMPSA had a positive impact during the first maize period, reducing the NO3− leaching without significant yield impact; however, it increased the nitrate leaching during the following autumn–winter period. On the other hand, the use of intercropping had no impact on the NO3− leaching during the first maize period but provided additional N to the soil during the next wheat cycle. Nevertheless, it was the combination of DMPSA and the introduction of vetch intercropping treatment that improved both lower nitrogen leaching along the entire maize–wheat cycle without a significant impact on the crop yield and the soil-available N.
Anyway, all the results observed in this study require further confirmation from additional experiments from different fields and laboratories to test not only the possible different behaviors, but also the different aspects that we were not able to demonstrate in this study, such as the impact of the treatments on the organic N stock fluctuations or other N losses (volatilization/denitrification) or the effect of the best adapted fertilization schedule and rates for DMPSA fertilizers.
Author Contributions
Conceptualization, J.L.G.; methodology, J.L.G. and D.M.-L.; formal analysis, J.L.G. and R.A.-M.; investigation, J.L.G., M.d.M.D., R.A.-M., D.M.-L. and M.A.P.; resources, J.L.G. and M.d.M.D.; data curation, J.L.G. and R.A.-M.; writing—original draft preparation, J.L.G. and R.A.-M.; writing—review and editing, J.L.G., R.A.-M., D.M.-L., M.A.P. and M.d.M.D.; visualization, J.L.G. and R.A.-M.; supervision, J.L.G.; project administration, J.L.G.; funding acquisition, J.L.G. and M.d.M.D. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the MCIN/AEI/10.13039/501100011033/(AGL2017-83283-C2-1/2-R), the Community of Madrid (AGRISOST-CM S2018/BAA-4330), and European Structural funding 2014-2020 (ERDF y ESF).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors wish to thank the work done by La Canaleja field staff (David Sanmartín and José Silveria), the laboratory staff (Mar Albarrán and Álvaro Moreno) and EuroChem Agro Iberia S.L: for facilitating the products for the experiment and their collaboration.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Sigurdarson, J.J.; Svane, S.; Karring, H. The molecular processes of urea hydrolysis in relation to ammonia emissions from agriculture. Rev. Environ. Sci. Biotechnol. 2018, 17, 241–258. [Google Scholar] [CrossRef]
- Mulvaney, R.L.; Khan, S.A.; Ellsworth, T.R. Synthetic Nitrogen Fertilizers Deplete Soil Nitrogen: A Global Dilemma for Sustainable Cereal Production. J. Environ. Qual. 2009, 38, 2295–2314. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing nitrogen for sustainable development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.D.; Lassaletta, L.; Runck, B.C.; Billen, G.; Garnier, J.; Gerber, J.S. Declining spatial efficiency of global cropland nitrogen allocation. Glob. Biogeochem. Cycles 2017, 31, 245–257. [Google Scholar] [CrossRef]
- Cameron, K.C.; Di, H.J.; Moir, J.L. Nitrogen losses from the soil/plant system: A review. Ann. Appl. Biol. 2013, 162, 145–173. [Google Scholar] [CrossRef]
- Quemada, M.; Gabriel, J.L. Approaches for increasing nitrogen and water use efficiency simultaneously. Glob. Food Sec. 2016, 9, 29–35. [Google Scholar] [CrossRef]
- Prasad, R.; Power, J.F. Nitrification Inhibitors for Agriculture, Health, and the Environment. Adv. Agron. 1995, 54, 233–281. [Google Scholar] [CrossRef]
- Ladha, J.K.; Pathak, H.; Krupnik, T.J.; Six, J.; van Kessel, C. Efficiency of Fertilizer Nitrogen in Cereal Production: Retrospects and Prospects. Adv. Agron. 2005, 87, 85–156. [Google Scholar] [CrossRef]
- Umar, W.; Ayub, M.A.; ur Rehman, M.Z.; Ahmad, H.R.; Farooqi, Z.U.R.; Shahzad, A.; Rehman, U.; Mustafa, A.; Nadeem, M. Nitrogen and Phosphorus Use Efficiency in Agroecosystems. In Resources Use Efficiency in Agriculture; Kumar, S., Meena, R.S., Jhariya, M.K., Eds.; Springer: Singapore, 2020; pp. 213–257. ISBN 978-981-15-6953-1. [Google Scholar]
- Salac, L.; Chaillou, S.; Morot-Gaudry, J.-F.; Lesaint, C.; Jolivet, E. Nitrate and ammonium nutrition in plants. Plant Physiol. Biochem. 1987, 25, 805–812. [Google Scholar]
- Vandermeer, J. The Ecology of Agroecosystems; Jones & Bartlett Learning: Sudbury, MA, USA, 2011; ISBN 0763771538. [Google Scholar]
- Brooker, R.W.; Bennett, A.E.; Cong, W.F.; Daniell, T.J.; George, T.S.; Hallett, P.D.; Hawes, C.; Iannetta, P.P.M.; Jones, H.G.; Karley, A.J.; et al. Improving intercropping: A synthesis of research in agronomy, plant physiology and ecology. New Phytol. 2015, 206, 107–117. [Google Scholar] [CrossRef]
- Panda, S.K.; Panda, P.; Pramanick, B.; Shankar, T.; Praharaj, S.; Saren, B.; Gitari, H.; Brahmachari, K.; Hossain, A.; Maitra, S. Advantages of Cotton Based Intercropping System: A Review. Int. J. Bioresour. Sci. 2020, 7, 51–57. [Google Scholar] [CrossRef]
- Hauggaard-Nielsen, H.; Ambus, P.; Jensen, E.S. The comparison of nitrogen use and leaching in sole cropped versus intercropped pea and barley. Nutr. Cycl. Agroecosyst. 2003, 65, 289–300. [Google Scholar] [CrossRef]
- Nie, S.; Eneji, A.E.; Chen, Y.; Sui, P.; Huang, J.; Huang, S. Nitrate Leaching from Maize Intercropping Systems with N Fertilizer Over-Dose. J. Integr. Agric. 2012, 11, 1555–1565. [Google Scholar] [CrossRef]
- Manevski, K.; Børgesen, C.D.; Andersen, M.N.; Kristensen, I.S. Reduced nitrogen leaching by intercropping maize with red fescue on sandy soils in North Europe: A combined field and modeling study. Plant Soil 2015, 388, 67–85. [Google Scholar] [CrossRef]
- Bedoussac, L.; Journet, E.P.; Hauggaard-Nielsen, H.; Naudin, C.; Corre-Hellou, G.; Jensen, E.; Prieur, L.; Justes, E. Ecological principles underlying the increase of productivity achieved by cereal-grain legume intercrops in organic farming. A review. Agron. Sustain. Dev. 2015, 35, 911–935. [Google Scholar] [CrossRef]
- Chen, P.; Song, C.; Liu, X.M.; Zhou, L.; Yang, H.; Zhang, X.; Zhou, Y.; Du, Q.; Pang, T.; Fu, Z.D.; et al. Yield advantage and nitrogen fate in an additive maize-soybean relay intercropping system. Sci. Total Environ. 2019, 657, 987–999. [Google Scholar] [CrossRef]
- Alonso-Ayuso, M.; Gabriel, J.L.; Pancorbo, J.L.; Quemada, M. Interseeding cover crops into maize: Characterization of species performance under Mediterranean conditions. Field Crops Res. 2020, 249, 107762. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, C.; Li, J.; Wu, X.; Long, Y.; Su, Y. Intercropping Vicia sativa L. Improves the Moisture, Microbial Community, Enzyme Activity and Nutrient in Rhizosphere Soils of Young Kiwifruit Plants and Enhances Plant Growth. Horticulturae 2021, 7, 335. [Google Scholar] [CrossRef]
- Lithourgidis, A.S.; Dhima, K.V.; Vasilakoglou, I.B.; Dordas, C.A.; Yiakoulaki, M.D. Sustainable production of barley and wheat by intercropping common vetch. Agron. Sustain. Dev. 2007, 27, 95–99. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. FAOSTAT Database: Rome, Italy, 2019. Available online: https://www.fao.org/faostat/en/#home (accessed on 1 December 2021).
- Gabriel, J.L.; Zarco-Tejada, P.J.; López-Herrera, P.J.; Pérez-Martín, E.; Alonso-Ayuso, M.; Quemada, M. Airborne and ground level sensors for monitoring nitrogen status in a maize crop. Biosyst. Eng. 2017, 160, 124–133. [Google Scholar] [CrossRef]
- Quemada, M.; Gabriel, J.L.; Zarco-Tejada, P. Airborne hyperspectral images and ground-level optical sensors as assessment tools for maize nitrogen fertilization. Remote Sens. 2014, 6, 2940–2962. [Google Scholar] [CrossRef]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop evapotranspiration-Guidelines for computing crop water requirements-FAO Irrigation and drainage paper 56. Fao Rome 1998, 300, D05109. [Google Scholar]
- Martínez-Cob, A. Use of thermal units to estimate corn crop coefficients under semiarid climatic conditions. Irrig. Sci. 2008, 26, 335–345. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Cerovic, Z.G. Sensing crop nitrogen status with fluorescence indicators. A review. Agron. Sustain. Dev. 2012, 32, 451–464. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef]
- Gabriel, J.L.; Quemada, M.; Alonso-Ayuso, M.; Lizaso, J.I.; Martín-Lammerding, D. Predicting N status in maize with clip sensors: Choosing sensor, leaf sampling point, and timing. Sensors 2019, 19, 3881. [Google Scholar] [CrossRef] [PubMed]
- Keeney, D.R.; Nelson, D.W. Nitrogen—Inorganic Forms. In Methods of Soil Analysis; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1983; pp. 643–698. ISBN 9780891189770. [Google Scholar]
- Solorzano, L. Determination of ammonia in natural waters by the phenolhypoclorite method. Limnol. Ocean. 1969, 14, 799–801. [Google Scholar]
- Vanclooster, M.; Viaene, P.; Diels, J.; Christiaens, K. WAVE: A mathematical model for simulating water and agrochemicals in the soil and vadose environment. In Reference and User’s Manual (Release 2.0); Institute for Land and Water Management: Leuven, Belgium, 1996. [Google Scholar]
- Lord, E.I.; Shepherd, M.A. Developments in the use of porous ceramic cups for measuring nitrate leaching. J. Soil Sci. 1993, 44, 435–449. [Google Scholar] [CrossRef]
- Nasar, J.; Shao, Z.; Arshad, A.; Jones, F.G.; Liu, S.; Li, C.; Khan, M.Z.; Khan, T.; Banda, J.S.K.; Zhou, X.; et al. The effect of maize–alfalfa intercropping on the physiological characteristics, nitrogen uptake and yield of maize. Plant Biol. 2020, 22, 1140–1149. [Google Scholar] [CrossRef]
- De Souza, R.T.; De Assis Valadão, F.C.; Júnior, D.D.V.; Guimarães, P.R.; De Paula, V.R.R. Maize-crotalaria intercropping systems. Semin. Agrar. 2019, 40, 1455–1467. [Google Scholar] [CrossRef]
- Dong, Y.J.; He, M.R.; Wang, Z.L.; Chen, W.F.; Hou, J.; Qiu, X.K.; Zhang, J.W. Effects of new coated release fertilizer on the growth of maize. J. Soil Sci. Plant Nutr. 2016, 16, 637–649. [Google Scholar] [CrossRef][Green Version]
- Yang, W.; Miao, J.; Wang, X.; Xu, J.; Lu, M.; Li, Z. Corn-soybean intercropping and nitrogen rates affected crop nitrogen and carbon uptake and C:N ratio in upland red soil. J. Plant Nutr. 2018, 41, 1890–1902. [Google Scholar] [CrossRef]
- Huérfano, X.; Estavillo, J.M.; Fuertes-Mendizábal, T.; Torralbo, F.; González-Murua, C.; Menéndez, S. DMPSA and DMPP equally reduce N2O emissions from a maize-ryegrass forage rotation under Atlantic climate conditions. Atmos. Environ. 2018, 187, 255–265. [Google Scholar] [CrossRef]
- Guardia, G.; Cangani, M.T.; Andreu, G.; Sanz-Cobena, A.; García-Marco, S.; Álvarez, J.M.; Recio-Huetos, J.; Vallejo, A. Effect of inhibitors and fertigation strategies on GHG emissions, NO fluxes and yield in irrigated maize. Field Crops Res. 2017, 204, 135–145. [Google Scholar] [CrossRef]
- De Antoni Migliorati, M.; Scheer, C.; Grace, P.R.; Rowlings, D.W.; Bell, M.; McGree, J. Influence of different nitrogen rates and DMPP nitrification inhibitor on annual N2O emissions from a subtropical wheat–maize cropping system. Agric. Ecosyst. Environ. 2014, 186, 33–43. [Google Scholar] [CrossRef]
- Ashraf, M.; Aziz, T.; Maqsood, M.; Bilal, H.; Raza, S.; Zia, M.; Mustafa, A.; Xu, M.; Wang, Y.; Ashraf, M.N.; et al. Evaluating Organic Materials Coating on Urea as Potential Nitrification Inhibitors for Enhanced Nitrogen Recovery and Growth of Maize (Zea mays L.). Int. J. Agric. Biol. 2019, 22, 1102–1108. [Google Scholar] [CrossRef]
- Allende-Montalbán, R.; Martín-Lammerding, D.; Del Mar Delgado, M.; Porcel, M.A.; Gabriel, J.L. Urease Inhibitors Effects on the Nitrogen Use Efficiency in a Maize–Wheat Rotation with or without Water Deficit. Agriculture 2021, 11, 684. [Google Scholar] [CrossRef]
- Martínez, E.; Maresma, A.; Biau, A.; Cela, S.; Berenguer, P.; Santiveri, F.; Michelena, A.; Lloveras, J. Long-Term Effects of Mineral Nitrogen Fertilizer on Irrigated Maize and Soil Properties. Agron. J. 2017, 109, 1880–1890. [Google Scholar] [CrossRef]
- López-Córcoles, H.; de Juan, J.A.; Picornell, M.R. Biomass production and yield in irrigated maize at different rates of nitrogen in a semi-arid climate. NJAS Wagening. J. Life Sci. 2020, 92, 100321. [Google Scholar] [CrossRef]
- Bundy, L.G.; Andraski, T.W. Recovery of fertilizer nitrogen in crop residuesand cover crops on an irrigated sandy soil. Soil Sci. Soc. Am. J. 2005, 69, 640–648. [Google Scholar] [CrossRef]
- Normand, B.; Recous, S.; Vachaud, G.; Kengni, L.; Garino, B. Nitrogen-15 tracers combined with tension-neutronic method to estimate the nitrogen balance of irrigated maize. Soil Sci. Soc. Am. J. 1997, 61, 1508–1518. [Google Scholar] [CrossRef]
- Alonso-Ayuso, M.; Gabriel, J.L.; Quemada, M. Nitrogen use efficiency and residual effect of fertilizers with nitrification inhibitors. Eur. J. Agron. 2016, 80, 1–8. [Google Scholar] [CrossRef]
- Quemada, M.; Lassaletta, L.; Jensen, L.S.; Godinot, O.; Brentrup, F.; Buckley, C.; Foray, S.; Hvid, S.K.; Oenema, J.; Richards, K.G.; et al. Exploring nitrogen indicators of farm performance among farm types across several European case studies. Agric. Syst. 2020, 177, 102689. [Google Scholar] [CrossRef]
- Corrochano-Monsalve, M.; Huérfano, X.; Menéndez, S.; Torralbo, F.; Fuertes-Mendizábal, T.; Estavillo, J.M.; González-Murua, C. Relationship between tillage management and DMPSA nitrification inhibitor efficiency. Sci. Total Environ. 2020, 718, 134748. [Google Scholar] [CrossRef]
- Recio, J.; Alvarez, J.M.; Rodriguez-Quijano, M.; Vallejo, A. Nitrification inhibitor DMPSA mitigated N2O emission and promoted NO sink in rainfed wheat. Environ. Pollut. 2019, 245, 199–207. [Google Scholar] [CrossRef]
- Scalise, A.; Tortorella, D.; Pristeri, A.; Petrovičová, B.; Gelsomino, A.; Lindström, K.; Monti, M. Legume–barley intercropping stimulates soil N supply and crop yield in the succeeding durum wheat in a rotation under rainfed conditions. Soil Biol. Biochem. 2015, 89, 150–161. [Google Scholar] [CrossRef]
- Monti, M.; Pellicanò, A.; Pristeri, A.; Badagliacca, G.; Preiti, G.; Gelsomino, A. Cereal/grain legume intercropping in rotation with durum wheat in crop/livestock production systems for Mediterranean farming system. Field Crops Res. 2019, 240, 23–33. [Google Scholar] [CrossRef]
- Sharma, N.K.; Singh, R.J.; Mandal, D.; Kumar, A.; Alam, N.M.; Keesstra, S. Increasing farmer’s income and reducing soil erosion using intercropping in rainfed maize-wheat rotation of Himalaya, India. Agric. Ecosyst. Environ. 2017, 247, 43–53. [Google Scholar] [CrossRef]
- Gabriel, J.L.; Quemada, M. Replacing bare fallow with cover crops in a maize cropping system: Yield, N uptake and fertiliser fate. Eur. J. Agron. 2011, 34, 133–143. [Google Scholar] [CrossRef]
- Quemada, M.; Alonso-Ayuso, M.; Castellano-Hinojosa, A.; Bedmar, E.J.; Gabriel, J.L.; García González, I.; Valentín, F.; Calvo, M. Residual effect of synthetic nitrogen fertilizers and impact on Soil Nitrifiers. Eur. J. Agron. 2019, 109, 125917. [Google Scholar] [CrossRef]
- Raya-Sereno, M.D.; Alonso-Ayuso, M.; Pancorbo, J.L.; Gabriel, J.L.; Camino, C.; Zarco-Tejada, P.J.; Quemada, M. Residual Effect and N Fertilizer Rate Detection by High-Resolution VNIR-SWIR Hyperspectral Imagery and Solar-Induced Chlorophyll Fluorescence in Wheat. IEEE Trans. Geosci. Remote Sens. 2022, 60, 4404017. [Google Scholar] [CrossRef]
- Gabriel, J.L.; Muñoz-Carpena, R.; Quemada, M. The role of cover crops in irrigated systems: Water balance, nitrate leaching and soil mineral nitrogen accumulation. Agric. Ecosyst. Environ. 2012, 155, 50–61. [Google Scholar] [CrossRef]
- Thorup-Kristensen, K.; Magid, J.; Jensen, L.S. Cover crops and green manures as biological tools in nitrogen management in temperate zones. Adv. Agron. 2003, 79, 227–302. [Google Scholar] [CrossRef]
- Menneer, J.C.; Ledgard, S.; Sprosen, M. Soil N process inhibitors alter nitrogen leaching dynamics in a pumice soil. Soil Res. 2008, 46, 323–331. [Google Scholar] [CrossRef]
- Di, H.J.; Cameron, K.C. Nitrate leaching losses and pasture yields as affected by different rates of animal urine nitrogen returns and application of a nitrification inhibitor—A lysimeter study. Nutr. Cycl. Agroecosyst. 2007, 79, 281–290. [Google Scholar] [CrossRef]
- Souza, E.F.C.; Rosen, C.J.; Venterea, R.T. Co-application of DMPSA and NBPT with urea mitigates both nitrous oxide emissions and nitrate leaching during irrigated potato production. Environ. Pollut. 2021, 284, 117124. [Google Scholar] [CrossRef]
- Long, G.; Li, L.; Wang, D.; Zhao, P.; Tang, L.; Zhou, Y.; Yin, X. Nitrogen levels regulate intercropping-related mitigation of potential nitrate leaching. Agric. Ecosyst. Environ. 2021, 319, 107540. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).