Impact of Ecological Factors on the Occurrence and Spatial-Taxonomic Structure of Keratinophilic Fungi and Their Co-Occurrence in Arable Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Soil Samples
2.2. Chemical Determinations
2.3. Mycological Analyses
2.3.1. Substrate
2.3.2. Isolation of Keratinophilic Fungi
2.3.3. Identification of Fungi
2.4. Result Analysis
2.5. Statistical Analyses
3. Results
3.1. Growth Indices of Keratinophilic and Non-Keratinophilic Fungi in the Analyzed Soils
3.2. General Characteristics of Keratinophilic and Non-Keratinophilic Species Composition
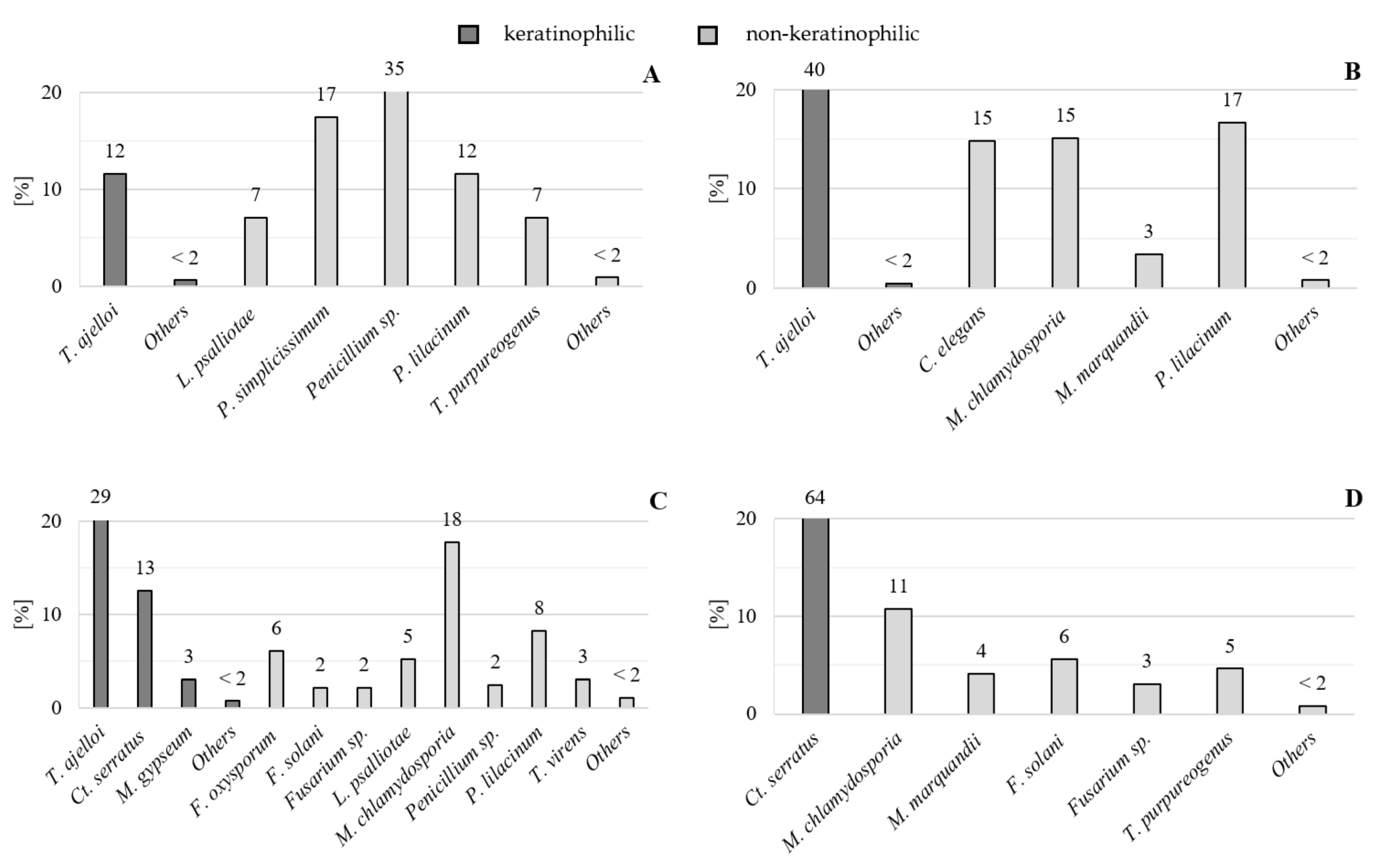
3.3. Species Dominance Coefficients and the Taxonomic and Spatial Structure of Fungi Colonizing Native Keratin
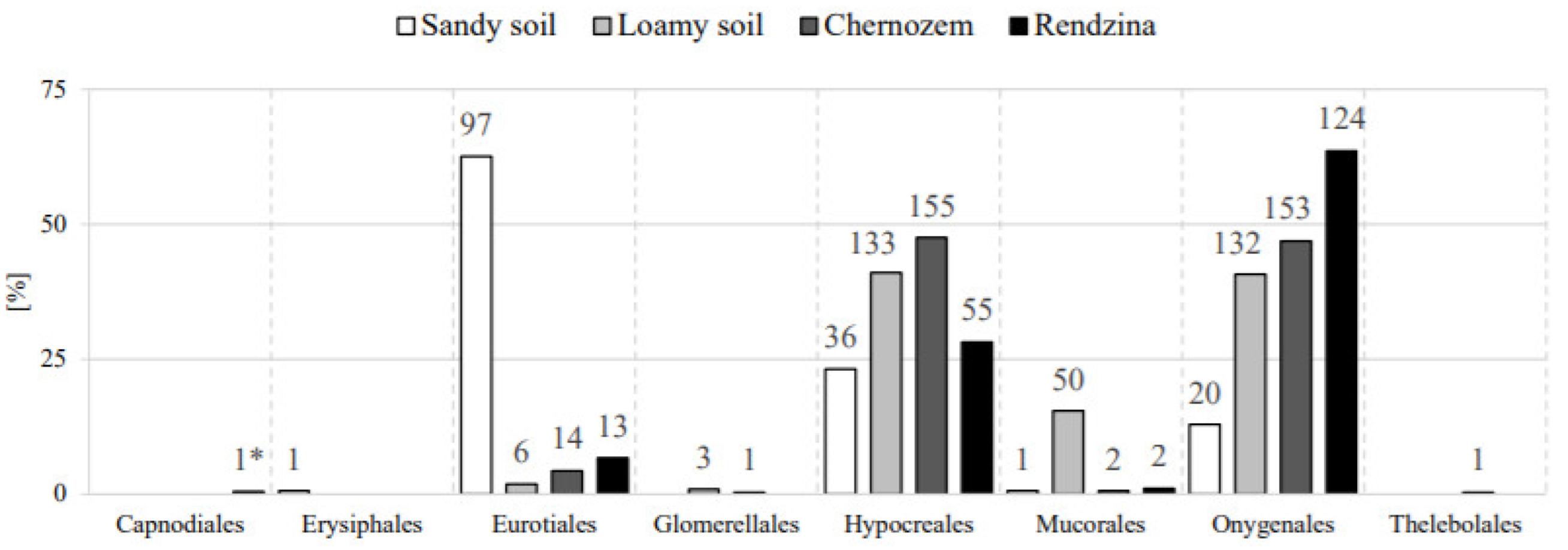
3.4. Species Similarity and Diversity in the Analyzed Fungal Communities
3.5. Correlations between the Frequency of Occurrence of Fungi and Soil Properties
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chmel, L.; Vláčilíková, A. The ecology of keratinophilic fungi in different depths of soil. Sabouraudia 1975, 13, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Korniłłowicz-Kowalska, T.; Bohacz, J. Some correlations between the occurrence frequency of keratinophilic fungi and selected soil properties. Acta Mycol. 2002, 37, 101–116. [Google Scholar] [CrossRef]
- Korniłłowicz-Kowalska, T.; Bohacz, J. Biodegradation of keratin waste: Theory and practical aspects. Waste Manag. 2011, 31, 1689–1701. [Google Scholar] [CrossRef] [PubMed]
- Javoreková, S.; Labuda, R.; Maková, J.; Novák, J.; Medo, J.; Majerčíková, K. Keratinophilic fungi isolated from soils of long-term fold-grazed, degraded pastures in National Parks of Slovakia. Mycopathologia 2012, 174, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Kačinová, J.; Tančinová, D.; Labuda, R. Keratinophilic fungi in soils stressed by occurrence of animals. J. Microbiol. Biotechnol. Food Sci. 2013, 2, 1436–1446. [Google Scholar]
- Volleková, A. Keratinophilic fungi in rodents’ burrows and in their adjoining surroundings. Czech Mycol. 1985, 39, 97–105. [Google Scholar]
- Moallaei, H.; Zaini, F.; Pihet, M.; Mahmoudi, M.; Hashemi, J. Isolation of keratinophilic fungi from soil samples of forests and farm yards. Iran. J. Public Health 2006, 35, 62–69. [Google Scholar]
- Deshmukh, S.K.; Verekar, S.A.; Chavan, Y.G. Incidence of Keratinophilic fungi from the selected soils of Kaziranga National Park, Assam (India). Mycopathologia 2017, 182, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Filipello-Marchisio, V. Keratinophilic fungi: Their role in nature and degradation of keratinic substrates. In Biology of Dermatophytes and Other Keratinophilic Fungi; Kushwaha, R.K.S., Guarro, J., Eds.; Revista Iberoamericana de Micología: Bilbao, Spain, 2000; Volume 17, pp. 86–92. [Google Scholar]
- Otčenášek, M.; Dvořák, J. Ecological classification of dermatophytes. Mycoses 1975, 18, 425–434. [Google Scholar] [CrossRef]
- Van Oorschot, C.A.N. A revision of Chrysosporium and allied genera. Stud. Mycol. 1980, 20, 1–89. [Google Scholar] [CrossRef]
- Simpanya, M.F. Dermatophytes: Their taxonomy, ecology and pathogenicity. In Biology of Dermatophytes and Other Keratinophilic Fungi; Kushwaha, R.K.S., Guarro, J., Eds.; Revista Iberoamericana de Micología: Bilbao, Spain, 2000; Volume 12, pp. 1–12. [Google Scholar]
- Korniłłowicz, T. The frequency of occurrence and distribution of keratinophilic fungi in some arable soils. Acta Mycol. 1993, 28, 3–17. [Google Scholar] [CrossRef]
- Kunert, J. Physiology of keratinophilic fungi. In Biology of Dermatophytes and Other Keratinophilic Fungi; Kushwaha, R.K.S., Guarro, J., Eds.; Revista Iberoamericana de Micología: Bilbao, Spain, 2000; Volume 17, pp. 77–85. [Google Scholar]
- Korniłłowicz-Kowalska, T. Studies on the decomposition of keratin wastes by saprotrophic microfungi. I. Criteria for evaluating keratinolytic activity. Acta Mycol. 1997, 32, 51–79. [Google Scholar] [CrossRef]
- Bohacz, J. Biodegradation of feather waste keratin by a keratinolytic soil fungus of the genus Chrysosporium and statistical optimization of feather mass loss. World J. Microbiol. Biotechnol. 2017, 33, 13. [Google Scholar] [CrossRef]
- Takiuchi, I.; Sei, Y.; Takagi, H.; Negi, M. Partial characterization of the extracellular keratinase from Microsporum canis. Sabouraudia 1984, 22, 219–224. [Google Scholar] [CrossRef]
- Bohacz, J.; Korniłłowicz-Kowalska, T. Species diversity of keratinophilic fungi in various soil types. Cent. Eur. J. Biol. 2012, 7, 259–266. [Google Scholar] [CrossRef]
- Garg, A.P.; Gandotra, S.; Mukrji, K.G.; Pugh, G.J.F. Ecology of keratinophilic fungi. Proc. Plant Sci. 1985, 94, 149–163. [Google Scholar] [CrossRef]
- Al-Musallam, A.A. Distribution of keratinophilic fungi in desert soil of Kuwait. Mycoses 1989, 32, 296–302. [Google Scholar] [CrossRef]
- Ciesielska, A.; Bohacz, J.; Korniłłowicz-Kowalska, T.; Stączek, P. Microsatellite-primed PCR for intra-species genetic relatedness in Trichophyton ajelloi strains isolated in Poland from various soil samples. Microbes Environ. 2014, 29, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Böhme, H.; Ziegler, H. The distribution of geophilic dermatophytes and other keratinophilic fungi in relation to the pH of the soil. Mycopathol. Mycol. Appl. 1969, 38, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Swati, K. Prevalence of keratinophilic fungi at various pH in different areas of Jaipur, Rajasthan. J. Microbiol. Biotechnol. Res. 2014, 4, 17–21. [Google Scholar]
- Kumawat, T.K.; Sharma, A.; Sharma, V.; Chandra, S.; Bhadauria, S. A study on the prevalence of keratinophilic fungal biota of semi-arid region of Rajasthan, India. J. King Saud Univ. Sci. 2020, 32, 1014–1020. [Google Scholar] [CrossRef]
- Chmel, L.; Hasilíková, A.; Hrašco, J.; Vláčilíková, A. The influence of some ecological factors on keratinophilic fungi in the soil. Sabouraudia 1972, 10, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Korniłłowicz, T. Studies on mycoflora colonizing raw keratin wastes in arable soil. Acta Mycol. 1992, 27, 231–245, In Polish. [Google Scholar] [CrossRef][Green Version]
- IUSS Working Group WRB. World Reference Base of Soil Resources. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; Update 2015; World Soil Recourses Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Domsch, K.H.; Gams, W.; Anderson, T.H. Compendium of Soil Fungi, 2nd ed.; IHW: Eching, Germany, 2007. [Google Scholar]
- Kwaśna, H.; Chełkowski, J.; Zajkowski, P. Polish Flora. Fungi (Mycota) v.22 (Deuteromycetes), (Hyphomycetales), (Fusarium); Polish Scientific Publishing House: Warsaw-Krakow, Poland, 1991. (In Polish) [Google Scholar]
- Nelson, P.E.; Toussoun, T.A.; Morasas, W.F.O. Fusarium Species: An Illustrated Manual for Identification; The Pennsylvania State University Press: University Park, PA, USA, 1983. [Google Scholar]
- Trojan, P. General Ecology; Polish Scientific Publishing House: Warsaw, Poland, 1975. (In Polish) [Google Scholar]
- Krebs, C.J. Ecology. The Experimental Analysis of Distribution and Abundance, 4th ed.; Harper Collins: New York, NY, USA, 1994. [Google Scholar]
- Szewczyk, W. Soil fungi communities from young Scots pine plantations affected with root rot. Acta Mycol. 2007, 42, 239–244. [Google Scholar] [CrossRef]
- Korniłłowicz-Kowalska, T.; Wojdyło-Kotwica, B.; Bohacz, J.; Możejko, M.T. Biodiversity of saprotrophic fungi of the root zone of grasses and clovers in a permanent meadow in a post-bog habitat in relation to the growing season and fertilization (Zaklęsłość Sosnowicka, Western Polesye). In Environmental Engineering in Polesye, Polish Polesye; Urban, D., Dobrowolski, R., Jeznach, J., Eds.; International Scientific Publications: Brest, Belarus; Rivne, Ukraine; Warsaw, Poland; Ryazan, Russia, 2020; pp. 485–538. (In Polish) [Google Scholar]
- Anane, S.; Hashim, M.; Al-Yasiri, Y.; Normand, A.C.; Ranque, S. Distribution of keratinophilic fungi in soil across Tunisia: A descriptive study and review of the literature. Mycopathologia 2015, 180, 61–68. [Google Scholar] [CrossRef]
- Anbu, P.; Hilda, A.; Gopinath, S.C.B. Keratinophilic fungi of poultry and feather dumping soil in Tamil Nadu, India. Mycopathologia 2004, 158, 303–309. [Google Scholar] [CrossRef]
- Agrawal, S.; Nandeibam, J.; Devi, I. Danger of exposure to keratinophilic fungi and other dermatophytes in recreational place in the northeast region of India. Aerobiologia 2021, 37, 755–766. [Google Scholar] [CrossRef]
- Ajello, I. Soil as a natural reservoir for human pathogenic fungi. Science 1956, 123, 876–879. [Google Scholar] [CrossRef]
- De Jong, R.; Campbell, C.A.; Nicholaichuk, W. Water retention equations and their relationship to soil organic matter and particle size distribution for disturbed samples. Can. J. Soil Sci. 1983, 63, 291–302. [Google Scholar] [CrossRef]
- Owczarzak, W.; Dębicki, R.; Mocek, A. Physical properties of soils. In Soil Science, 1st ed.; Mocek, A., Ed.; Polish Scientific Publishing House: Warsaw, Poland, 2015; pp. 131–188. (In Polish) [Google Scholar]
- Hubálek, Z. Keratinophilic fungi associated with free living mammals and birds. In Biology of Dermatophytes and Other Keratinophilic Fungi; Kushwaha, R.K.S., Guarro, J., Eds.; Revista Iberoamericana de Micología: Bilbao, Spain, 2000; Volume 17, pp. 104–108. [Google Scholar]
- Zarrin, M.; Haghgoo, R. Survey of keratinophilic fungi from soils in Ahvaz, Iran. Jundishapur J. Microbiol. 2011, 4, 191–194. [Google Scholar]
- Grant, C.; Hunter, C.A.; Flannigan, B.; Bravery, A.F. The moisture requirements of moulds isolated from domestic dwellings. Int. Biodeterior. 1989, 25, 259–284. [Google Scholar] [CrossRef]
- Korniłłowicz, T. Studies on the mycroflora colonizing keratin-bark-urea manure. Acta Mycol. 1993, 28, 19–30. [Google Scholar] [CrossRef]
- Bohacz, J.; Korniłłowicz-Kowalska, T. Fungal diversity and keratinolytic activity of fungi from lignocellulosic composts with chicken feathers. Process. Biochem. 2019, 80, 119–128. [Google Scholar] [CrossRef]
- Bohacz, J. Changes in mineral forms of nitrogen and sulfur and enzymatic activities during composting of lignocellulosic waste and chicken feathers. Environ. Sci. Pollut. Res. 2019, 26, 10333–10342. [Google Scholar] [CrossRef] [PubMed]
- Bohacz, J. Composts and water extracts of lignocellulosic composts in the aspect of fertilization, humus-forming, sanitary, phytosanitary and phytotoxicity value assessment. Waste Biomass Valorization 2019, 10, 2837–2850. [Google Scholar] [CrossRef]
- Krasowicz, S.; Oleszek, W.; Horabik, J.; Dębicki, R.; Jankowiak, J.; Stuczyński, T.; Jadczyszyn, J. Racjonalne gospodarowanie środowiskiem glebowym Polski. Pol. J. Agron. 2011, 7, 43–58. (In Polish) [Google Scholar]

| Parameter | Soil Type/Particle Size Distribution | |||
|---|---|---|---|---|
| Cambisol/Loamy Sand | Cambisol/Sandy Loam | Chernozem/Silt Loam | Leptosol/Silt Loam | |
| C org. [%] | 0.59 | 1.02 | 1.45 | 3.16 |
| Organic matter [%] | 1.01 | 1.76 | 2.5 | 5.44 |
| N tot. [%] | 0.059 | 0.107 | 0.154 | 0.301 |
| C tot. [%] | 0.665 | 1.196 | 1.692 | 10.289 |
| CaCO3 [%] | 0.00 | 0.00 | 0.00 | 57.96 |
| P2O5 [mg kg−1] | 102.00 | 67.00 | 114.00 | 212.00 |
| K2O [mg kg−1] | 80.00 | 140.00 | 207.00 | 472.00 |
| pHKCl | 3.4 | 5.4 | 5.0 | 6.9 |
| P [mg kg−1] | 500.88 | 481.44 | 773.73 | 994.65 |
| Mg [mg kg−1] | 575.68 | 2851.90 | 2214.59 | 19,300.12 |
| K [mg kg−1] | 1079.87 | 5158.00 | 3886.99 | 7562.13 |
| Ca [mg kg−1] | 578.78 | 2422.73 | 3604.02 | 170,000.50 |
| Ø 2–0.05 mm [%] | 84.08 | 54.57 | 17.37 | 31.93 |
| Ø 0.05–0.002 mm [%] | 14.69 | 39.83 | 75.58 | 58.07 |
| Ø < 0.002 mm [%] | 1.23 | 5.61 | 7.04 | 9.99 |
| Growth Indices | Soil | |||||||
|---|---|---|---|---|---|---|---|---|
| Sandy | Loamy | Chernozem | Rendzina | |||||
| Number of Plates/Samples Colonized by: | ||||||||
| Keratinophilic fungi | 12 | 46 | 49 | 45 | ||||
| Including geophilic dermatophytes | 11 | 45 | 46 | 0 | ||||
| Chrysosporium group | 2 | 1 | 32 | 45 | ||||
| Non-keratinophilic fungi | 41 | 50 | 49 | 46 | ||||
| Total | 41 | 50 | 50 | 50 | ||||
| Number of Isolated | ||||||||
| Genera | ||||||||
| Keratinophilic | 2 | 3 | 3 | 1 | ||||
| Non-keratinophilic | 12 | 15 | 11 | 14 | ||||
| Total | 14 | 18 | 16 | 15 | ||||
| Species | ||||||||
| Geophilic dermatophytes | 1 | 2 | 2 | 0 | ||||
| Chrysosporium group | 2 | 1 | 5 | 1 | ||||
| Non-keratinophilic | 14 | 16 | 14 | 15 | ||||
| Total | 17 | 19 | 21 | 16 | ||||
| Strains | ||||||||
| Geophilic dermatophytes | 18 | 20 b | 131 | 132 a | 103 | 154 c | 0 | 124a |
| Chrysosporium group | 2 | 1 | 51 | 124 | ||||
| Non-keratinophilic | 135 b | 192 d | 172 c | 71 a | ||||
| Total | 155 | 324 | 326 | 195 | ||||
| Number of Species per Plate/Sample | ||||||||
| Geophilic dermatophytes | 0.36 | 2.62 | 2.06 | 0 | ||||
| Chrysosporium group | 0.04 | 0.02 | 1.02 | 2.48 | ||||
| Total | 0.4 | 2.64 | 3.08 | 2.48 | ||||
| Non-keratinophilic | 2.7 | 3.84 | 3.44 | 1.42 | ||||
| No. | Fungal Species | Soil | |||||
|---|---|---|---|---|---|---|---|
| Sandy | Loamy | Chernozem | Rendzina | Total | |||
| Species Name Acc. to Index Fungorum | Species Name | Number of Records (Isolates) | |||||
| Keratinophilic fungi | |||||||
| Chrysosporium group | |||||||
| 1. | Chrysosporium sp. (Onygenales) | Chrysosporium sp. (Onygenales) | 1 | 0 | 3 | 0 | 4 |
| 2. | Chrysosporium tropicum J.W. Carmich. (Onygenales) | Chrysosporium tropicum J.W. Carmich. (Onygenales) | 1 | 0 | 5 | 0 | 6 |
| 3. | Ctenomyces serratus Eidam (Onygenales) | Ctenomyces serratus Eidam (Onygenales) | 0 | 1 | 41 | 124 | 166 |
| 4. | Ctenomyces vellereus (Sacc. & Speg.) P.M. Kirk (Onygenales) | Myceliophthora vellerea (Sacc. & Speg.) Oorschot (Sordariales) | 0 | 0 | 1 | 0 | 1 |
| 5. | Pseudogymnoascus pannorum (Link) Minnis & D.L. Lindner (Thelebolales) | Chrysosporium pannorum (Link) S. Hughes (Onygenales) | 0 | 0 | 1 | 0 | 1 |
| Geophilic dermatophytes | |||||||
| 6. | Microsporum gypseum (E. Bodin) Guiart & Grigoraki (Onygenales) | Microsporum gypseum (E. Bodin) Guiart & Grigoraki (Onygenales) | 0 | 2 | 10 | 0 | 12 |
| 7. | Trichophyton ajelloi (Vanbreus.) Ajello (Onygenales) | Trichophyton ajelloi (Vanbreus.) Ajello (Onygenales) | 18 | 129 | 93 | 0 | 240 |
| Total keratinophilic | 20 | 132 | 154 | 124 | 430 | ||
| Non-keratinophilic fungi | |||||||
| 1. | Acremonium rutilum W. Gams (Hypocreales) | Acremonium rutilum W. Gams (Hypocreales) | 1 | 0 | 0 | 1 | 1 |
| 2. | Akanthomyces lecanii (Zimm.) Spatafora, Kepler & B. Shrestha (Hypocreales) | Verticillium lecanii (Zimm.) Viégas (Glomerellales) | 1 | 2 | 0 | 0 | 3 |
| 3. | Cladosporium cladosporioides (Fresen.) G.A. de Vries (Capnodiales) | Cladosporium cladosporioides (Fresen.) G.A. de Vries (Capnodiales) | 0 | 0 | 0 | 1 | 1 |
| 4. | Clonostachys rosea (Link) Schroers, Samuels, Seifert & W. Gams (Hypocreales) | Gliocladium roseum Bainier (Hypocreales) | 2 | 5 | 6 | 2 | 15 |
| 5. | Cunninghamella elegans Lendn. (Mucorales) | Cunninghamella elegans Lendn. (Mucorales) | 1 | 48 | 2 | 2 | 53 |
| 6. | Fusarium oxysporum Schltdl. (Hypocreales) | Fusarium oxysporum Schltdl. (Hypocreales) | 0 | 2 | 20 | 0 | 22 |
| 7. | Fusarium solani (Mart.) Sacc. (Hypocreales) | Fusarium solani (Mart.) Sacc. (Hypocreales) | 0 | 0 | 7 | 11 | 18 |
| 8. | Fusarium sp. (Hypocreales) | Fusarium sp. (Hypocreales) | 0 | 5 | 7 | 6 | 18 |
| 9. | Gliocladium sp. (Hypocreales) | Gliocladium sp. (Hypocreales) | 0 | 3 | 0 | 0 | 3 |
| 10. | Lecanicillium psalliotae (Treschew) Zare & W. Gams (Hypocreales) | Verticillium psalliotae Treschew (Glomerellales) | 11 | 1 | 17 | 2 | 31 |
| 11. | Metacordyceps chlamydosporia (H.C. Evans) G.H. Sung, J.M. Sung, Hywel-Jones & Spatafora (Hypocreales) | Verticillium chlamydosporium Goddard (Glomerellales) | 1 | 49 | 58 | 21 | 129 |
| 12. | Metarhizium marquandii (Massee) Kepler, S.A. Rehner & Humber (Hypocreales) | Paecilomyces marquandii (Massee) S. Hughes (Eurotiales) | 2 | 11 | 0 | 8 | 21 |
| 13. | Oidiodendron griseum Robak (Erysiphales) | Oidiodendron griseum Robak (Erysiphales) | 1 | 0 | 0 | 0 | 1 |
| 14. | Paecilomyces sp. (Eurotiales) | Paecilomyces sp. (Eurotiales) | 3 | 1 | 1 | 1 | 6 |
| 15. | Penicillium glabrum (Wehmer) Westling (Eurotiales) | Penicillium frequentans Westling (Eurotiales) | 1 | 0 | 0 | 0 | 1 |
| 16. | Penicillium simplicissimum (Oudem.) Thom (Eurotiales) | Penicillium janthinellum Biourge (Eurotiales) | 27 | 0 | 0 | 0 | 27 |
| 17. | Penicillium sp. (Eurotiales) | Penicillium sp. (Eurotiales) | 55 | 4 | 8 | 3 | 70 |
| 18. | Purpureocillium lilacinum (Thom) Luangsa-ard, Houbraken, Hywel-Jones & Samson (Hypocreales) | Paecilomyces lilacinus (Thom) Samson (Eurotiales) | 18 | 54 | 27 | 2 | 101 |
| 19. | Rhizopus stolonifer (Ehrenb.) Vuill. (Mucorales) | Rhizopus nigricans Ehrenb. (Mucorales) | 0 | 2 | 0 | 0 | 2 |
| 20. | Sarocladium kiliense (Grütz) Summerb. (Hypocreales) | Acremonium kiliense Grütz (Hypocreales) | 0 | 1 | 0 | 0 | 1 |
| 21. | Sarocladium strictum(W. Gams) Summerb. (Hypocreales) | Acremonium strictum W. Gams (Hypocreales) | 0 | 0 | 0 | 1 | 1 |
| 22. | Talaromyces purpureogenus Samson, N. Yilmaz, Houbraken, Spierenb., Seifert, Peterson, Varga & Frisvad (Eurotiales) | Penicillium purpureogenum Stoll (Eurotiales) | 11 | 1 | 5 | 9 | 26 |
| 23. | Trichoderma sp. (Hypocreales) | Trichoderma sp. (Hypocreales) | 1 | 0 | 3 | 1 | 5 |
| 24. | Trichoderma virens (J.H. Mill., Giddens & A.A. Foster) Arx (Hypocreales) | Gliocladium virens J.H. Mill., Giddens & A.A. Foster (Hypocreales) | 0 | 0 | 10 | 0 | 10 |
| 25. | Verticillium sp. (Glomerellales) | Verticillium sp. (Glomerellales) | 0 | 3 | 1 | 0 | 4 |
| Total non-keratinophilic | 135 | 192 | 172 | 71 | 570 | ||
| TOTAL | 155 | 324 | 326 | 195 | 1000 | ||
| Compared Habitats | Marczewski–Steinhaus Similarity Index (S) |
|---|---|
| Soil I–Soil II | 44.00% |
| Soil I–Soil III | 46.20% |
| Soil I–Soil IV | 43.50% |
| Soil II–Soil III | 53.80% |
| Soil II–Soil IV | 45.80% |
| Soil III–Soil IV | 48.00% |
| Sandy Soil | Loamy Soil | Chernozem | Rendzina |
|---|---|---|---|
| Keratinophilic and non-keratinophilic fungi (total) | |||
| 0.8057 | 0.7667 | 0.8533 | 0.5753 |
| Keratinophilic fungi | |||
| 0.1850 | 0.0447 | 0.5587 | 0.000 |
| Non-keratinophilic fungi | |||
| 0.7617 | 0.7873 | 0.8269 | 0.8467 |
| Parameters | Keratinophilic | Non-keratinophilic | Geophilic dermatophytes | Chrysosporium group | Trichophyton ajelloi | Ctenomyces serratus |
|---|---|---|---|---|---|---|
| C org. | 0.460 | −0.758 ** | −0.453 | 0.976 *** | −0.463 | 0.986 *** |
| Organic matter | 0.461 | −0.759 ** | −0.452 | 0.975 *** | −0.462 | 0.985 *** |
| N tot. | 0.509 | −0.721 ** | −0.408 | 0.974 *** | −0.420 | 0.980 *** |
| C tot. | 0.267 | −0.864 *** | −0.603 * | 0.938 *** | −0.603 * | 0.962 *** |
| pH KCl | 0.698 * | −0.450 | −0.073 | 0.801 ** | −0.072 | 0.818 ** |
| P2O5 | 0.133 | −0.924 *** | −0.738 ** | 0.948 *** | −0.752 ** | 0.955 *** |
| K2O | 0.446 | −0.770 ** | −0.469 | 0.978 *** | −0.478 | 0.988 *** |
| CaCO3 | 0.184 | −0.895 *** | −0.657 * | 0.913 *** | −0.653 * | 0.942 *** |
| P | 0.464 | −0.720 ** | −0.460 | 0.981 *** | −0.490 | 0.965 *** |
| Mg | 0.281 | −0.841 *** | −0.571 | 0.918 *** | −0.567 | 0.946 *** |
| K | 0.695 * | −0.415 | −0.037 | 0.757 ** | −0.031 | 0.778 ** |
| Ca | 0.197 | −0.889 *** | −0.648 * | 0.914 *** | −0.644 * | 0.942 *** |
| ø 2–0.05 | −0.897 *** | −0.064 | −0.250 | −0.651* | −0.208 | −0.604 * |
| ø 0.05–0.002 | 0.891 *** | −0.020 | 0.282 | 0.605 * | 0.238 | 0.554 |
| ø < 0.002 | 0.798 ** | −0.409 | −0.032 | 0.856 *** | −0.049 | 0.849 *** |
| Parameters | Capnodiales | Erysiphales | Eurotiales | Glomerellales | Hypocreales | Mucorales | Onygenales | Thelebolales |
|---|---|---|---|---|---|---|---|---|
| C org. | 0.672 * | −0.570 | −0.530 | −0.343 | −0.174 | −0.302 | 0.460 | −0.062 |
| Organic matter | 0.693 * | −0.573 | −0.533 | −0.335 | −0.171 | −0.302 | 0.463 | −0.061 |
| N tot. | 0.694 * | −0.612 * | −0.570 | −0.307 | −0.112 | −0.292 | 0.510 | −0.008 |
| C tot. | 0.729 ** | −0.407 | −0.373 | −0.417 | −0.375 | −0.318 | 0.270 | −0.257 |
| pH KCl | 0.613 * | −0.821 ** | −0.808 ** | 0.086 | 0.132 | 0.119 | 0.702 * | −0.081 |
| P2O5 | 0.723 ** | −0.232 | −0.177 | −0.638 * | −0.452 | −0.597 * | 0.133 | −0.104 |
| K2O | 0.656 * | −0.557 | −0.517 | −0.349 | −0.184 | −0.312 | 0.449 | −0.068 |
| CaCO3 | 0.718 ** | −0.333 | −0.301 | −0.447 | −0.453 | −0.323 | 0.188 | −0.333 |
| P | 0.609 * | −0.508 | −0.446 | −0.485 | −0.089 | −0.544 | 0.464 | 0.234 |
| Mg | 0.699 * | −0.430 | −0.401 | −0.363 | −0.363 | −0.245 | 0.285 | −0.306 |
| K | 0.543 | −0.824 *** | −0.817 ** | 0.143 | 0.145 | 0.199 | 0.701 * | −0.132 |
| Ca | 0.746 ** | −0.346 | −0.313 | −0.441 | −0.440 | −0.320 | 0.200 | −0.322 |
| ø 2–0.05 | −0.239 | 0.848 *** | 0.805 ** | −0.048 | −0.604* | 0.155 | −0.891 *** | −0.677 * |
| ø 0.05–0.002 | 0.218 | −0.827 *** | −0.780 ** | 0.047 | 0.639* | −0.165 | 0.882 *** | 0.729 ** |
| ø < 0.002 | 0.533 | −0.865 *** | −0.833 *** | −0.009 | 0.266 | −0.044 | 0.796 ** | 0.196 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohacz, J.; Możejko, M.; Korniłłowicz-Kowalska, T.; Siebielec, G. Impact of Ecological Factors on the Occurrence and Spatial-Taxonomic Structure of Keratinophilic Fungi and Their Co-Occurrence in Arable Soils. Agriculture 2022, 12, 194. https://doi.org/10.3390/agriculture12020194
Bohacz J, Możejko M, Korniłłowicz-Kowalska T, Siebielec G. Impact of Ecological Factors on the Occurrence and Spatial-Taxonomic Structure of Keratinophilic Fungi and Their Co-Occurrence in Arable Soils. Agriculture. 2022; 12(2):194. https://doi.org/10.3390/agriculture12020194
Chicago/Turabian StyleBohacz, Justyna, Michał Możejko, Teresa Korniłłowicz-Kowalska, and Grzegorz Siebielec. 2022. "Impact of Ecological Factors on the Occurrence and Spatial-Taxonomic Structure of Keratinophilic Fungi and Their Co-Occurrence in Arable Soils" Agriculture 12, no. 2: 194. https://doi.org/10.3390/agriculture12020194
APA StyleBohacz, J., Możejko, M., Korniłłowicz-Kowalska, T., & Siebielec, G. (2022). Impact of Ecological Factors on the Occurrence and Spatial-Taxonomic Structure of Keratinophilic Fungi and Their Co-Occurrence in Arable Soils. Agriculture, 12(2), 194. https://doi.org/10.3390/agriculture12020194









