Studies on the Possibilities of Processing Buckwheat Husks and Ash in the Production of Environmentally Friendly Fertilizers
Abstract
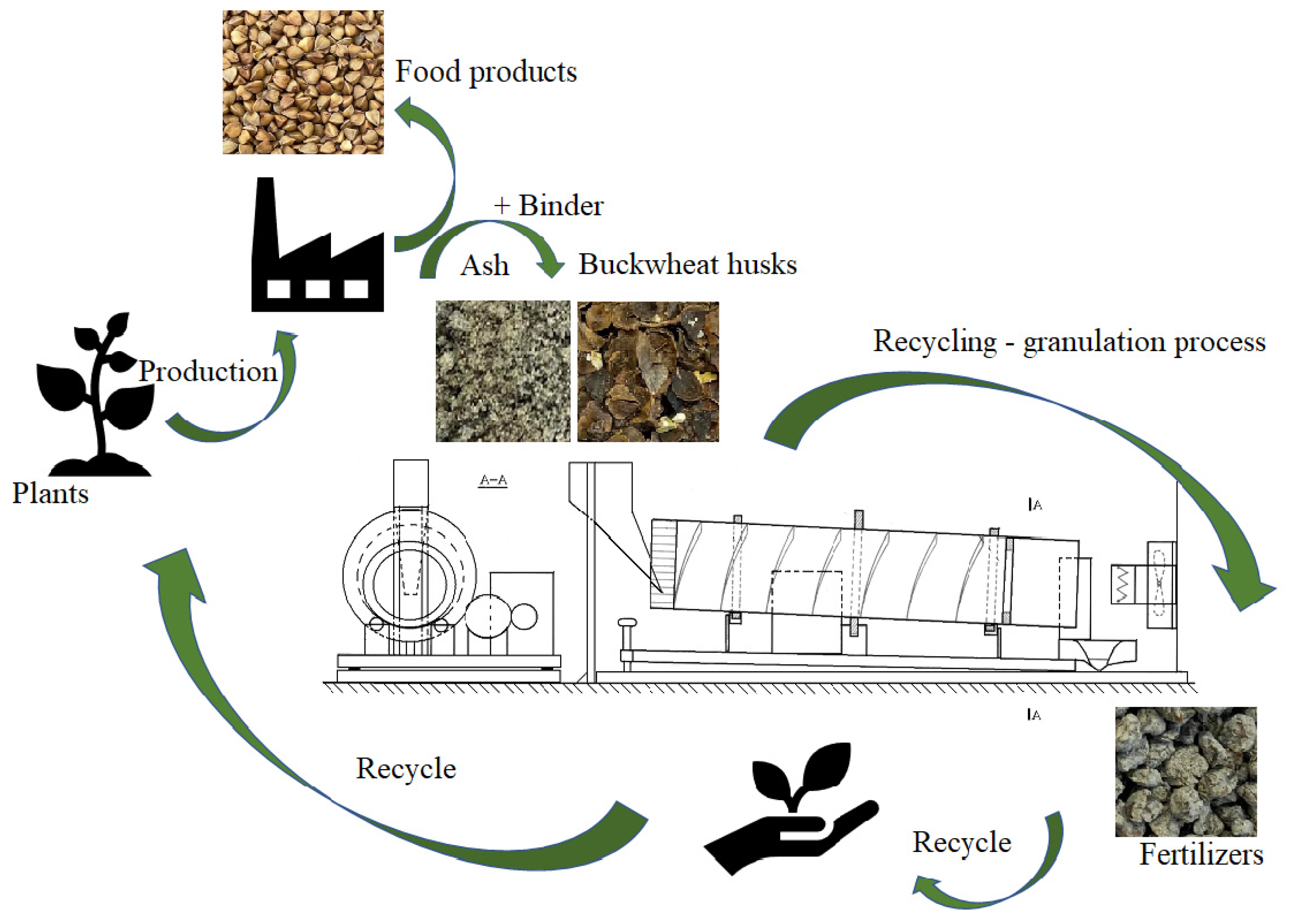
1. Introduction
2. Methods and Materials
2.1. Materials
2.2. Analyte Preparation
2.3. Chemical Analysis
2.4. Instrumental Analysis
2.5. Granulation
2.6. Physical Analysis
2.7. Statistical Data Analysis
3. Results and Discussion
3.1. The Chemical and Structural Composition of the Buckwheat Husks Ash and Uncleaned Buckwheat Husks
3.2. Granulation of the Buckwheat Husk Ash and Uncleaned Buckwheat Husks
3.3. The Properties of the Granular Buckwheat Husks Ash and Uncleaned Buckwheat Husks Fertilizers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wijngaard, H.H.; Arendt, E.K. Buckwheat. Cereal Chem. J. 2006, 83, 391–401. [Google Scholar] [CrossRef]
- Pomeranz, Y.; Lorenz, K. Buckwheat: Structure, composition, and utilization. Crit. Rev. Food Sci. Nutr. 1983, 19, 213–258. [Google Scholar] [CrossRef] [PubMed]
- Kayashita, J.; Shimaoka, I.; Nakajoh, M.; Yamazaki, M.; Kato, N. Consumption of Buckwheat Protein Lowers Plasma Cholesterol and Raises Fecal Neutral Sterols in Cholesterol-Fed Rats Because of Its Low Digestibility. J. Nutr. 1997, 127, 1395–1400. [Google Scholar] [CrossRef] [PubMed]
- Tolaini, V.; Del Fiore, A.; Nobili, C.; Presenti, O.; De Rossi, P.; Procacci, S.; Vitali, F.; Brunori, A. Exploitation of Tartary Buckwheat as Sustainable Ingredient for Healthy Foods Production. Agric. Agric. Sci. Procedia 2016, 8, 455–460. [Google Scholar] [CrossRef][Green Version]
- Khanh, T.D.; Chung, M.I.; Xuan, T.D.; Tawata, S. The Exploitation of Crop Allelopathy in Sustainable Agricultural Production. J. Agron. Crop Sci. 2005, 191, 172–184. [Google Scholar] [CrossRef]
- Corporate Statistical Database (FAOSTAT). Buckwheat Production in 2019, Crops/Regions/World list/Production Quantity (Pick Lists). Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 28 January 2022).
- Kojima, Y.; Obara, Y. Utilization of Buckwheat Husks through a Two-stage Cooking and Carbonization Treatment. J. Jpn. Soc. Waste Manag. Expert 2007, 18, 137–144. [Google Scholar] [CrossRef][Green Version]
- Wronkowska, M.; Haros, M. Wet-milling of buckwheat with hull and dehulled—The properties of the obtained starch fraction. J. Cereal Sci. 2014, 60, 477–483. [Google Scholar] [CrossRef]
- Zielinska, D.; Szawara-Nowak, D.; Zielinski, H. Antioxidative and Anti-Glycation Activity of Buckwheat Hull Tea Infusion. Int. J. Food Prop. 2013, 16, 228–239. [Google Scholar] [CrossRef]
- Whittaker, C.; Borrion, A.L.; Newnes, L.; McManus, M. The renewable energy directive and cereal residues. Appl. Energy 2014, 122, 207–215. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. Utilization of rice husk ash as novel adsorbent: A judicious recycling of the colloidal agricultural waste. Adv. Colloid Interface Sci. 2009, 152, 39–47. [Google Scholar] [CrossRef]
- McDonough, W.; Braungart, M. Cover of Cradle to Cradle: Remaking the Way We Make Things; North Point Press: New York, NY, USA, 2002. [Google Scholar]
- Zhang, H.; Frey, M.; Navizaga, C.; Lenzo, C.; Taborda, J.; Taifan, W.; Sadeghnejad, A.; Sviklas, A.M.; Baltrusaitis, J. Dairy Wastewater for Production of Chelated biodegradable Zn Micronutrient Fertilizers. ACS Sustain. Chem. Eng. 2016, 4, 1722–1727. [Google Scholar] [CrossRef]
- Chemical Book. Available online: https://www.chemicalbook.com/ProductChemicalPropertiesCB3700594_EN.htm (accessed on 17 September 2021).
- Schumacher, B.A. Methods for the determination of total organic carbon (TOC) in soils and sediments. Ecol. Risk Assess Support Cent. 2002, 1–23. [Google Scholar]
- Fulton, J. Physical Properties of Granular Fertilizers and Impact on Spreading. CFAES. 2016. Available online: https://ohioline.osu.edu/factsheet/fabe-5501 (accessed on 4 October 2021).
- Lazdovica, K.; Kampars, V.; Liepina, L.; Vilka, M. Comparative study on thermal pyrolysis of buckwheat and wheatstraws by using TGA-FTIR and Py-GC/MS methods. J. Anal. Appl. Pyrolysis 2017, 124, 1–15. [Google Scholar] [CrossRef]
- Mudunkotuwa, I.A.; Minshid, A.A.; Grassian, V.H. ATR-FTIR spectroscopy as a tool to probe surface adsorption on nanoparticles at the liquid–solid interface in environmentally and biologically relevant media. Analyst 2014, 139, 870–881. [Google Scholar] [CrossRef] [PubMed]
- Brian, C.S. Fourier Transforma Infrared Spectroscopy; CRC Press Taylor & Francis Group: Darmstadt, Germany, 2011; p. 193. [Google Scholar]
- Hummel, D. Atlas of Plastics additives. Analysis by Spectrometric Methods; Springer: Berlin/Heidelberg, Germany, 2002; p. 537. [Google Scholar]
- Baltrusaitis, J.; Schuttlefield, J.; Zeitler, E.; Grassian, V.H. Carbon dioxide adsorption on oxide nanoparticle surfaces. Chem. Eng. J. 2011, 170, 471–481. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Raman Spectra of Inorganic and Coordination Compounds, Part B: Applications in Coordination, Organometallic, and Bioinorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 9780470405840. [Google Scholar]
- Vaickelionis, G.; Valančienė, V. Lightweight Concrete with an Agricultural Waste—Buckwheat Husk. Mater. Sci. 2016, 22, 98–104. [Google Scholar] [CrossRef]
- Peys, A.; Mobili, A.; Arnout, L.; Rahier, H.; Blanpain, B.; Pontikes, Y. One-part inorganic polymers from residues only: Biomass ash activation of Fe-rich slag. In Proceedings of the 5th International Conference on Industrial and Hazardous Waste Management, Chania, Greece, 27–30 September 2016; ISBN 978-960-8475-24-3. [Google Scholar]
- Vaiciukynienė-Palubinskaitė, D.; Nizeviciene, D.; Kantautas, A.; Bocullo, V.; Kiele, A. Alkali Activated Paste and Concrete Based on of Biomass Bottom Ash with Phosphogypsum. Appl. Sci. 2020, 10, 5190. [Google Scholar] [CrossRef]
- Baek, C.; Junhyung, S.; Choi, M.; Cho, J.; Ahn, J.; Cho, K. Utilization of CFBC Fly Ash as a Binder to Produce In-Furnace Desulfurization Sorbent. Sustainability 2018, 10, 4854. [Google Scholar] [CrossRef]
- Lai, L.W.; Ibrahim, M.; Rahim, N.M.; Hashim, E.F.; Ya’cob, M.Z.; Idris, A.; Akhtar, J. Study on composition, structural and property changes of oil palm frond biomass under different pretreatments. Cellulose Chem. Technol. 2016, 50, 951–959. [Google Scholar]
- Demeyer, A.; Voundi Nkana, J.C.; Verloo, M.G. Characteristics of wood ash and influence on soil properties and nutrient uptake: An overview. Bioresour. Technol. 2001, 77, 287–295. [Google Scholar] [CrossRef]
- Paleckiene, R.; Sviklas, A.M.; Slinksiene, R.; Streimikis, V. Processing of rape straw ash into compound fertilizers using sugar factory waste. Polish J. Environ. Stud. 2012, 21, 993–999. [Google Scholar]
- Niu, Y.; Li, H. Controlled Release of Urea Encapsulated by Starch-g-poly(vinyl acetate). Ind. Eng. Chem. Res. 2012, 51, 12173–12177. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Adhikari, R.; Casey, P.; Muster, T.; Gill, H.; Adhikari, B. Enhanced efficiency fertilisers: A review of formulation and nutrient release patterns. J. Sci. Food Agric. 2015, 95, 1131–1142. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.I.; da Cruz, C.C.T.; Solomon, A.; Le, A.; Cavigelli, M.A.; Ribeiro, C. Novel Slow-Release Nanocomposite Nitrogen Fertilizers: The Impact of Polymers on Nanocomposite Properties and Function. Ind. Eng. Chem. Res. 2015, 54, 3717–3725. [Google Scholar] [CrossRef]
- Lubkowski, K.; Smorowska, A.; Grzmil, B.; Kozłowska, A. Controlled-Release Fertilizer Prepared Using a Biodegradable Aliphatic Copolyester of Poly(butylene succinate) and Dimerized Fatty Acid. J. Agric. Food Chem. 2015, 63, 2597–2605. [Google Scholar] [CrossRef]
- Schneider Teixeira, A.; Deladino, L.; Zaritzky, N. Yerba Mate (Ilex paraguariensis) Waste and Alginate as a Matrix for the Encapsulation of N Fertilizer. ACS Sustain. Chem. Eng. 2016, 4, 2449–2458. [Google Scholar] [CrossRef]
- Du, C.; Zhou, J.; Shaviv, A. Release Characteristics of Nutrients from Polymer-coated Compound Controlled Release Fertilizers. J. Polym. Environ. 2006, 14, 223–230. [Google Scholar] [CrossRef]
- Frodeson, S.; Lindén, P.; Henriksson, G.; Berghel, J. Compression of Biomass Substances—A Study on Springback Effects and Color Formation in Pellet Manufacture. Appl. Sci. 2019, 9, 4302. [Google Scholar] [CrossRef]







| Sample | Primary and Secondary Macronutrients, % | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | K2O | P2O5 | CaO | MgO | C | |||||||
| W | HCl | W | HCl | W | HCl | W | HCl | |||||
| BHA | – | 0.66 ± 0.09 | 35.92 ± 1.62 | 38.63 ± 1.82 | 0.30 ± 0.06 | 5.84 ± 0.43 | 0.17 ± 0.05 | 12.18 ± 0.38 | 0.92 ± 0.39 | 3.56 ± 0.18 | – | 29.53 ± 0.50 |
| UBH | – | 2.28 ± 0.27 | 4.56 ± 0.46 | 7.60 ± 0.21 | 0.28 ± 0.06 | 0.41 ± 0.05 | 0.09 ± 0.01 | 0.29 ± 0.07 | 0.47 ± 0.08 | 0.88 ± 0.09 | – | 54.35 ± 0.58 |
| Microelements, mg/kg | ||||||||||||
| Zn | Mn | Cu | Fe | Co | Mo | |||||||
| BHA | – | 541.13 ± 2.86 | – | 547.88 ± 1.64 | – | 385.63 ± 2.56 | – | 1331.25 ± 0.93 | – | 7.60 ± 0.06 | – | 9.30 ± 0.13 |
| UBH | 16.13 ± 0.15 | 86.63 ± 1.01 | 15.13 ± 0.40 | 28.63 ± 0.39 | – | 3.50 ± 0.22 | 0.88 ± 0.04 | 212.38 ± 0.92 | – | – | – | – |
| Heavy Metals, mg/kg | ||||||||||||
| Cd | Pb | Hg | Cr | Ni | Si | |||||||
| BHA | – | 1.80 ± 0.09 | – | 2.88 ± 0.19 | – | 0.01 ± 0.008 | 58.25 ± 0.72 | 76.63 ± 0.74 | – | 15.70 ± 0.89 | – | 8.03 ± 0.35 |
| UBH | – | – | – | – | – | – | – | – | – | – | – | – |
| Sample No. | Raw Materials Humidity, % | Granules Size Distribution, % | pH of 10% Solution | Granule Crushing Strength, N/gran. | |||||
|---|---|---|---|---|---|---|---|---|---|
| >5 mm | 4–5 mm | 3.15–4 mm | 2–3.15 mm | 1–2 mm | <1 mm | ||||
| 100% BHA | |||||||||
| 1 | 41.2 | 8.9 ± 0.14 | 1.5 ± 0.14 | 4.1 ± 0.14 | 2.7 ± 0.25 | 44.7 ± 0.29 | 38.1 ± 0.29 | 12.0 ± 0.38 | 6.30 ± 0.19 |
| 2 | 44.4 | 2.2 ± 0.38 | 3.4 ± 0.87 | 3.9 ± 0.52 | 7.1 ± 0.52 | 35.3 ± 0.89 | 48.1 ± 0.63 | 12.0 ± 0.87 | 6.18 ± 0.42 |
| 3 | 47.4 | 10.1 ± 0.87 | 6.8 ± 0.66 | 9.6 ± 0.49 | 10.4 ± 0.63 | 18.8 ± 0.38 | 44.3 ± 0.49 | 11.5 ± 0.76 | 6.36 ± 0.32 |
| 4 | 50.0 | 16.6 ± 0.76 | 8.1 ± 0.25 | 10.3 ± 0.19 | 12.4 ± 0.52 | 21.9 ± 0.38 | 30.7 ± 0.63 | 11.5 ± 0.25 | 6.47 ± 0.21 |
| 5 | 52.4 | 10.0 ± 0.31 | 11.9 ± 0.14 | 7.1 ± 0.56 | 19.3 ± 0.38 | 39.2 ± 0.38 | 12.5 ± 0.25 | 12.0 ± 0.71 | 6.45 ± 0.11 |
| 6 | 53.5 | 16.3 ±0.49 | 10.8 ± 0.38 | 16.3± 0.52 | 21.5 ± 0.38 | 29.7 ± 0.75 | 5.4 ± 0.14 | 11.5 ± 0.38 | 6.82 ± 0.13 |
| 7 | 54.4 | 37.0 ±0.76 | 18.5 ± 0.38 | 18.4 ± 0.75 | 16.3 ± 0.29 | 8.1 ± 0.52 | 1.7 ± 0.25 | 11.0 ± 0.45 | 6.91 ± 0.17 |
| 8 | 55.6 | 30.6 ± 0.25 | 20.3 ± 0.63 | 19.3 ± 0.14 | 14.0 ± 0.38 | 12.8 ± 0.38 | 3.0 ± 0.38 | 11.5 ± 0.75 | 7.39 ± 0.41 |
| 9 | 56.5 | 64.4 ± 0.49 | 21.6 ± 0.52 | 7.8 ± 0.38 | 4.4 ± 0.49 | 1.1 ± 0.25 | 0.7 ± 0.19 | 11.0 ± 0.63 | 6.61 ± 0.25 |
| 80% BHA and 20% UBH | |||||||||
| 10 | 23.1 | 2.6 ± 0.63 | 2.4 ± 0.14 | 3.4 ± 0.25 | 10.3 ± 0.63 | 27.1 ± 0.38 | 54.2 ± 0.38 | 10.5 ± 0.25 | Plastic deformation at 6–7 N/gran. |
| 11 | 28.6 | 17.5 ± 0.63 | 7.4 ± 0.38 | 8.7 ± 0.38 | 15.4 ± 0.49 | 29.6 ± 0.87 | 21.4 ± 0.87 | 10.5 ± 0.25 | |
| 12 | 31.0 | 36.4 ± 0.63 | 13.4 ± 0.38 | 14.9 ± 0.29 | 23.1 ± 0.25 | 7.8 ± 0.52 | 4.4 ± 0.49 | 10.5 ± 0.43 | |
| 13 | 33.3 | 18.0 ± 0.66 | 13.6 ± 0.49 | 15.1 ± 0.66 | 25.5 ± 0.38 | 16.4 ± 0.25 | 11.4 ± 0.25 | 10.5 ± 0.38 | |
| 14 | 35.5 | 60.6 ± 0.52 | 16.7 ± 0.43 | 11.2 ± 0.25 | 4.7 ± 0.25 | 1.5 ± 0.38 | 5.3 ± 0.14 | 10.0 ± 0.52 | |
| 15 | 37.5 | 57.9 ± 0.38 | 16.9 ± 0.89 | 7.0 ± 0.25 | 2.8 ± 0.25 | 4.0 ± 0.38 | 11.4 ± 0.29 | 10.0 ± 0.49 | |
| 60% BHA and 40% UBH | |||||||||
| 16 | 28.6 | 1.1 ± 0.38 | 2.6 ± 0.38 | 4.8 ± 0.52 | 10.5 ± 0.52 | 25.8 ± 0.52 | 55.2 ± 0.25 | 10.3 ± 0.49 | Plastic deformation at 5–6 N/gran. |
| 17 | 33 | 4.4 ± 0.38 | 7.0 ± 0.49 | 10.3 ± 0.38 | 23.8 ± 0.38 | 32.6 ± 0.25 | 21.9 ± 0.29 | 10.3 ± 0.14 | |
| 18 | 37.5 | 4.9 ± 0.25 | 8.0 ± 0.49 | 14.1 ± 0.75 | 28.7 ± 0.25 | 24.1 ± 0.52 | 20.2 ± 0.52 | 10.3 ± 0.52 | |
| 19 | 39.4 | 8.1 ± 0.52 | 13.7 ± 0.38 | 17.8 ± 0.52 | 28.4 ± 0.38 | 16.5 ± 0.49 | 15.5 ± 0.66 | 10.0 ± 0.63 | |
| 20 | 41.2 | 17.8 ± 0.38 | 16.5 ± 0.14 | 19.7 ± 0.38 | 16.9 ± 0.52 | 8.4 ± 0.38 | 20.7 ± 0.38 | 10.1 ± 0.14 | |
| 21 | 42.9 | 12.9 ± 0.52 | 16.6 ± 0.29 | 15.6 ± 0.29 | 11.9 ± 0.63 | 9.3 ± 0.63 | 33.7 ± 0.38 | 10.0 ± 0.29 | |
| 22 | 44.4 | 15.1 ± 0.38 | 15.9 ± 0.25 | 13.9 ± 0.25 | 10.7 ± 0.38 | 9.2 ± 0.25 | 35.2 ± 0.25 | 10.0 ± 0.14 | |
| 40% BHA and 60% UBH | |||||||||
| 23 | 37.5 | 0.0 ± 0.52 | 0.9 ± 0.49 | 3.1 ± 0.25 | 14.2 ± 0.38 | 40.7 ± 0.76 | 41.1 ± 0.25 | 10.1 ± 0.14 | Plastic deformation at 5–6 N/gran. |
| 24 | 44.4 | 4.2 ± 0.25 | 9.0 ± 0.49 | 16.1 ± 0.38 | 25.9 ± 0.63 | 18.1 ± 0.52 | 26.7 ± 0.49 | 10.1 ± 0.14 | |
| 25 | 47.4 | 12.9 ± 0.49 | 16.1 ± 0.38 | 9.7 ± 0.52 | 29.2 ± 0.49 | 15.4 ± 0.38 | 16.7 ± 0.38 | 10.0 ± 0.25 | |
| 26 | 48.7 | 10.2 ± 0.38 | 17.3 ± 0.38 | 19.3 ± 0.52 | 22.5 ± 0.49 | 11.7 ± 0.25 | 19.0 ± 0.49 | 10.0 ± 0.29 | |
| 27 | 50.0 | 7.4 ± 0.38 | 13.3 ± 0.63 | 15 ± 0.38 | 16.2 ± 0.49 | 12.4 ± 0.38 | 35.3 ± 0.38 | 9.9 ± 0.14 | |
| 20% BHA and 80% UBH | |||||||||
| 28 | 47.4 | 9.3 ± 0.38 | 11.7 ± 0.49 | 15 ± 0.38 | 30.8 ± 0.79 | 19.7 ± 0.38 | 13.2 ± 0.25 | 9.9 ± 0.14 | Plastic deformation at 5 N/gran. |
| 29 | 48.7 | 9.3 ± 0.38 | 12.2 ± 0.29 | 15.9 ± 0.66 | 29.3 ± 0.63 | 19.3 ± 0.25 | 14.0 ± 0.52 | 9.8 ± 0.29 | |
| 30 | 50.0 | 10.5 ± 0.38 | 16.6 ± 0.25 | 15.3 ± 0.38 | 25.8±0.38 | 18.1 ± 0.52 | 13.7 ± 0.25 | 9.8 ± 0.14 | |
| 31 | 51.2 | 26.0 ± 0.63 | 21.4 ± 0.49 | 18 ± 0.57 | 20.0 ± 0.63 | 6.8 ± 0.52 | 7.8 ±0.52 | 9.7 ± 0.29 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pocienė, O.; Šlinkšienė, R. Studies on the Possibilities of Processing Buckwheat Husks and Ash in the Production of Environmentally Friendly Fertilizers. Agriculture 2022, 12, 193. https://doi.org/10.3390/agriculture12020193
Pocienė O, Šlinkšienė R. Studies on the Possibilities of Processing Buckwheat Husks and Ash in the Production of Environmentally Friendly Fertilizers. Agriculture. 2022; 12(2):193. https://doi.org/10.3390/agriculture12020193
Chicago/Turabian StylePocienė, Odeta, and Rasa Šlinkšienė. 2022. "Studies on the Possibilities of Processing Buckwheat Husks and Ash in the Production of Environmentally Friendly Fertilizers" Agriculture 12, no. 2: 193. https://doi.org/10.3390/agriculture12020193
APA StylePocienė, O., & Šlinkšienė, R. (2022). Studies on the Possibilities of Processing Buckwheat Husks and Ash in the Production of Environmentally Friendly Fertilizers. Agriculture, 12(2), 193. https://doi.org/10.3390/agriculture12020193






