Influence of Pulsed, Scanning and Constant (16- and 24-h) Modes of LED Irradiation on the Physiological, Biochemical and Morphometric Parameters of Lettuce Plants (Lactuca sativa L.) while Cultivated in Vertical Farms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of Lettuce Varieties
2.2. Cultivation Conditions
2.3. Irradiation Conditions
2.4. Biometric Measurements of Lettuce Plants
2.5. Spectrophotometric Measurements of the Pigment Composition of Leaves
2.6. Reflection Measurements in Lettuce Leaves
2.7. Measurement of Chlorophyll Fluorescence Parameters
2.8. Raman Spectroscopy of Lettuce Leaves
2.9. Statistical Data Processing
3. Results and Discussion
3.1. Morphological Parameters of Lettuce Plants
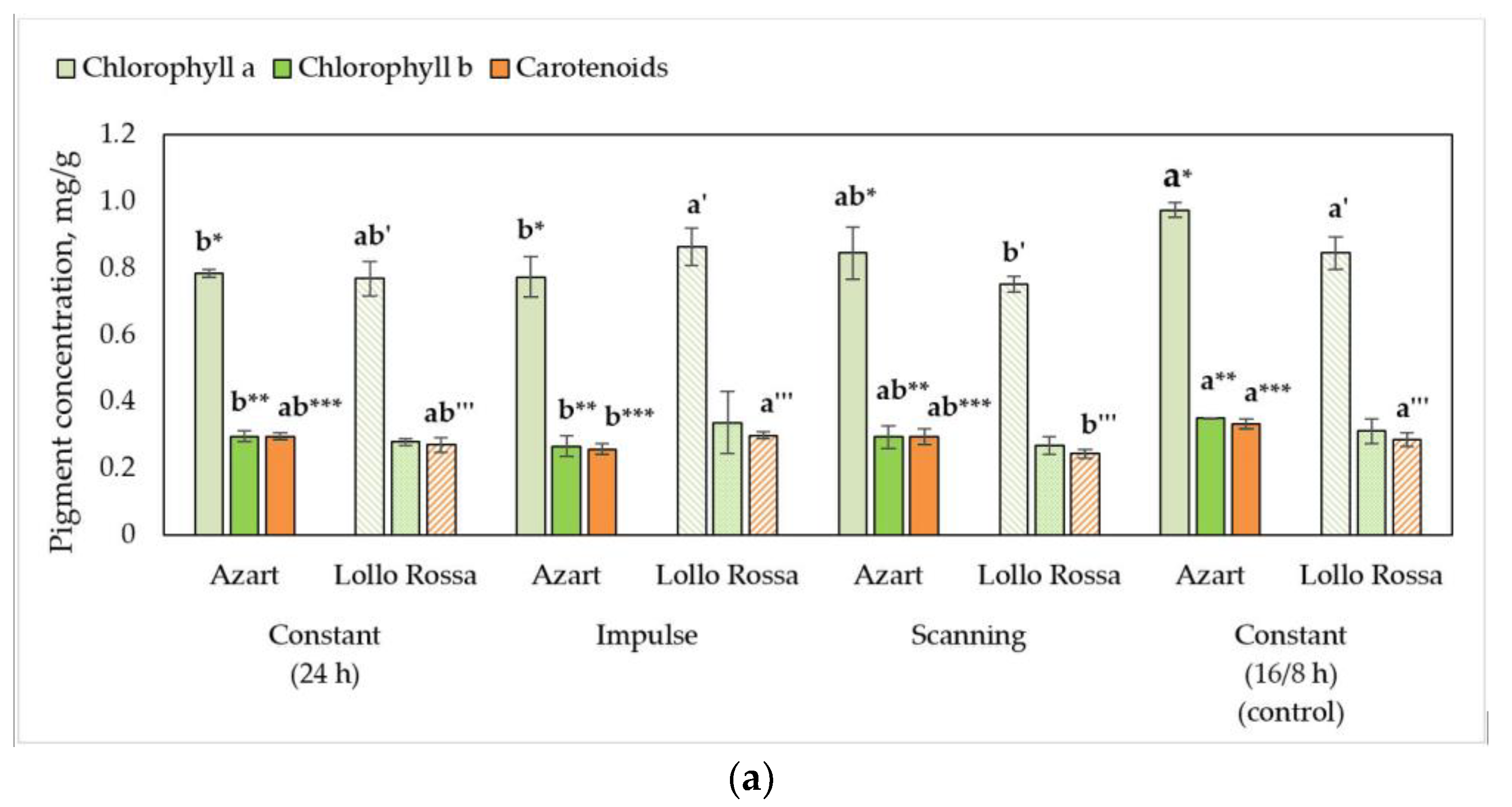
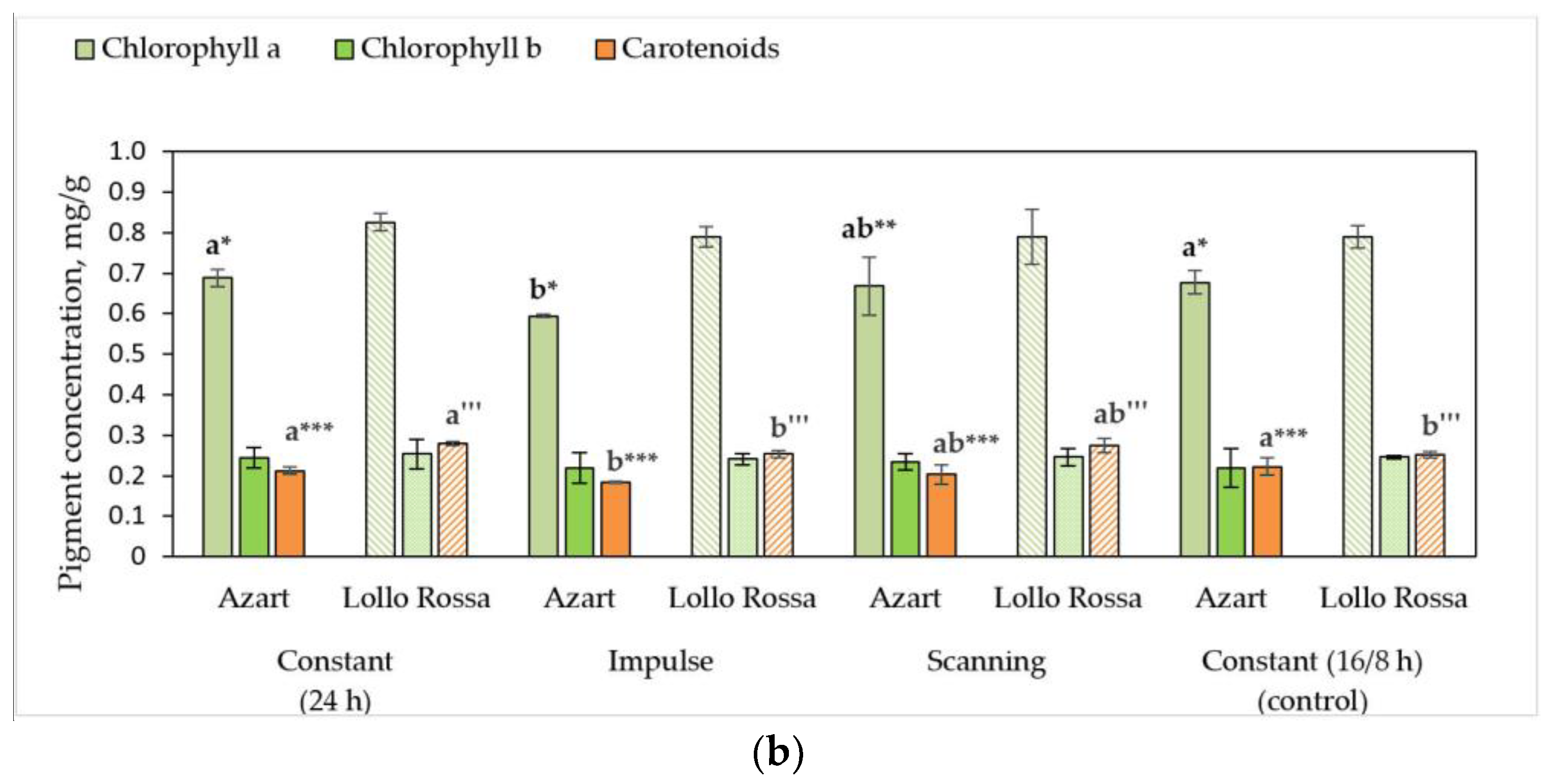
3.2. Biochemical Parameters of Lettuce Plants
3.3. Chlorophyll Fluorescence Parameters
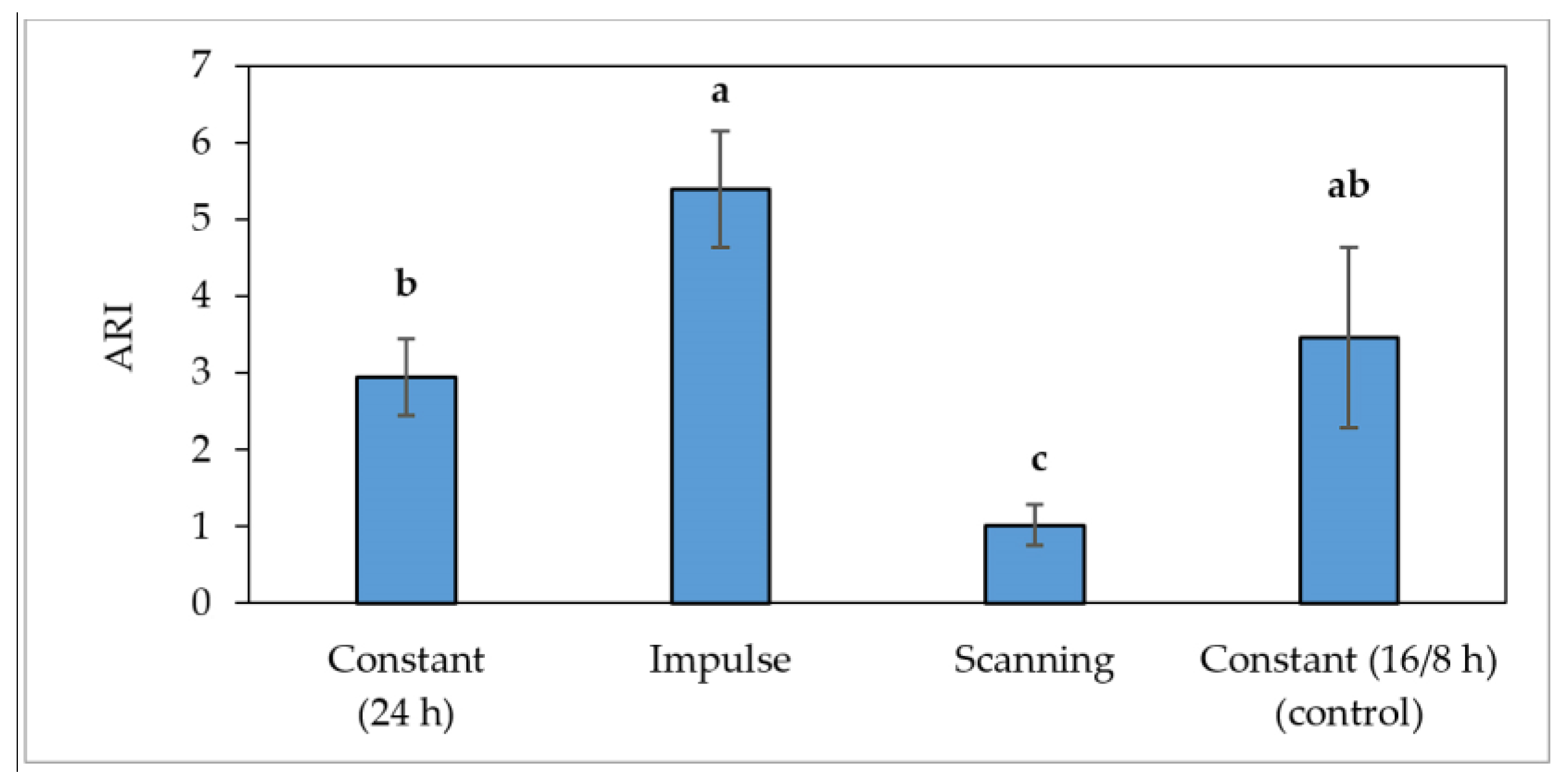
3.4. Study of the Influence of the Light Regime during Cultivation on Leaf Reflectance Indices
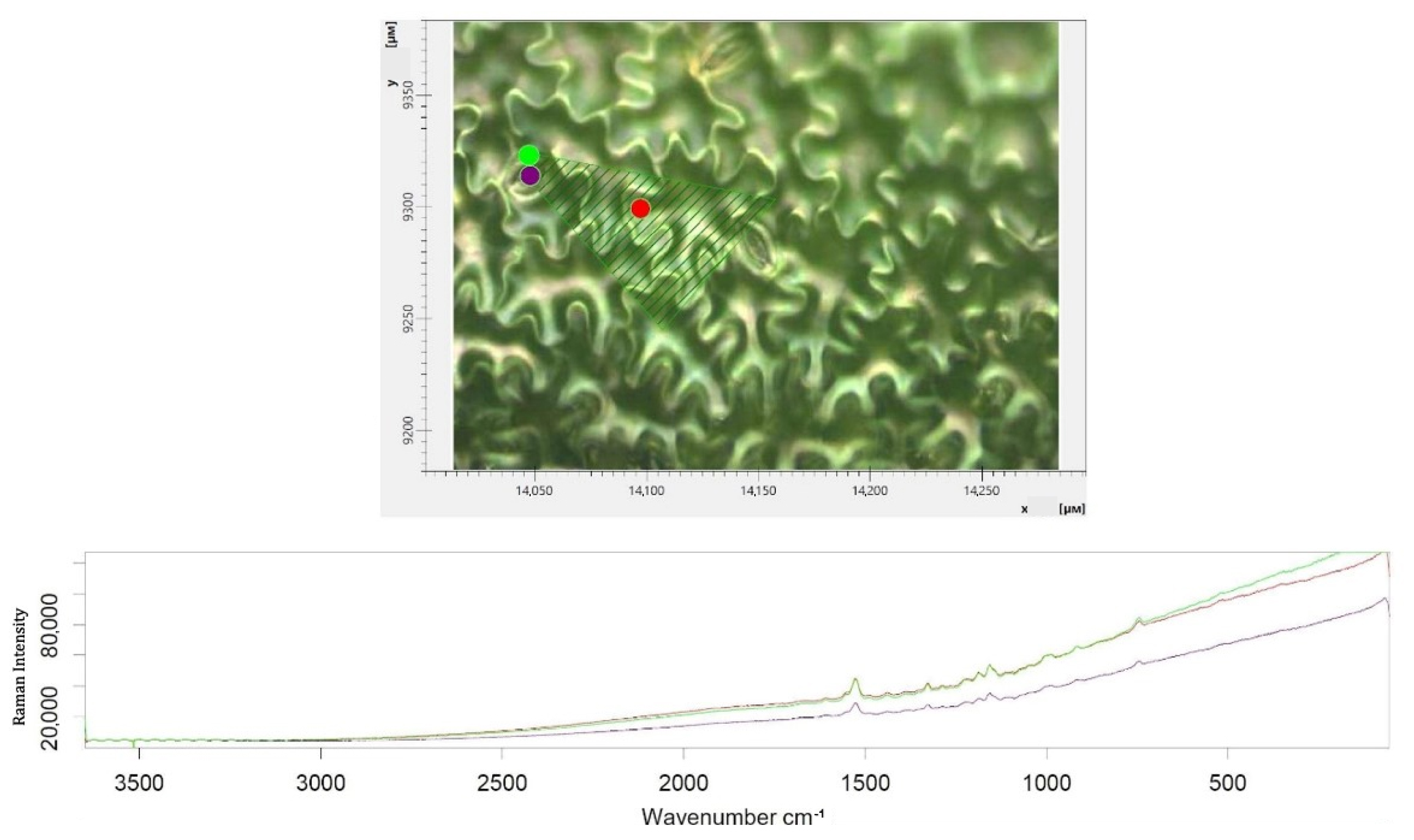
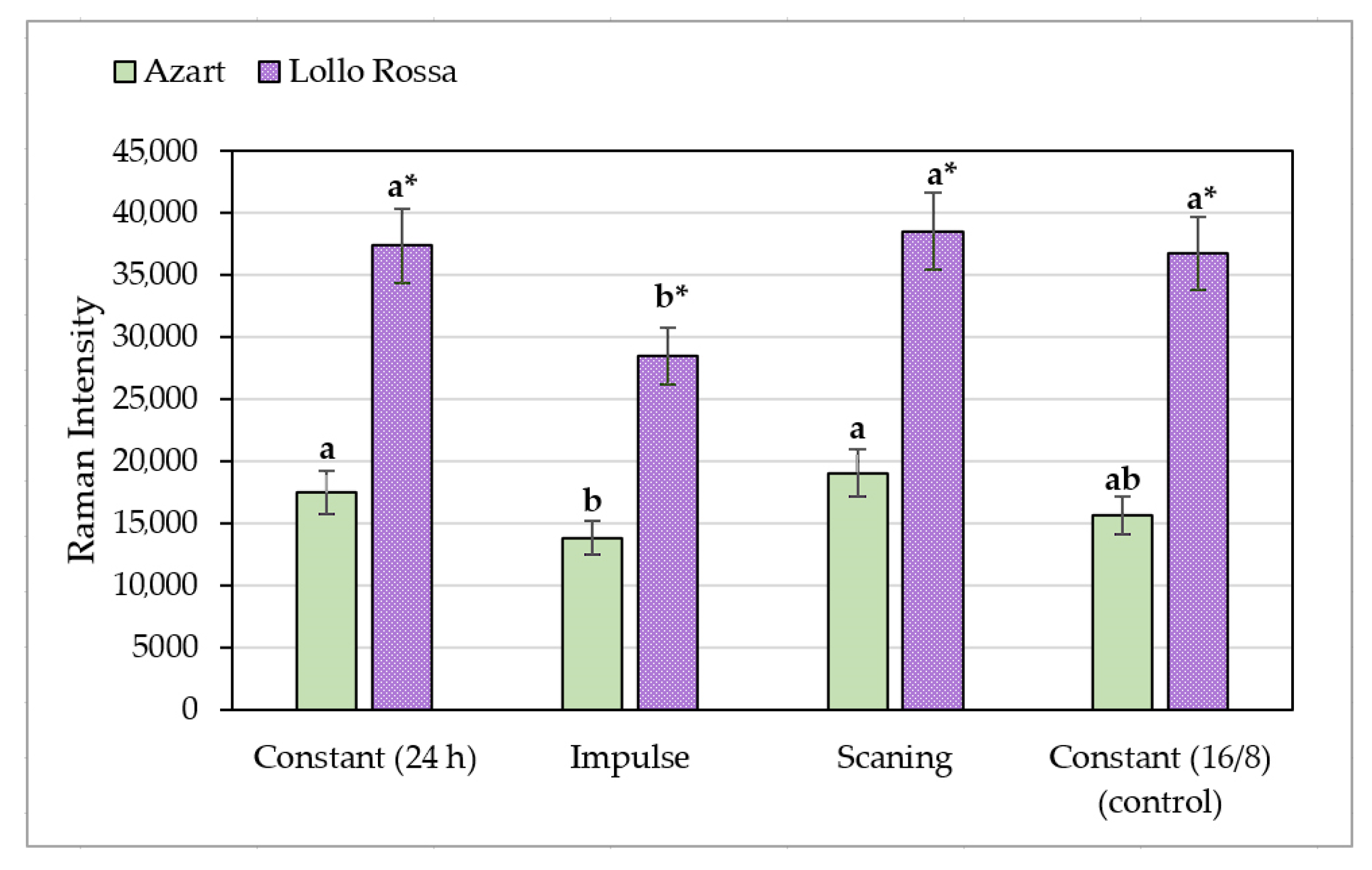
3.5. Raman Spectroscopy of Lettuce Leaves
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olvera-Gonzalez, E.; Escalante-Garcia, N.; Myers, D.; Ampim, P.; Obeng, E.; Alaniz-Lumbreras, D.; Castaño, V. Pulsed LED-Lighting as an Alternative Energy Savings Technique for Vertical Farms and Plant Factories. Energies 2021, 14, 1603. [Google Scholar] [CrossRef]
- Son, K.H.; Jeon, Y.M.; Oh, M.M. Application of supplementary white and impulse light-emitting diodes to lettuce grown in a plant factory with artificial lighting. Hortic. Environ. Biotechnol. 2016, 57, 560–572. [Google Scholar] [CrossRef]
- Skripnichenko, A. Pulsed power supply of Cree XLamp LEDs with increased current. Semicond. Lighting Eng. 2011, 1, 16–19. [Google Scholar]
- Avgoustaki, D.D.; Bartzanas, T.; Xydis, G. Minimising the energy footprint of indoor food production while maintaining a high growth rate: Introducing disruptive cultivation protocols. Food Control 2021, 130, 108290. [Google Scholar] [CrossRef]
- Avgoustaki, D.D.; Li, J.Y.; Xydis, G. Basil plants grown under intermittent light stress in a small-scale indoor environment: Introducing energy demand reduction intelligent technologies. Food Control 2020, 118, 107389. [Google Scholar] [CrossRef]
- Li, Y.T.; Yang, C.; Zhang, Z.S.; Zhao, S.J.; Gao, H.Y. Photosynthetic acclimation strategies in response to intermittent exposure to high light intensity in wheat (Triticum aestivum L.). Environ. Exp. Bot. 2021, 181, 104275. [Google Scholar] [CrossRef]
- Dong, C.; Shao, L.Z.; Liu, G.H.; Wang, M.J.; Liu, H.; Xie, B.Z.; Li, B.W.; Fu, Y.M.; Liu, H. Photosynthetic characteristics, antioxidant capacity and biomass yield of wheat exposed to intermittent light irradiation with millisecond-scale periods. J. Plant Physiol. 2015, 184, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, Y. Photochemical activities of etiochloroplasts isolated from plants greened under intermittent light.2. Photobleaching of pigments. Physiologia Plantarum 1976, 37, 55–61. [Google Scholar] [CrossRef]
- Kotik, M.I.; Andriychuk, V.A.; Kostik, L.N.; Gerts, N.V.; Gerts, A.I. Pulse light stimulation of pepper sprouts cultivation. Light Eng. 2019, 27, 84–91. [Google Scholar]
- Tennessen, D.J.; Bula, R.J.; Sharkey, T.D. The efficiency of photosynthesis in continuous and impulse light emitting diode irradiation. Photosynth. Res. 1995, 44, 261–269. [Google Scholar] [CrossRef]
- Kurata, H.; Mochizuki, A.; Okuda, N.; Seki, M.; Furusaki, S. Intermittent light irradiation with second- or hour-scale periods controls anthocyanin production by strawberry cells. Enzyme Microb. Technol. 2000, 26, 621–629. [Google Scholar] [CrossRef]
- Pinker, I.M. Chopper-light for shoot cultures. Acta Hortic. 2000, 520, 195–202. [Google Scholar] [CrossRef]
- Chow, W.S.; Funk, C.; Hope, A.B. Greening of intermittent-light-grown bean plants in continuous light: Thylakoid components in relation to photosynthetic performance and capacity for photoprotection. Ind. J. Biochem. Biophys. 2000, 37, 395–404. [Google Scholar]
- Grobbelaar, J.U.; Nedbal, L.; Tichý, V. Influence of high frequency light/dark fluctuations on photosynthetic characteristics of microalgae photoacclimated to different light intensities and implications for mass algal cultivation. J. Appl. Phycol. 1996, 8, 335–343. [Google Scholar] [CrossRef]
- Jao, R.C.; Fang, W. Effects of frequency and duty ratio on the growth of potato plantlets in vitro using light-emitting diodes. Hortsci. 2004, 39, 375–379. [Google Scholar] [CrossRef] [Green Version]
- Kanechi, M.; Maekawa, A.; Nishida, Y.; Miyashita, E. Effects of impulse lighting based light-emitting diodes on the growth and photosynthesis of lettuce leaves. Acta Hort. 2016, 1134, 207–214. [Google Scholar] [CrossRef]
- Chazdon, R.L.; Pearcy, R.W. The Importance of Sunflecks for Forest Understory. Plants. BioScience. 1991, 41, 760–766. [Google Scholar] [CrossRef]
- Marissen, N.; Snel, J.; Elings, A.; Warmenhoven, M. Mobile light in roses. Acta Hortic. 2006, 711, 189–194. [Google Scholar] [CrossRef]
- Blom, T.J.; Zheng, Y.B. The effect of moving high intensity lights on potted Campanula haylodgensis. Acta Hortic. 2006, 711, 157–164. [Google Scholar] [CrossRef]
- Semenova, N.A.; Smirnov, A.A.; Grishin, A.A.; Pishchalnikov, R.Y.; Chesalin, D.D.; Gudkov, S.V.; Chilingaryan, N.O.; Skorokhodova, A.N.; Dorokhov, A.S.; Izmailov, A.Y. The effect of plant growth compensation by adding silicon-containing fertilizer under light stress conditions. Plants 2021, 10, 1287. [Google Scholar] [CrossRef]
- Velez-Ramirez, A.I.; van Ieperen, W.; Vreugdenhil, D.; Millenaar, F.F. Plants under continuous light. Trends Plant Sci. 2011, 16, 310–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proietti, S.; Moscatello, S.; Riccio, F.; Downey, P.; Battistelli, A. Continuous Lighting Promotes Plant Growth, Light Conversion Efficiency, and Nutritional Quality of Eruca vesicaria (L.) Cav. in Controlled Environment with Minor Effects Due to Light Quality. Front. Plant Sci. 2021, 12, 730119. [Google Scholar] [CrossRef] [PubMed]
- Son, K.H.; Lee, S.R.; Oh, M.M. Comparison of Lettuce Growth under Continuous and Impulse Irradiation Using Light-Emitting Diodes. Hortic. Sci. Technol. 2018, 36, 542–551. [Google Scholar] [CrossRef]
- Wettstein, D. Chlorophyll-letale und der submikroskopische Formwechsel der Plastiden. Exp. Cell Res. 1957, 12, 427–434. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, H.R.; Deering, D.W.; Schell, J.A.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation (Green Wave Effect) of Natural Vegetation; NASA/GSFC Type III Final Rep.; The Goddard Space Flight Center: Greenbelt, MD, USA, 1974; 371p. [Google Scholar]
- Zarco-Tejada, P.J.; Berjón, A.; López-Lozano, R.; Miller, J.R.; Martín, P.; Cachorro, V.; González, M.R.; de Frutos, A. Assessing vineyard condition with hyperspectral indices: Leaf and canopy reflectance simulation in a row-structured discontinuous canopy. Remote Sens. Environ. 2005, 99, 271–287. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N. Remote estimation of chlorophyll content in higher plant leaves. Int. J. Remote Sens. 1997, 18, 2691–2697. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing carotenoid content in plant leaves with reflectance spectroscopy. Photochem. Photobiol. 2002, 75, 272–281. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Merzlyak, M.N.; Chivkunova, O.B. Optical properties and nondestructive estimation of anthocyanin content in plant leaves. Photochem. Photobiol. 2001, 74, 38–45. [Google Scholar] [CrossRef]
- Yudina, L.; Sukhova, E.; Mudrilov, M.; Nerush, V.; Pecherina, A.; Smirnov, A.A.; Dorokhov, A.S.; Chilingaryan, N.O.; Vodeneev, V.; Sukhov, V. Ratio of Intensities of Blue and Red Light at Cultivation Influences Photosynthetic Light Reactions, Respiration, Growth, and Reflectance Indices in Lettuce. Biology 2022, 11, 60. [Google Scholar] [CrossRef]
- Proshkin, Y.A.; Smirnov, A.A.; Semenova, N.A.; Dorokhov, A.S.; Burynin, D.A.; Ivanitskikh, A.S.; Panchenko, V.A. Assessment of Ultraviolet Impact on Main Pigment Content in Purple Basil (Ocimum basilicum L.) by the Spectrometric Method and Hyperspectral Images Analysis. Appl. Sci. 2021, 11, 8804. [Google Scholar] [CrossRef]
- Chivkunova, O.; Dalke, I.; Dymova, O.; Elsakov, V.; Fiedor, L.; Fujita, M.; Garmash, E.; Hasanuzzaman, M.; Jedynak, P.; Jemiola-Rzeminska, M.; et al. Photosynthetic Pigments: Chemical Structure, Biological Function and Ecology; Komi Scientific Centre of the Ural Branch of the Russian Academy of Sciences: Syktyvkar, Russia, 2014. [Google Scholar]
- Song, J.; Huang, H.; Song, S.; Zhang, Y.; Su, W.; Liu, H. Effects of Photoperiod Interacted with Nutrient Solution Concentration on Nutritional Quality and Antioxidant and Mineral Content in Lettuce. Agronomy 2020, 10, 920. [Google Scholar] [CrossRef]
- Shao, M.J.; Liu, W.K.; Zhou, C.B.; Wang, Q.; Li, B.S. Alternation of temporally overlapped red and blue light under continuous irradiation affected yield, antioxidant capacity and nutritional quality of purple-leaf lettuce. Sci. Hortic. 2022, 295, 110864. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Hogewoning, S.W.; Wientjes, E.; Douwstra, P.; Trouwborst, G.; van Ieperen, W.; Croce, R.; Harbinson, J. Photosynthetic quantum yield dynamics: From photosystems to leaves. Plant Cell. 2012, 24, 1921–1935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kior, A.; Sukhov, V.; Sukhova, E. Application of Reflectance Indices for Remote Sensing of Plants and Revealing Actions of Stressors. Photonics 2021, 8, 582. [Google Scholar] [CrossRef]
- De Matos, Y.M.L.S.; Vasconcelos, D.L.M.; Barreto, A.C.H.; Rocha, J.E.; De Araújo-Neto, J.B.; Campina, F.F.; Da Silva, M.M.C.; Al Yafawi, T.T.; Sobral-Souza, C.E.; Pinheiro, J.C.A.; et al. Protection against the Phytotoxic Effect of Mercury Chloride by Catechin and Quercetin. J. Chem. 2022, 2022, 3770935. [Google Scholar] [CrossRef]
- De Paiva Pinheiro, S.K.; Rangel Miguel, T.B.A.; Chaves, M.D.M.; Barros, F.C.D.F.; Farias, C.P.; De Moura, T.A.; Ferreira, O.P.; Paschoal, A.R.; Souza Filho, A.G.; De Castro Miguel, E. Silver nanoparticles (AgNPs) internalization and passage through the Lactuca sativa (Asteraceae) outer cell wall. Fun. Plant Biol. 2021, 48, 1113–1123. [Google Scholar] [CrossRef]
- Uzu, G.; Sobanska, S.; Sarret, G.; Muñoz, M.; Dumat, C. Foliar Lead uptake by lettuce exposed to atmospheric fallouts. Environ. Sci. Technol. 2010, 44, 1036–1042. [Google Scholar] [CrossRef]
- Baranska, M.; Schulz, H. Application of infrared and Raman spectroscopy for analysis of selected medicinal and spice plants: A review. J. Med. Spice Plants 2006, 11, 72–80. [Google Scholar]



| Irradiation Mode | Fresh Mass, g | Dry Mass, % | Leaf Surface Area, sm2 | Stem Height, sm |
|---|---|---|---|---|
| ‘Azart’ | ||||
| Constant (24 h) | 42.17 (a) ± 11.46 | 6.31 (a) ± 1.05 | 954.74 (a) ± 188.3 | 3.65 (ab) ± 1.28 |
| Impulse | 36.22 (a) ± 9.17 | 6.56 (a) ± 0.65 | 816.66 (a) ± 174.68 | 2.75 (b) ± 0.69 |
| Scanning | 35.06 (a) ± 7.07 | 4.87 (b) ± 0.47 | 869.36 (a) ± 146.38 | 5.69 (a) ± 1.62 |
| Constant (16/8 h) (control) | 36.77 (a) ± 7.61 | 5.12 (ab) ± 0.62 | 893.38 (a) ± 165.66 | 3.19 (b) ± 0.85 |
| ‘Lollo Rossa’ | ||||
| Constant (24 h) | 31.42 (ab) ± 7.22 | 6.48 (a) ± 0.55 | 937.51 (ab) ± 163.05 | 2.8 (a) ± 0.54 |
| Impulse | 40.39 (a) ± 10.2 | 6.53 (a) ± 0.56 | 1073.66 (a) ± 241.45 | 2.47 (a) ± 0.68 |
| Scanning | 25.76 (b) ± 5.33 | 5.9 (b) ± 0.33 | 747.13 (b) ± 145.44 | 1.53 (b) ± 0.29 |
| Constant (16/8 h) (control) | 32.6 (ab) ± 5.84 | 6.04 (ab) ± 0.55 | 945.78 (ab) ± 158.25 | 1.36 (b) ± 0.44 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smirnov, A.A.; Semenova, N.A.; Dorokhov, A.S.; Proshkin, Y.A.; Godyaeva, M.M.; Vodeneev, V.; Sukhov, V.; Panchenko, V.; Chilingaryan, N.O. Influence of Pulsed, Scanning and Constant (16- and 24-h) Modes of LED Irradiation on the Physiological, Biochemical and Morphometric Parameters of Lettuce Plants (Lactuca sativa L.) while Cultivated in Vertical Farms. Agriculture 2022, 12, 1988. https://doi.org/10.3390/agriculture12121988
Smirnov AA, Semenova NA, Dorokhov AS, Proshkin YA, Godyaeva MM, Vodeneev V, Sukhov V, Panchenko V, Chilingaryan NO. Influence of Pulsed, Scanning and Constant (16- and 24-h) Modes of LED Irradiation on the Physiological, Biochemical and Morphometric Parameters of Lettuce Plants (Lactuca sativa L.) while Cultivated in Vertical Farms. Agriculture. 2022; 12(12):1988. https://doi.org/10.3390/agriculture12121988
Chicago/Turabian StyleSmirnov, Alexandr A., Natalya A. Semenova, Alexey S. Dorokhov, Yuri A. Proshkin, Maria M. Godyaeva, Vladimir Vodeneev, Vladimir Sukhov, Vladimir Panchenko, and Narek O. Chilingaryan. 2022. "Influence of Pulsed, Scanning and Constant (16- and 24-h) Modes of LED Irradiation on the Physiological, Biochemical and Morphometric Parameters of Lettuce Plants (Lactuca sativa L.) while Cultivated in Vertical Farms" Agriculture 12, no. 12: 1988. https://doi.org/10.3390/agriculture12121988
APA StyleSmirnov, A. A., Semenova, N. A., Dorokhov, A. S., Proshkin, Y. A., Godyaeva, M. M., Vodeneev, V., Sukhov, V., Panchenko, V., & Chilingaryan, N. O. (2022). Influence of Pulsed, Scanning and Constant (16- and 24-h) Modes of LED Irradiation on the Physiological, Biochemical and Morphometric Parameters of Lettuce Plants (Lactuca sativa L.) while Cultivated in Vertical Farms. Agriculture, 12(12), 1988. https://doi.org/10.3390/agriculture12121988







