Screening Winter Wheat Genotypes for Resistance Traits against Rhizoctonia cerealis and Rhizoctonia solani Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Susceptibility Tests of Wheat Cultivars Conducted under Field Conditions
2.2. Susceptibility Study of Wheat Cultivars Conducted in the Paper Test
2.3. Statistical Analysis
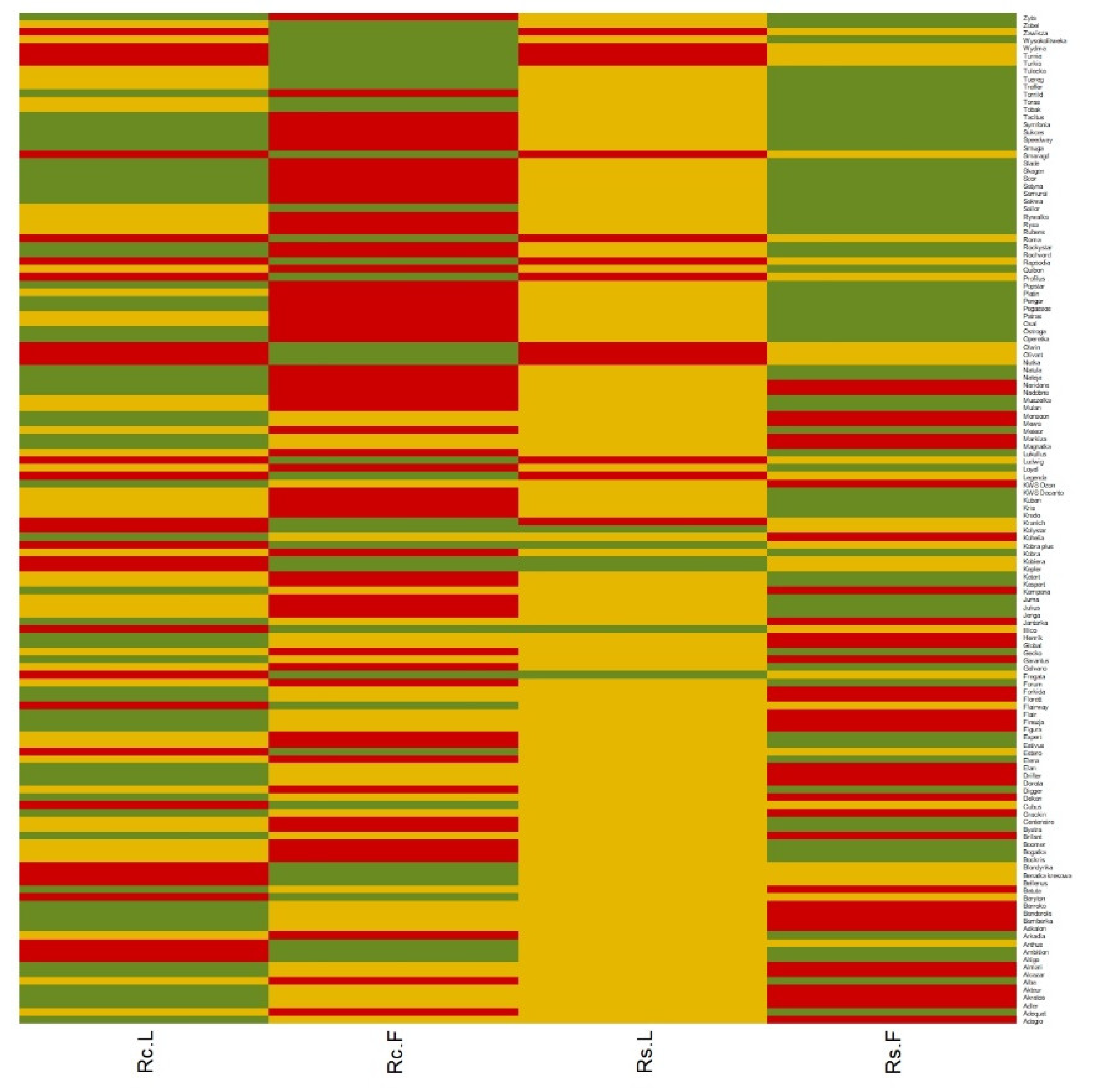
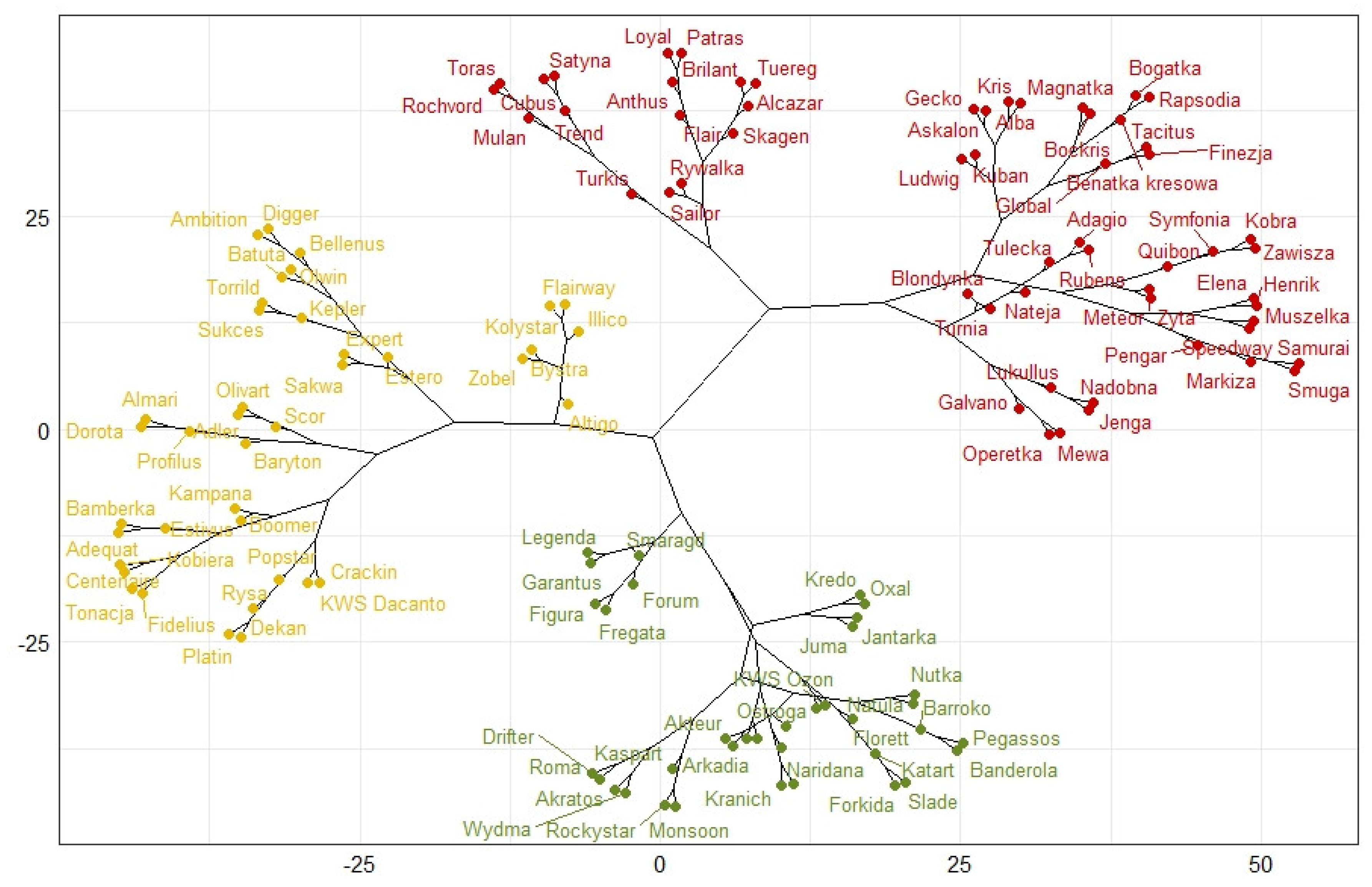
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, Y.X.; Liu, Q.H.; Ng, T.B.; Wang, H.X. Isarfelin, a peptide with antifungal and insecticidal activities from Isaria felina. Peptides 2005, 26, 2384–2391. [Google Scholar] [CrossRef] [PubMed]
- Hamada, M.S.; Yanni, Y.; Ma, Z. Sensitivity to iprodione, difenoconazole and fludioxonil of Rhizoctonia cerealis isolates collected from wheat in China. Crop Prot. 2011, 30, 1028–1033. [Google Scholar] [CrossRef]
- Lemańczyk, G.; Kwaśna, H. Effects of sharp eyespot (Rhizoctonia cerealis) on yield and grain quality of winter wheat. Eur. J. Plant Pathol. 2013, 135, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Al-Abdalall, A.H.A. Assessment of yield loss caused by root rots in wheat and barley. J. Food. Agric. Environ. 2010, 8, 638–641. [Google Scholar]
- Shamim, M.D.; Deepak, K.; Deepti, S.; Pramila, P.; Singh, K.N. Evaluation of major cereal crops for resistance against Rhizoctonia solani under green house and field conditions. Indian Phytopath. 2014, 67, 42–48. [Google Scholar]
- Liu, J.; Mundt, C.C. Genetic structure and population diversity in the wheat sharp eyespot pathogen Rhizoctonia cerealis in the Willamette Valley, Oregon, USA. Plant Pathol. 2020, 69, 101–111. [Google Scholar] [CrossRef]
- Rachoń, L.; Szumiło, G.; Stankowski, S. Porównanie wybranych wskaźników wartości technologicznej pszenicy zwyczajnej (Triticum aestivum ssp. vulgare), twardej (Triticum durum) i orkiszowej (Triticum aestivum spp. spelta). Fragm. Agron. 2011, 28, 52–59. [Google Scholar]
- Okabura, P.A.; Steber, C.M.; DeMacon, V.L.; Walter, N.L.; Paulitz, T.C.; Kidwell, K.K. Scarlet-Rz1, an EMS-generated hexaploid wheat with tolerance to the soilborne necrotrophic pathogens Rhizoctonia solani AG-8 and R. oryzae. Theor. Appl. Genet. 2009, 119, 293–303. [Google Scholar] [CrossRef]
- Doornik, A.W. Effect of storage duration and temperature on the survival of Rhizoctonia solani in tulip and iris bulbs. Neth. J. Plant. Pathol. 1982, 88, 185–190. [Google Scholar] [CrossRef]
- Arakawa, M.; Inagaki, K. Molecular markers for genotyping anastomosis groups and understanding the population biology of Rhizoctonia species. J. Gen. Plant Pathol. 2014, 80, 401–407. [Google Scholar] [CrossRef]
- Maculewicz, D. Binucleate Rhizoctonia spp. as a biocontrol agents against plant pathogens. Ecol. Chem. Eng. A. 2015, 22, 195–203. [Google Scholar]
- Jiang, J.H.; Tam, S.L.; Toda, T.; Chen, L.C. Controlling Rhizoctonia damping-off of chinese mustard by using endomycorrhizal Rhizoctonia spp. isolated from orchid mycorrhizae. Plant Dis. 2016, 100, 85–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choupannejad, R.; Sharifnabi, B.; Fadaei Tehrani, A.A.; Gholami, J. Rhizoctonia solani AG4 associated with foliar blight symptoms on barley in Iran. Australas. Plant Path. 2017, 12, 2. [Google Scholar] [CrossRef] [Green Version]
- Inokuti, E.M.; Thiery-Lanfranchi, D.; Edel-Hermann, V.; Gautheron, N.; Fayolle, L.; Michereff, S.J.; Steinberg, C. Genetic and pathogenic variability of Rhizoctonia solani causing crown and root rot on sugar beet in France. J. Plant Pathol. 2019, 101, 907–916. [Google Scholar] [CrossRef]
- Kazempour, M.N. Biological control of Rhizoctonia solani, the causal agent of rice sheath blight by antagonistics bacteria in greenhouse and field conditions. J. Plant Pathol. 2004, 3, 88–96. [Google Scholar]
- Herr, L.J. Biological control of Rhizoctonia solani by binucleate Rhizoctonia spp. and hypovirulent R. solani agents. Crop Prot. 1995, 14, 179–186. [Google Scholar] [CrossRef]
- Gutiérrez, S.A.; Cúndom, M.A.; Barrera, V.; Gasoni, L. First record of Rhizoctonia zeae on corn in Argentina. Australas. Plant Dis. Notes. 2007, 2, 137–138. [Google Scholar] [CrossRef] [Green Version]
- Rosa, D.D.; Ohto, C.T.; Basseto, M.A.; Furtado, E.L.; de Souza, N.L. First report of Rhizoctonia solani AG 4 HG-II attacking Gazania rigens plants in Brazil. Australas. Plant Dis. Notes 2008, 3, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Lakshmidevi, N.; Sudisha, J.; Mahadevamurthy, S.; Prakash, H.S.; Shekar Shetty, H. First report of the seed-borne nature of root and collar rot disease caused by Rhizoctonia solani in sunflower from India. Australas. Plant Dis. Notes. 2010, 5, 11–13. [Google Scholar] [CrossRef] [Green Version]
- Lemańczyk, G. Susceptibility of winter triticale cultivars to Rhizoctonia cerealis (sharp eyespot) and R. solani. J. Plant. Prot. Res. 2012, 52, 421–433. [Google Scholar] [CrossRef] [Green Version]
- Erper, I.; Kilicoglu MTurkkan, C.M.; Onder, H. Characterization and pathogenicity of Rhizoctonia spp. isolated from winter squash in the Black Sea region of Turkey. Eur. J. Plant Pathol. 2016, 146, 683–697. [Google Scholar] [CrossRef]
- Misawa, T.; Kayamori, M.; Kurose, D.; Sasaki, J.; Toda, T. First report of Rhizoctonia disease of lily caused by Rhizoctonia solani AG-11 in Japan. J. Gen. Plant Pathol. 2017, 83, 406–409. [Google Scholar] [CrossRef]
- Moliszewska, E.; Nabrdalik, M.; Ziembik, Z. Rhizoctonia solani AG 11 isolated for the first time from sugar beet in Poland. Saudi J. Biolo. Sci. 2020, 27, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Mahoney, A.K.; Babiker, E.M.; See, D.R.; Paulitz, T.C.; Okubara, P.A.; Hulbert, S.H. Analysis and mapping of Rhizoctonia root rot resistance traits from the synthetic wheat (Triticum aestivum L.) line SYN-172. Mol. Breeding 2017, 37, 130. [Google Scholar] [CrossRef]
- Kannaiyan, S. Effect of certain fungicides on the production of enzymes by Rhizoctonia solani. Plant. Soil. 1988, 108, 299–302. [Google Scholar] [CrossRef]
- Kurowski, T.P.; Marks, M.; Makowski, P.; Jaźwińska, E. Zdrowotność pszenicy ozimej w stanowiskach po różnych sposobach dwuletniego ugorowania. Fragm. Agron. 2009, 26, 102–108. [Google Scholar]
- Townsend, G.R.; Heuberger, J.W. Methods for estimating losses caused by diseases in fungicide experiments. Plant Dis. Rep. 1943, 24, 340–343. [Google Scholar]
- Kassambara, A. Practical Guide to Cluster Analysis in R. Unsupervised Machine Learning, 1st ed.; STHDA; CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA, 2017. [Google Scholar]
- Kassambara, A. Practical Guide to Principal Component Methods in R, 1st ed.; STHDA; CreateSpace Independent Publishing Platform: Scotts Valley, CA, USA, 2017. [Google Scholar]
- Smith, J.D.; Kidwell, K.K.; Evans, M.A.; Cook, R.J.; Smiley, R.W. Assessment of spring wheat genotypes for disease reaction to Rhizoctonia solani AG-8 in controlled environment and direct-seeded field evaluations. Crop Sci. 2003, 43, 694–700. [Google Scholar] [CrossRef]
- Smith, J.D.; Kidwell, K.K.; Evans, M.A.; Cook, R.J.; Smiley, R.W. Evaluation of spring cereal grains and wild Triticum germplasm for resistance to Rhizoctonia solani AG-8. Crop Sci. 2003, 43, 701–709. [Google Scholar] [CrossRef]
- Cromey, M.G.; Butler, R.C.; Munro, C.A.; Shorter, S.C. Susceptibility of New Zealand wheat cultivars to sharp eyespot. N. Z. Plant Protect. 2005, 58, 268–272. [Google Scholar] [CrossRef]
- Cromey, M.G.; Hide, C.C.L.; Meenken, E.D. Resistance to sharp eyespot in wheat. N. Z. Plant Protect. 2012, 65, 204–212. [Google Scholar] [CrossRef]
- Płonka, K.J.; Roj, J. Wrażliwość pięciu odmian pszenicy ozimej na Rhizoctonia cerealis i R. solani. Prog. Plant Prot. 2012, 52, 657–662. [Google Scholar]
- Bai, G.; Shaner, G. Management and resistance in wheat and barley to Fusarium head blight. Annu. Rev. Phytopathol. 2004, 42, 135–161. [Google Scholar] [CrossRef] [PubMed]




| Laboratory Test | Field Test | |||||
|---|---|---|---|---|---|---|
| Pathogen | Roots | Stem Base | Leaves | Disease Index | Percentage | Damping Off |
| R. cerealis | 32.1 ± 0.8 | 76.9 ± 0.7 | 52.3 ± 1.3 | 28.0 ±1.0 | 49.7 ± 1.5 | 30.8 ± 1.7 |
| R. solani | 15.5 ± 1.2 | 96.1 ± 0.5 | 18.1 ± 2.1 | 7.9 ± 0.6 | 18.4 ± 1.2 | 12.8 ± 0.7 |
| Type of Experiment-Cluster | Potentially Resistant (Green Cluster) | Intermediate (Yellow Cluster) | Susceptible (Red Cluster) |
|---|---|---|---|
| R. cerealis Laboratory | 57 | 45 | 30 |
| R. cerealis Field | 38 | 33 | 61 |
| R. solani Laboratory | 6 | 112 | 14 |
| R. solani Field | 69 | 28 | 35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisiecki, K.; Lemańczyk, G.; Piesik, D.; Mayhew, C.A. Screening Winter Wheat Genotypes for Resistance Traits against Rhizoctonia cerealis and Rhizoctonia solani Infection. Agriculture 2022, 12, 1981. https://doi.org/10.3390/agriculture12121981
Lisiecki K, Lemańczyk G, Piesik D, Mayhew CA. Screening Winter Wheat Genotypes for Resistance Traits against Rhizoctonia cerealis and Rhizoctonia solani Infection. Agriculture. 2022; 12(12):1981. https://doi.org/10.3390/agriculture12121981
Chicago/Turabian StyleLisiecki, Karol, Grzegorz Lemańczyk, Dariusz Piesik, and Chris A. Mayhew. 2022. "Screening Winter Wheat Genotypes for Resistance Traits against Rhizoctonia cerealis and Rhizoctonia solani Infection" Agriculture 12, no. 12: 1981. https://doi.org/10.3390/agriculture12121981
APA StyleLisiecki, K., Lemańczyk, G., Piesik, D., & Mayhew, C. A. (2022). Screening Winter Wheat Genotypes for Resistance Traits against Rhizoctonia cerealis and Rhizoctonia solani Infection. Agriculture, 12(12), 1981. https://doi.org/10.3390/agriculture12121981







