Combined Reproductive Effects of Imidacloprid, Acetochlor and Tebuconazole on Zebrafish (Danio rerio)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Test Chemicals and Reagents
2.2. Zebrafish Maintenance
2.3. Zebrafish Combined Chronic Exposure
2.4. Adult Fish Producing F1 Offspring Embryos
2.5. Histology Microphotographs of Testes and Ovaries
2.6. Reproductive Hormones Assay
2.7. Reproductive Genes Expression Assay
2.8. Statistical Analysis
3. Results
3.1. Reproductive Effects
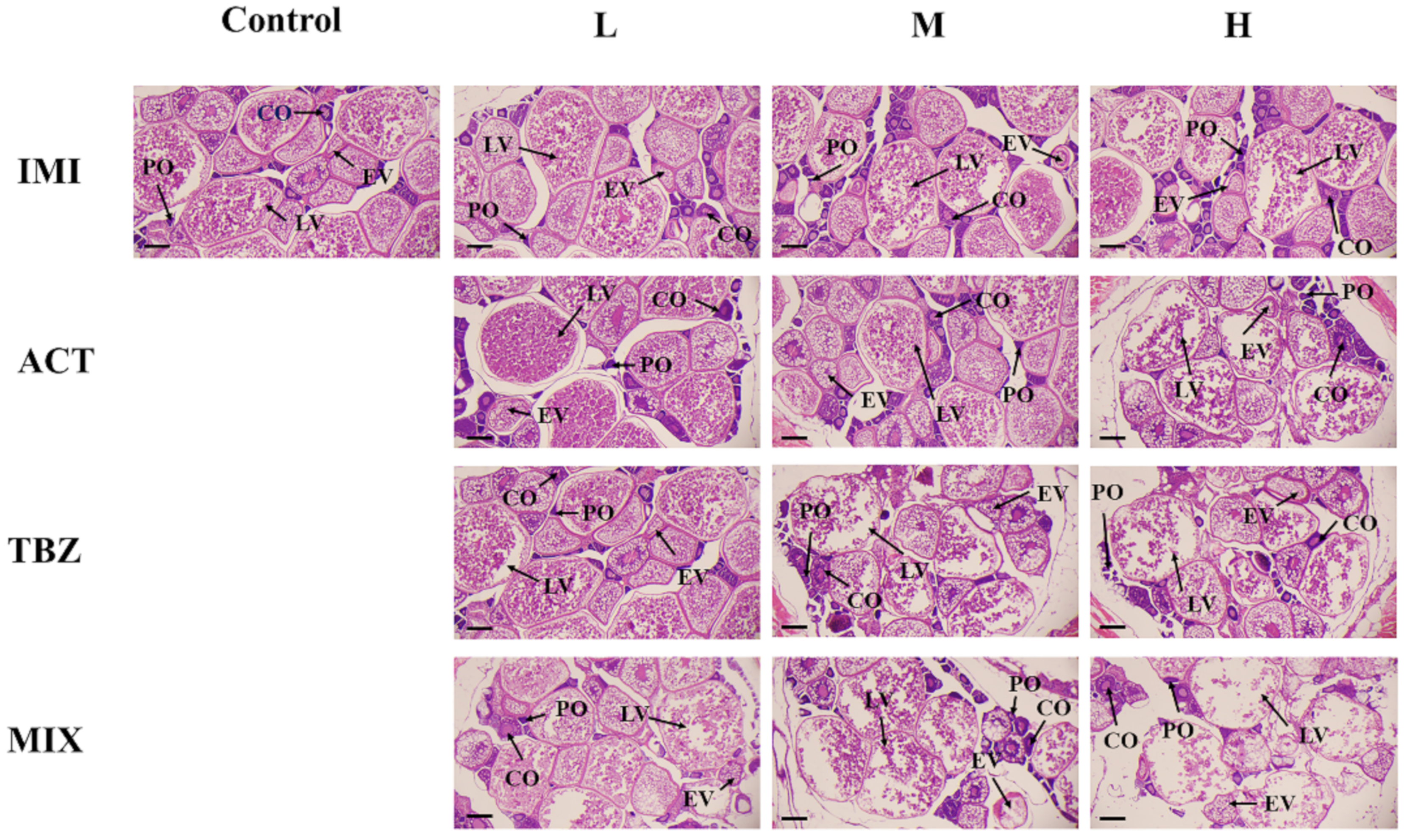
3.2. Histological Alterations in the Gonads
3.3. Reproductive Hormones Effects
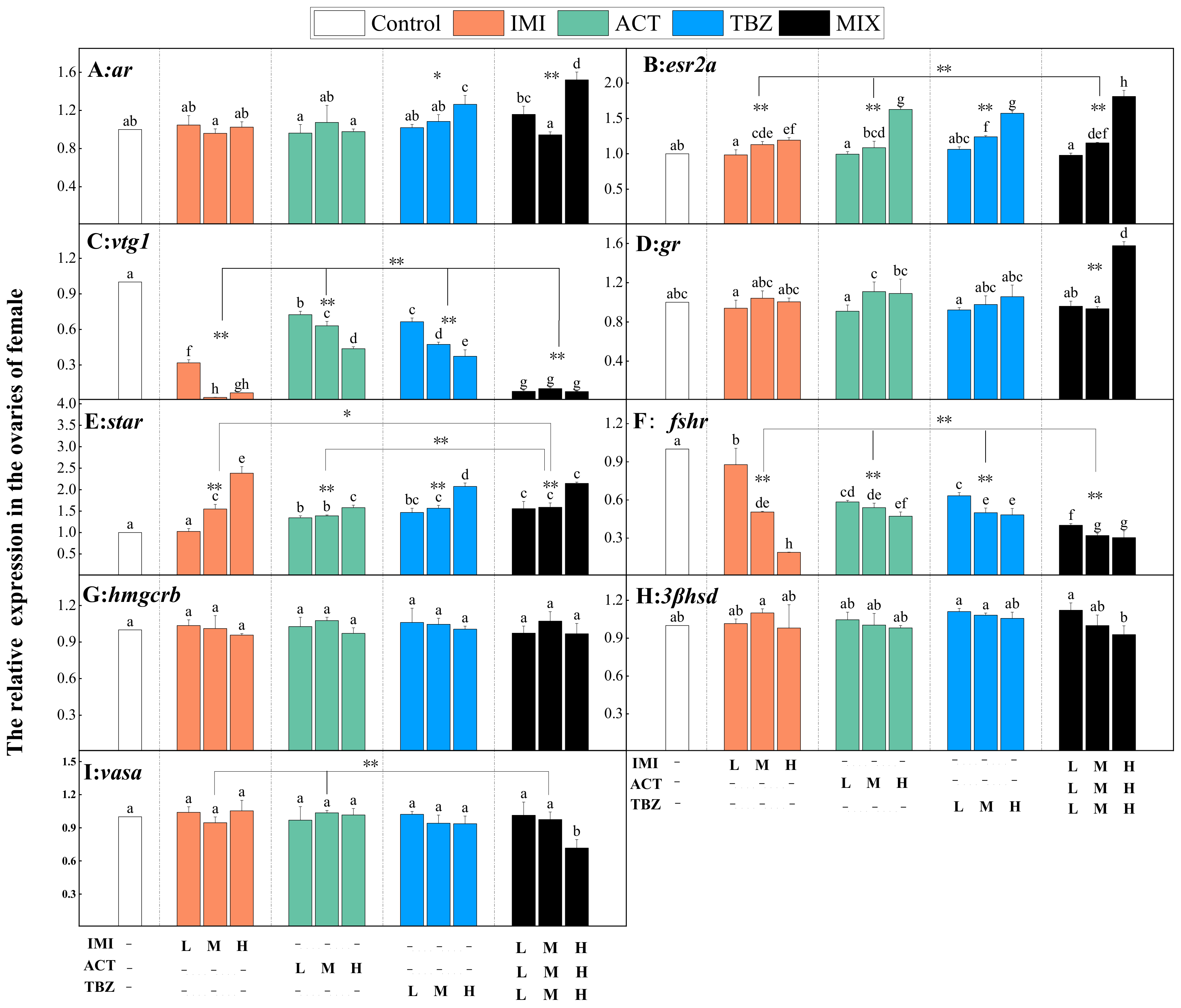
3.4. Reproductive Genes Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Ethics Statements
References
- Hladik, M.L.; Kolpin, D.W.; Kuivila, K.M. Widespread occurrence of neonicotinoid insecticides in streams in a high corn and soybean producing region, USA. Environ. Pollut. 2014, 193, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Li, Q.; Zhang, H.; Wu, C.; Zhao, S.; Deng, X.; Li, Y. Pesticide residues in agricultural topsoil from the Hainan tropical riverside basin: Determination, distribution, and relationships with planting patterns and surface water. Sci. Total Environ. 2020, 722, 137856. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Liu, L.; Yan, S.; Sun, W.; Jia, M.; Tian, S.; Huang, S.; Zhou, Z.; Zhu, W. Gut microbiota: A key factor in the host health effects induced by pesticide exposure? J. Agric. Food Chem. 2020, 68, 10517–10531. [Google Scholar] [CrossRef] [PubMed]
- Grung, M.; Lin, Y.; Zhang, H.; Steen, A.O.; Huang, J.; Zhang, G.; Larssen, T. Pesticide levels and environmental risk in aquatic environments in China—A review. Environ. Int. 2015, 81, 87–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uwizeyimana, H.; Wang, M.; Chen, W.; Khan, K. The eco-toxic effects of pesticide and heavy metal mixtures towards earthworms in soil. Environ. Toxicol. Pharmacol. 2017, 55, 20–29. [Google Scholar] [CrossRef]
- Shahjahan, M.; Rahman, M.S.; Islam, S.M.; Uddin, M.; Al-Emran, M. Increase in water temperature increases acute toxicity of sumithion causing nuclear and cellular abnormalities in peripheral erythrocytes of zebrafish Danio rerio. Environ. Sci. Pollut. Res. 2019, 26, 36903–36912. [Google Scholar] [CrossRef]
- Luo, T.; Weng, Y.; Huang, Z.; Zhao, Y.; Jin, Y. Combined hepatotoxicity of imidacloprid and microplastics in adult zebrafish: Endpoints at gene transcription. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 246, 109043. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, C.; Wang, D.; Weng, H.; Yang, G.; Guo, D.; Yu, R.; Wang, X.; Wang, Q. Combined toxic effects of fludioxonil and triadimefon on embryonic development of zebrafish (Danio rerio). Environ. Pollut. 2020, 260, 114105. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, D.; Yu, Y.; Yang, G.; Shen, W.; Wang, Q.; Weng, H.; Zhao, X. Evaluation of joint effects of cyprodinil and kresoxim-methyl on zebrafish, Danio rerio. J. Hazard. Mater. 2018, 352, 80–91. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, H.; Zhou, J.; Liu, J.; Liu, W. Joint toxicity of permethrin and cypermethrin at sublethal concentrations to the embryo-larval zebrafish. Chemosphere 2013, 96, 146–154. [Google Scholar] [CrossRef]
- Kongtip, P.; Nankongnab, N.; Kallayanatham, N.; Pundee, R.; Yimsabai, J.; Woskie, S. Longitudinal study of metabolic biomarkers among conventional and organic farmers in Thailand. Int. J. Environ. Res. Public Heal. 2020, 17, 4178. [Google Scholar] [CrossRef]
- Graham, K.K.; Milbrath, M.O.; Zhang, Y.; Soehnlen, A.; Baert, N.; McArt, S.; Isaacs, R. Identities, concentrations, and sources of pesticide exposure in pollen collected by managed bees during blueberry pollination. Sci. Rep. 2021, 11, 16857. [Google Scholar] [CrossRef]
- Bradford, B.Z.; Huseth, A.; Groves, R.L. Widespread detections of neonicotinoid contaminants in central Wisconsin groundwater. PLoS ONE 2018, 13, e0201753. [Google Scholar] [CrossRef]
- Sánchez-Bayo, F.; Hyne, R.V. Detection and analysis of neonicotinoids in river waters—Development of a passive sampler for three commonly used insecticides. Chemosphere 2014, 99, 143–151. [Google Scholar] [CrossRef]
- Anderson, J.; Dubetz, C.; Palace, V. Neonicotinoids in the Canadian aquatic environment: A literature review on current use products with a focus on fate, exposure, and biological effects. Sci. Total Environ. 2015, 505, 409–422. [Google Scholar] [CrossRef]
- Mineau, P. Neonicotinoids in California. Their Use and Threats to the State’s Aquatic Ecosystems and Pollinators, with a Focus on Neon-ic-Treated Seeds. Pierre Mineau Consulting. 2020. Available online: https://www.nrdc.org/sites/default/files (accessed on 29 September 2022).
- Zhang, J.-G.; Ma, D.-D.; Xiong, Q.; Qiu, S.-Q.; Huang, G.-Y.; Shi, W.-J.; Ying, G.-G. Imidacloprid and thiamethoxam affect synaptic transmission in zebrafish. Ecotoxicol. Environ. Saf. 2021, 227, 112917. [Google Scholar] [CrossRef]
- Luo, T.; Wang, X.; Jin, Y. Low concentrations of imidacloprid exposure induced gut toxicity in adult zebrafish (Danio rerio). Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 241, 108972. [Google Scholar] [CrossRef]
- Baysal, M.; Atlı-Eklioğlu, Ö. Comparison of the toxicity of pure compounds and commercial formulations of imidacloprid and acetamiprid on HT-29 cells: Single and mixture exposure. Food Chem. Toxicol. 2021, 155, 112430. [Google Scholar] [CrossRef]
- Kolpin, D.W.; Nations, B.K.; Goolsby, D.A.; Thurman, E.M. Acetochlor in the hydrologic system in the midwestern United States, 1994. Environ. Sci. Technol. 1996, 30, 1459–1464. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Wang, M.; Chen, S.; Zhao, W.; Zhao, Y.; Wang, X.; Zhang, Y. A urinary metabonomics analysis of long-term effect of acetochlor exposure on rats by ultra-performance liquid chromatography/mass spectrometry. Pestic. Biochem. Physiol. 2016, 128, 82–88. [Google Scholar] [CrossRef]
- Li, H.; Feng, Y.; Li, X.; Zeng, D. Analytical confirmation of various herbicides in drinking water resources in sugarcane production regions of Guangxi, China. Bull. Environ. Contam. Toxicol. 2018, 100, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xue, W.; Long, R.; Yang, H.; Wei, W. Acetochlor affects zebrafish ovarian development by producing estrogen effects and inducing oxidative stress. Environ. Sci. Pollut. Res. 2020, 27, 27688–27696. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Dong, F.; Xu, J.; Liu, X.; Zheng, Y. Chiral bioaccumulation behavior of tebuconazole in the zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2016, 126, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Xu, H.; Yao, S.; He, Y.; Zhang, H.; Yu, Y. Chiral triazole fungicide tebuconazole: Enantioselective bioaccumulation, bioactivity, acute toxicity, and dissipation in soils. Environ. Sci. Pollut. Res. 2018, 25, 25468–25475. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, M.; Liu, Y.; Gui, W.; Zhu, G. Thyroid endocrine disruption in zebrafish larvae following exposure to hexaconazole and tebuconazole. Aquat. Toxicol. 2013, 138–139, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Berenzen, N.; Lentzen-Godding, A.; Probst, M.; Schulz, H.; Schulz, R.; Liess, M. A comparison of predicted and measured levels of runoff-related pesticide concentrations in small lowland streams on a landscape level. Chemosphere 2005, 58, 683–691. [Google Scholar] [CrossRef]
- Azevedo, R.D.S.; Falcão, K.V.G.; Assis, C.R.D.; Martins, R.M.G.; Araújo, M.C.; Yogui, G.T.; Neves, J.L.; Seabra, G.M.; Maia, M.B.S.; Amaral, I.P.G.; et al. Effects of pyriproxyfen on zebrafish brain mitochondria and acetylcholinesterase. Chemosphere 2021, 263, 128029. [Google Scholar] [CrossRef]
- Vieira, R.S.F.; Venâncio, C.A.S.; Félix, L.M. Behavioural impairment and oxidative stress by acute exposure of zebrafish to a commercial formulation of tebuconazole. Environ. Toxicol. Pharmacol. 2022, 91, 103823. [Google Scholar] [CrossRef]
- Chang, Y.; Mao, L.; Zhang, L.; Zhang, Y.; Jiang, H. Combined toxicity of imidacloprid, acetochlor, and tebuconazole to zebrafish (Danio rerio): Acute toxicity and hepatotoxicity assessment. Environ. Sci. Pollut. Res. 2020, 27, 10286–10295. [Google Scholar] [CrossRef]
- Brander, S.M.; Jeffries, K.M.; Cole, B.J.; DeCourten, B.M.; White, J.W.; Hasenbein, S.; Fangue, N.A.; Connon, R.E. Transcriptomic changes underlie altered egg protein production and reduced fecundity in an estuarine model fish exposed to bifenthrin. Aquat. Toxicol. 2016, 174, 247–260. [Google Scholar] [CrossRef]
- Chu, S.-H.; Liao, P.-H.; Chen, P.-J. Developmental exposures to an azole fungicide triadimenol at environmentally relevant concentrations cause reproductive dysfunction in females of medaka fish. Chemosphere 2016, 152, 181–189. [Google Scholar] [CrossRef]
- Cao, F.; Zhu, L.; Li, H.; Yu, S.; Wang, C.; Qiu, L. Reproductive toxicity of azoxystrobin to adult zebrafish (Danio rerio). Environ. Pollut. 2016, 219, 1109–1121. [Google Scholar] [CrossRef]
- Wang, H.; Dong, F.; Zhao, Y.; Fu, S.; Zhao, H.; Liu, S.; Zhang, W.; Hu, F. Exposure to diclofenac alters thyroid hormone levels and transcription of genes involved in the hypothalamic-pituitary-thyroid axis in zebrafish embryos/larvae. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2022, 257, 109335. [Google Scholar] [CrossRef]
- Lonare, M.; Kumar, M.; Raut, S.; More, A.; Doltade, S.; Badgujar, P.; Telang, A. Evaluation of ameliorative effect of curcumin on imidacloprid-induced male reproductive toxicity in wistar rats. Environ. Toxicol. 2015, 31, 1250–1263. [Google Scholar] [CrossRef]
- Bal, R.; Naziroğlu, M.; Türk, G.; Yilmaz, Ö.; Kuloğlu, T.; Etem, E.; Baydas, G. Insecticide imidacloprid induces morphological and DNA damage through oxidative toxicity on the reproductive organs of developing male rats. Cell Biochem. Funct. 2012, 30, 492–499. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wu, S.; Chen, J.; Zhang, C.; Xu, Z.; Li, G.; Cai, L.; Shen, W.; Wang, Q. Single and joint toxicity assessment of four currently used pesticides to zebrafish (Danio rerio) using traditional and molecular endpoints. Chemosphere 2018, 192, 14–23. [Google Scholar] [CrossRef]
- Altenhofen, S.; Nabinger, D.D.; Wiprich, M.T.; Pereira, T.C.B.; Bogo, M.R.; Bonan, C.D. Tebuconazole alters morphological, behavioral and neurochemical parameters in larvae and adult zebrafish (Danio rerio). Chemosphere 2017, 180, 483–490. [Google Scholar] [CrossRef]
- Li, S.; Wu, Q.; Sun, Q.; Coffin, S.; Gui, W.; Zhu, G. Parental exposure to tebuconazole causes thyroid endocrine disruption in zebrafish and developmental toxicity in offspring. Aquat. Toxicol. 2019, 211, 116–123. [Google Scholar] [CrossRef]
- Li, S.; Jiang, Y.; Sun, Q.; Coffin, S.; Chen, L.; Qiao, K.; Gui, W.; Zhu, G. Tebuconazole induced oxidative stress related hepatotoxicity in adult and larval zebrafish (Danio rerio). Chemosphere 2019, 241, 125129. [Google Scholar] [CrossRef]
- Ilyushina, N.A.; Egorova, O.V.; Masaltsev, G.V.; Averianova, N.S.; Revazova, Y.A.; Rakitskii, V.N.; Goumenou, M.; Vardavas, A.; Stivaktakis, P.; Tsatsakis, A. Genotoxicity of mixture of imidacloprid, imazalil and tebuconazole. Toxicol. Rep. 2020, 7, 1090–1094. [Google Scholar] [CrossRef]
- Mu, X.; Qi, S.; Liu, J.; Wang, H.; Yuan, L.; Qian, L.; Li, T.; Huang, Y.; Wang, C.; Guo, Y.; et al. Environmental level of bisphenol F induced reproductive toxicity toward zebrafish. Sci. Total Environ. 2021, 806, 149992. [Google Scholar] [CrossRef] [PubMed]
- Paten, A.M.; Colin, T.; Coppin, C.W.; Court, L.N.; Barron, A.B.; Oakeshott, J.G.; Morgan, M.J. Non-additive gene interactions underpin molecular and phenotypic responses in honey bee larvae exposed to imidacloprid and thymol. Sci. Total Environ. 2022, 814, 152614. [Google Scholar] [CrossRef] [PubMed]
- Mzid, M.; Ghlissi, Z.; Ben Salem, M.; Ben Khedir, S.; Chaabouni, K.; Ayedi, F.; Sahnoun, Z.; Hakim, A.; Rebai, T. Chemoprotective role of ethanol extract of Urtica urens L. against the toxicity of imidacloprid on endocrine disruption and ovarian morphometric in female rats, GC/MS analysis. Biomed. Pharmacother. 2018, 97, 518–527. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, D.; Yang, H.; Wang, F. Specific detection of acetamiprid with aptamer based on flexible and adhesive SERS membrane. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 270, 120801. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, Q.; Wu, Q.; Gui, W.; Zhu, G.; Schlenk, D. Endocrine disrupting effects of tebuconazole on different life stages of zebrafish (Danio rerio). Environ. Pollut. 2019, 249, 1049–1059. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Cao, J.; Chen, J.; Song, J.; Zhou, B.; Feng, C.; Wang, J. Waterborne fluoride exposure changed the structure and the expressions of steroidogenic-related genes in gonads of adult zebrafish (Danio rerio). Chemosphere 2016, 145, 365–375. [Google Scholar] [CrossRef]
- Kazeto, Y.; Kohara, M.; Miura, T.; Miura, C.; Yamaguchi, S.; Trant, J.M.; Adachi, S.; Yamauchi, K. Japanese eel follicle-stimulating hormone (Fsh) and luteinizing hormone (Lh): Production of biologically active recombinant Fsh and Lh by Drosophila S2 cells and their differential actions on the reproductive biology. Biol. Reprod. 2008, 79, 938–946. [Google Scholar] [CrossRef] [Green Version]
- Kime, D.E. ‘Classical’ and ‘non-classical’ reproductive steroids in fish. Rev. Fish Biol. Fish. 1993, 3, 160–180. [Google Scholar] [CrossRef]
- Vivar, O.I.; Zhao, X.; Saunier, E.F.; Griffin, C.; Mayba, O.S.; Tagliaferri, M.; Cohen, I.; Speed, T.P.; Leitman, D.C. Estrogen receptor β binds to and regulates three distinct classes of target genes. J. Biol. Chem. 2010, 285, 22059–22066. [Google Scholar] [CrossRef] [Green Version]
- Cui, J.; Shen, Y.; Li, R. Estrogen synthesis and signaling pathways during aging: From periphery to brain. Trends Mol. Med. 2013, 19, 197–209. [Google Scholar] [CrossRef]
- Celeghin, A.; Benato, F.; Pikulkaew, S.; Rabbane, G.; Colombo, L.; Valle, L.D. The knockdown of the maternal estrogen receptor 2a (esr2a) mRNA affects embryo transcript contents and larval development in zebrafish. Gen. Comp. Endocrinol. 2011, 172, 120–129. [Google Scholar] [CrossRef]
- Wu, S.M.; Tseng, Y.-J.; Chen, T.-H. Maternal effect and dietary supplementation of estradiol-17β on female zebrafish (Danio rerio) affects the swimming behavior and stress-coping styles of its offspring. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 252, 109211. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, A.; Lu, W. Corticotropin-releasing hormone reduces basal estradiol production in zebrafish follicular cells. Mol. Cell. Endocrinol. 2021, 527, 111222. [Google Scholar] [CrossRef]
- Orlando, E.F.; Kolok, A.S.; Binzcik, G.A.; Gates, J.L.; Horton, M.K.; Lambright, C.S.; Gray, L.E., Jr.; Soto, A.M.; Guillette, L.J., Jr. Endocrine disrupting effects of cattle feedlot effluent on an aquatic sentinel species, the fathead minnow. Environ. Health Perspect. 2004, 112, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Yuan, H.-X.; Xu, X.; Sima, Y.-H.; Xu, S.-Q. Reproductive toxicity effects of 4-nonylphenol with known endocrine disrupting effects and induction of vitellogenin gene expression in silkworm, Bombyx mori. Chemosphere 2013, 93, 263–268. [Google Scholar] [CrossRef]
- Aoki, J.-Y.; Nagae, M.; Takao, Y.; Hara, A.; Lee, Y.-D.; Yeo, I.-K.; Lim, B.-S.; Park, C.-B.; Soyano, K. Survey of contamination of estrogenic chemicals in Japanese and Korean coastal waters using the wild grey mullet (Mugil cephalus). Sci. Total Environ. 2010, 408, 660–665. [Google Scholar] [CrossRef] [Green Version]
- Jun, I.; Ryu, C.; Park, C.; Cho, H.; Kim, Y. 5α-reductase inhibition results in decreases of DHT, E2 and VTG in zebrafish larvae. Toxicol. Lett. 2021, 350, S70. [Google Scholar] [CrossRef]
- Wu, S.; Li, X.; Liu, X.; Yang, G.; An, X.; Wang, Q.; Wang, Y. Joint toxic effects of triazophos and imidacloprid on zebrafish (Danio rerio). Environ. Pollut. 2018, 235, 470–481. [Google Scholar] [CrossRef]
- Darvishi, M.; Safari, R.; Hoseinifar, S.H.; Shabani, A.; Dadar, M.; Jarayedi, Z.; Paolucci, M. Sublethal doses of diazinon affected reproductive, immune, and oxidative status in female zebrafish (Danio rerio). Aquac. Rep. 2021, 22, 100944. [Google Scholar] [CrossRef]
- Whirledge, S.; Cidlowski, J.A. Glucocorticoids and reproduction: Traffic control on the road to reproduction. Trends Endocrinol. Metab. 2017, 28, 399–415. [Google Scholar] [CrossRef]
- Faught, E.; Vijayan, M.M. Mechanisms of cortisol action in fish hepatocytes. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2016, 199, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, C.; Yang, G.; Wang, X.; Wang, Q.; Weng, H.; Zhang, Z.; Qian, Y. Combined lethal toxicity, biochemical responses, and gene expression variations induced by tebuconazole, bifenthrin and their mixture in zebrafish (Danio rerio). Ecotoxicol. Environ. Saf. 2021, 230, 113116. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, M.; Zuccarelli, M.D.; Nakamura, I.; Young, G. Steroidogenic acute regulatory protein in white sturgeon (Acipenser transmontanus): cDNA cloning, sites of expression and transcript abundance in corticosteroidogenic tissue after an acute stressor. Gen. Comp. Endocrinol. 2009, 162, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Alderman, S.L.; Bernier, N.J. Ontogeny of the corticotropin-releasing factor system in zebrafish. Gen. Comp. Endocrinol. 2009, 164, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Alderman, S.L.; Leishman, E.; Fuzzen, M.L.; Bernier, N.J. Corticotropin-releasing factor regulates caspase-3 and may protect developing zebrafish from stress-induced apoptosis. Gen. Comp. Endocrinol. 2018, 265, 207–213. [Google Scholar] [CrossRef]
- Lu, J.; Wu, Q.; Yang, Q.; Li, G.; Wang, R.; Liu, Y.; Duan, C.; Duan, S.; He, X.; Huang, Z.; et al. Molecular mechanism of reproductive toxicity induced by beta-cypermethrin in zebrafish. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 239, 108894. [Google Scholar] [CrossRef]
- Chu, L.; Li, J.; Liu, Y.; Cheng, C.H.K. Gonadotropin signaling in zebrafish ovary and testis development: Insights from gene knockout study. Mol. Endocrinol. 2015, 29, 1743–1758. [Google Scholar] [CrossRef] [Green Version]
- Ferraresso, S.; Bargelloni, L.; Babbucci, M.; Cannas, R.; Follesa, M.C.; Carugati, L.; Melis, R.; Cau, A.; Koutrakis, M.; Sapounidis, A.; et al. fshr: A fish sex-determining locus shows variable incomplete penetrance across flathead grey mullet populations. iScience 2020, 24, 101886. [Google Scholar] [CrossRef]
- Yang, R.; Wang, X.; Wang, J.; Chen, P.; Liu, Q.; Zhong, W.; Zhu, L. Insights into the sex-dependent reproductive toxicity of 2-ethylhexyl diphenyl phosphate on zebrafish (Danio rerio). Environ. Int. 2021, 158, 106928. [Google Scholar] [CrossRef]
- Cao, J.; Wang, G.; Wang, T.; Chen, J.; Wenjing, G.; Wu, P.; He, X.; Xie, L. Copper caused reproductive endocrine disruption in zebrafish (Danio rerio). Aquat. Toxicol. 2019, 211, 124–136. [Google Scholar] [CrossRef]
- Yu, M.; Zhang, X.; Guo, L.; Tian, H.; Wang, W.; Ru, S. Anti-estrogenic effect of semicarbazide in female zebrafish (Danio rerio) and its potential mechanisms. Aquat. Toxicol. 2016, 170, 262–270. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, D.; Dang, Y.; Yu, L.; Liu, C.; Wang, J. Reproduction impairment and endocrine disruption in adult zebrafish (Danio rerio) after waterborne exposure to TBOEP. Aquat. Toxicol. 2017, 182, 163–171. [Google Scholar] [CrossRef]
- Maharajan, K.; Muthulakshmi, S.; Karthik, C.; Nataraj, B.; Nambirajan, K.; Hemalatha, D.; Jiji, S.; Kadirvelu, K.; Liu, K.-C.; Ramesh, M. Pyriproxyfen induced impairment of reproductive endocrine homeostasis and gonadal histopathology in zebrafish (Danio rerio) by altered expression of hypothalamus-pituitary-gonadal (HPG) axis genes. Sci. Total Environ. 2020, 735, 139496. [Google Scholar] [CrossRef]
- Zhang, S.; Mo, J.; Wang, Y.; Ni, C.; Li, X.; Zhu, Q.; Ge, R.-S. Endocrine disruptors of inhibiting testicular 3β-hydroxysteroid dehydrogenase. Chem. Interact. 2019, 303, 90–97. [Google Scholar] [CrossRef]
- Ma, Y.; Han, J.; Guo, Y.; Lam, P.K.; Wu, R.S.; Giesy, J.P.; Zhang, X.; Zhou, B. Disruption of endocrine function in in vitro H295R cell-based and in in vivo assay in zebrafish by 2,4-dichlorophenol. Aquat. Toxicol. 2012, 106–107, 173–181. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, W.; Zhu, L.; Ye, D.; Zhu, X.; Wang, H.; Sun, Y.; Deng, F. Nanog suppresses the expression of vasa by directly regulating nlkl in the early zebrafish embryo. Biochimie 2017, 142, 93–101. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Komiya, T.; Kawabata, H.; Sato, M.; Fujimoto, H.; Furusawa, M.; Noce, T. Isolation of a DEAD-family protein gene that encodes a murine homolog of Drosophila vasa and its specific expression in germ cell lineage. Proc. Natl. Acad. Sci. USA 1994, 91, 12258–12262. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Kajiura-Kobayashi, H.; Nagahama, Y. Differential expression of vasa homologue gene in the germ cells during oogenesis and spermatogenesis in a teleost fish, tilapia, Oreochromis niloticus. Mech. Dev. 2000, 99, 139–142. [Google Scholar] [CrossRef]
- Yoshizaki, G.; Sakatani, S.; Tominaga, H.; Takeuchi, T. Cloning and characterization of a vasa-like gene in rainbow trout and its expression in the germ cell lineage. Mol. Reprod. Dev. 2000, 55, 364–371. [Google Scholar] [CrossRef]





| Gene | Sequence of the Primers (5′ →3′) | Accession No. |
|---|---|---|
| rpl8 | F: TTGTTGGTGTTGTTGCTGGT | NM_200713.1 |
| R: GGATGCTCAACAGGGTTCAT | ||
| ef1α | F: GATCACTGGTACTTCTCAGGCTGA | NM_131263.1 |
| R: GGTGAAAGCCAGGAGGGC | ||
| ar | F: GCGAATGGATGGATGTAAC | NM_001083123.1 |
| R: TCATCAGAGCAGATTAGGC | ||
| esr2a | F: CTCTGAACTCATCCGCCTTC | NM_180966.2 |
| R: AGCAGAGCGGGACTGTAAAA | ||
| vtg1 | F: CTCCCGAGTTCATTCAGA | NM_001044897.2 |
| R: ATGACAACTTCACGCAGA | ||
| gr | F: GAGCCAGACACCCTCTATGC | NM_001020711.3 |
| R: CCAGCCCAGTCCAAAAGACA | ||
| star | F: GAATGCCTGAGCAGAAGGGA | NM_131663.1 |
| R: CGTCTATACCCCCACCGGAT | ||
| fshr | F: GTCTGTCTGGGCAACAAGGT | NM_001001812.1 |
| R: CACCACTATTCTCTTCAGCTCGT | ||
| hmgrb | F: CCCGCCCAAAGCCATAAAAG | NM_001014292 |
| R: GCTTCCAGACGGTATCCCAG | ||
| 3βhsd | F: AACAAGCCCATTCTGCCCAT | AY279108 |
| R: TCCTCCCAGTCATACCGAGG | ||
| vasa | F: CAACAGGCTTCAACCCACG | BC129275.1 |
| R: GCCAGTTATTCCCATTCCTCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Chang, Y.; Zhang, Y.; Zhu, L.; Mao, L.; Zhang, L.; Liu, X.; Jiang, H. Combined Reproductive Effects of Imidacloprid, Acetochlor and Tebuconazole on Zebrafish (Danio rerio). Agriculture 2022, 12, 1979. https://doi.org/10.3390/agriculture12121979
Yang J, Chang Y, Zhang Y, Zhu L, Mao L, Zhang L, Liu X, Jiang H. Combined Reproductive Effects of Imidacloprid, Acetochlor and Tebuconazole on Zebrafish (Danio rerio). Agriculture. 2022; 12(12):1979. https://doi.org/10.3390/agriculture12121979
Chicago/Turabian StyleYang, Jin, Yiming Chang, Yanning Zhang, Lizhen Zhu, Liangang Mao, Lan Zhang, Xingang Liu, and Hongyun Jiang. 2022. "Combined Reproductive Effects of Imidacloprid, Acetochlor and Tebuconazole on Zebrafish (Danio rerio)" Agriculture 12, no. 12: 1979. https://doi.org/10.3390/agriculture12121979
APA StyleYang, J., Chang, Y., Zhang, Y., Zhu, L., Mao, L., Zhang, L., Liu, X., & Jiang, H. (2022). Combined Reproductive Effects of Imidacloprid, Acetochlor and Tebuconazole on Zebrafish (Danio rerio). Agriculture, 12(12), 1979. https://doi.org/10.3390/agriculture12121979









