Fermented Apple Pomace Improves Plasma Biochemical and Antioxidant Indicators and Fecal Microbiota of Weaned Pigs
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design, Animals, and Housing
2.2. Growth Performance and Diarrhea Rate of Weaned Pigs
2.3. Plasma Biochemical Indicators of Weaned Pigs
2.4. DNA Extraction and 16S RNA Gene Sequencing
2.5. Bioinformatics Analysis of Sequencing Data
2.6. Statistic Analysis
3. Results
3.1. Growth Performance and Diarrhea Rate of Weaned Pigs
3.2. Blood Biochemical, Antioxidant Stress and Immune Indicators
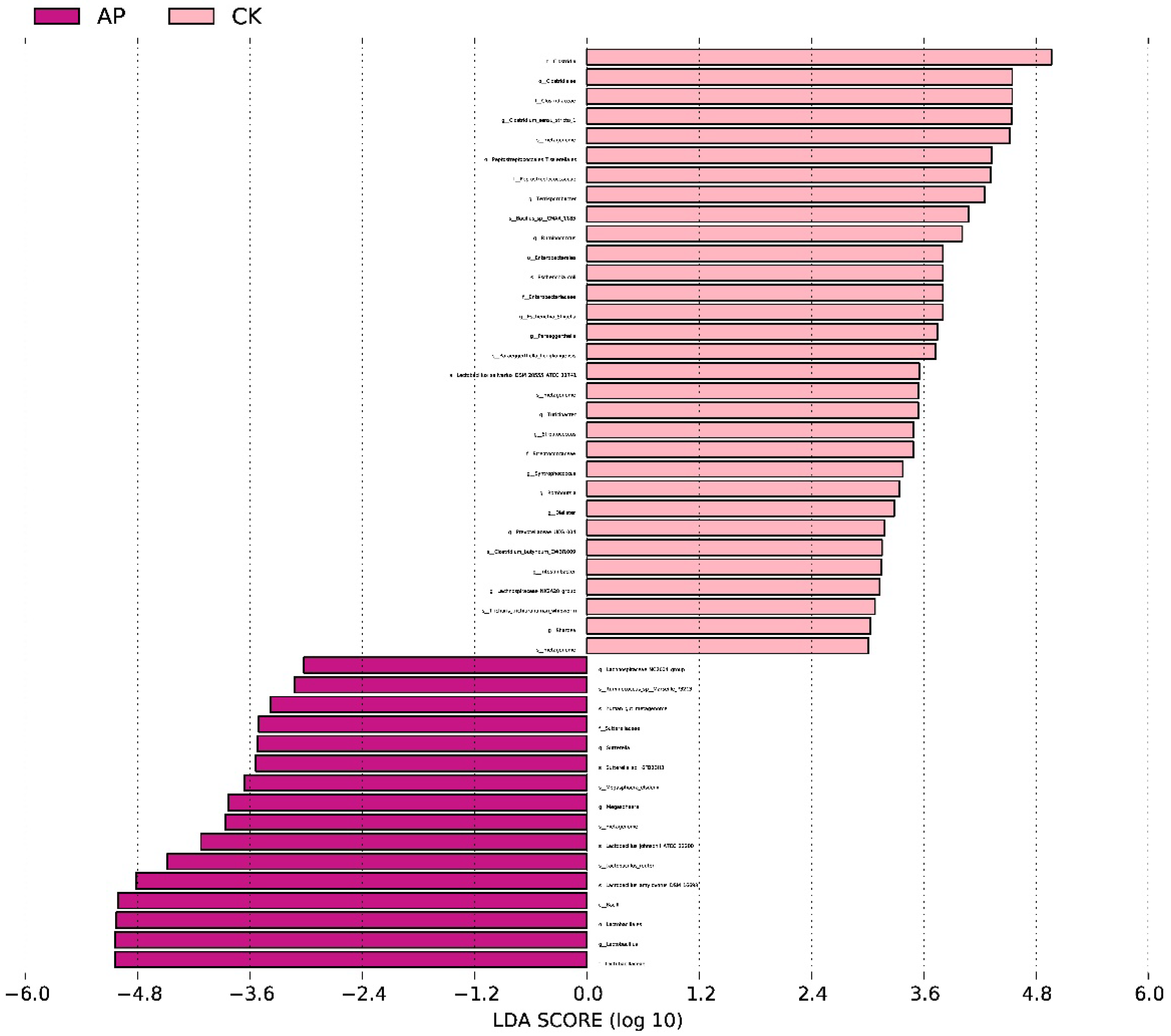
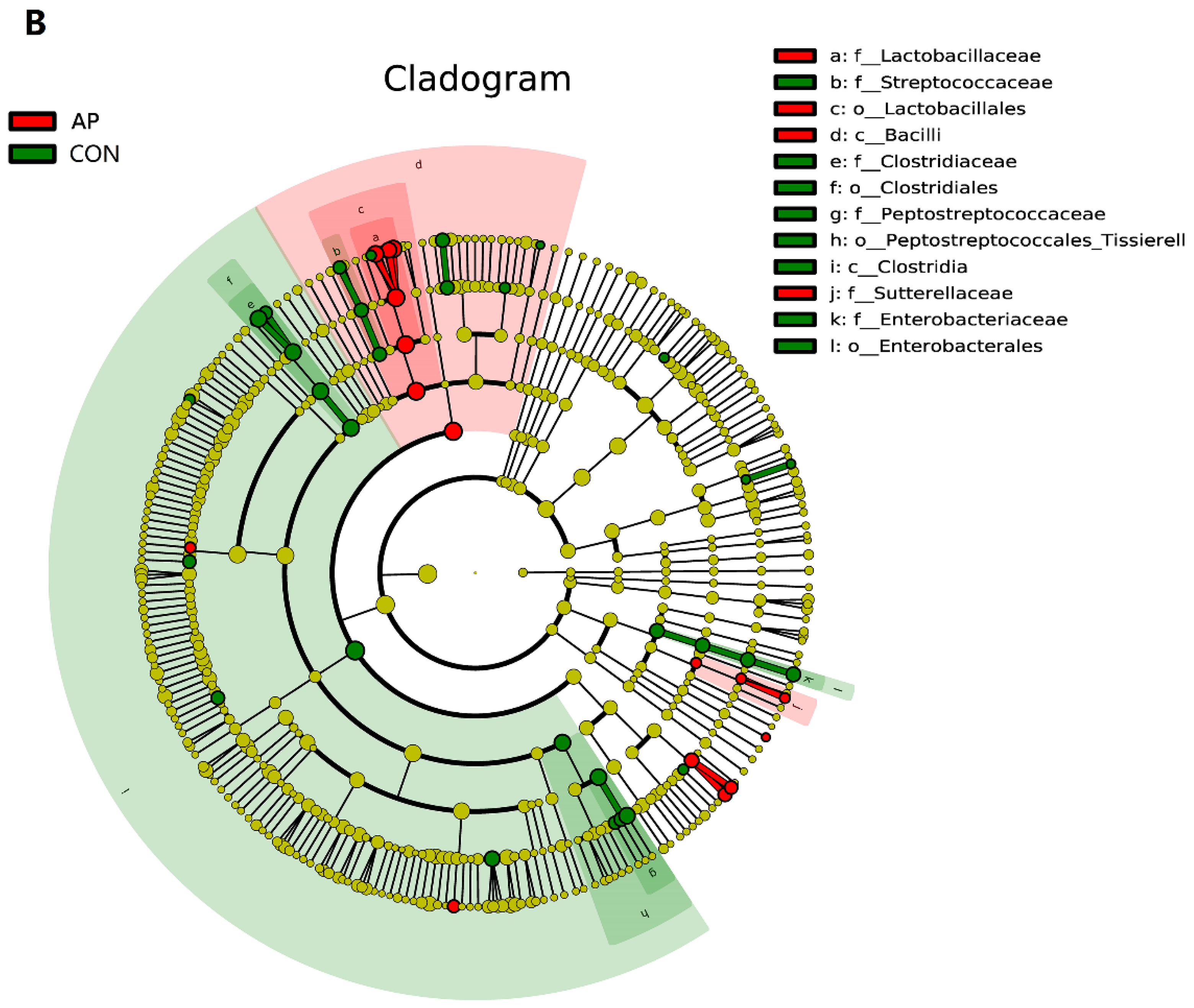
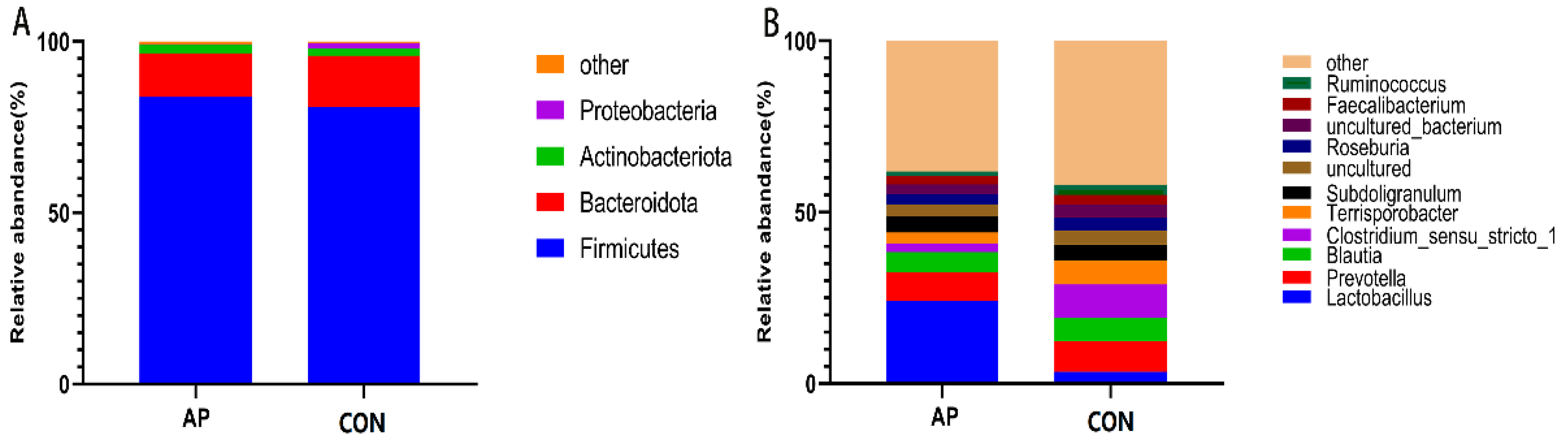
3.3. Gastrointestinal Microbiota Diversity
4. Discussion
4.1. Growth Performance of Pigs
4.2. Effects of Fermented AP on Plasma Biochemical, Antioxidant, and Immune Indicators of Weaned Pigs
4.3. Intestinal Microbiota of Weaned Pigs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koutsos, A.; Tuohy, K.M.; Lovegrove, J.A. Apples and cardiovascular health—Is the gut microbiota a core consideration? Nutrients 2015, 7, 3959–3998. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT. Food and Agriculture Organization of the United Nations. FAOSTAT Database. 2019. Available online: https://www.fao.org/faostat/zh/#data/QCL/visualize (accessed on 25 November 2021).
- Konrade, D.; Klava, D.; Gramatina, I. Cereal crispbread improvement with dietary fibre from apple by-products. In Proceedings of CBU International Conference Proceedings; Materials Science: Prague, Czech Republic; pp. 1143–1148.
- Bhushan, S.; Kalia, K.; Sharma, M.; Singh, B.; Ahuja, P.S. Processing of apple pomace for bioactive molecules. Crit. Rev. Biotechnol. 2008, 28, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, G.S.; Kaur, S.; Brar, S.K. Perspective of apple processing wastes as low-cost substrates for bioproduction of high value products: A review. Renew. Sustain. Energy Rev. 2013, 27, 789–805. [Google Scholar] [CrossRef]
- Shalini, R.; Gupta, D.K. Utilization of pomace from apple processing industries: A review. J. Food Sci. Technol. 2010, 47, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, F.; Albuquerque, P.M.; Streit, F.; Esposito, E.; Ninow, J.L. Apple pomace: A versatile substrate for biotechnological applications. Crit. Rev. Biotechnol. 2008, 28, 1–12. [Google Scholar] [CrossRef]
- Suárez, B.; Álvarez, Á.L.; García, Y.D.; Barrio, G.D.; Lobo, A.P.; Parra, F. Phenolic profiles, antioxidant activity and in vitro antiviral properties of apple pomace. Food Chem. 2010, 120, 339–342. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, X.; Zhao, G.; Hu, T.; Wang, Y. Potential and challenges of tannins as an alternative to in-feed antibiotics for farm animal production. Anim. Nutr. 2018, 4, 137–150. [Google Scholar] [CrossRef]
- Rahmat, H.; Hodge, R.A.; Manderson, G.J.; Yu, P.L. Solid-substrate fermentation ofKloeckera apiculata andCandida utilis on apple pomace to produce an improved stock-feed. World J. Microbiol. Biotechnol. 1995, 11, 168–170. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Bhalla, T.C.; Joshi, M. Protein enrichment of apple pomace by co-culture of cellulolytic moulds and yeasts. World J. Microbiol. Biotechnol. 1994, 10, 116–117. [Google Scholar] [CrossRef]
- Tosun, R.; YaŞAr, S. Fungal Fermantasyonu ile Elma Posasının Besin Madde İçeriğinin Zenginleştirilmesi. Kahramanmaraş Sütçü İmam Üniversitesi Tarım ve Doğa Dergisi 2020. [Google Scholar] [CrossRef]
- Kafantaris, I.; Stagos, D.; Kotsampasi, B.; Hatzis, A.; Kypriotakis, A.; Gerasopoulos, K.; Makri, S.; Goutzourelas, N.; Mitsagga, C.; Giavasis, I.; et al. Grape pomace improves performance, antioxidant status, fecal microbiota and meat quality of piglets. Animal 2018, 12, 246–255. [Google Scholar] [CrossRef]
- Noblet, J.; Perez, J.M. Prediction of digestibility of nutrients and energy values of pig diets from chemical analysis. J. Anim. Sci. 1993, 71, 3389–3398. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, K.S.; Toft, N. Intra- and inter-observer agreement when using a descriptive classification scale for clinical assessment of faecal consistency in growing pigs. Prev. Vet. Med. 2011, 98, 288–291. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef] [PubMed]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Lyu, F.; Luiz, S.F.; Azeredo, D.R.P.; Cruz, A.G.; Ajlouni, S.; Ranadheera, C.S. Apple Pomace as a Functional and Healthy Ingredient in Food Products: A Review. Processes 2020, 8, 319. [Google Scholar] [CrossRef]
- Devrajan, A.; Joshi, V.K.; Gupta, K.; Sheikher, C.; Lal, B.B. Evaluation of apple pomace based reconstituted feed in rats after solid state fermentation and ethanol recovery. Braz. Arch. Biol. Technol. 2004, 47, 93–106. [Google Scholar] [CrossRef]
- Lee, S.D.; Kim, H.Y.; Jung, H.J.; Ji, S.Y.; Chowdappa, R.; Ha, J.H.; Song, Y.M.; Park, J.C.; Moon, H.K.; Kim, I.C. The effect of fermented apple diet supplementation on the growth performance and meat quality in finishing pigs. Anim. Sci. J. 2009, 80, 79–84. [Google Scholar] [CrossRef]
- Joshi, V.K.; Sandhu, D.K. Preparation and evaluation of an animal feed byproduct produced by solid-state fermentation of apple pomace. Bioresour. Technol. 1996, 56, 251–255. [Google Scholar] [CrossRef]
- Liao, P.; Li, M.; Li, Y.; Tan, X.; Zhao, F.; Shu, X.; Yin, Y. Effects of dietary supplementation with cupreous N-carbamylglutamate (NCG) chelate and copper sulfate on growth performance, serum biochemical profile and immune response, tissue mineral levels and fecal excretion of mineral in weaning piglets. Food Agric. Immunol. 2017, 28, 1315–1329. [Google Scholar] [CrossRef]
- Tao, H.; Guo, F.; Tu, Y.; Si, B.W.; Xing, Y.C.; Huang, D.J.; Diao, Q.Y. Effect of weaning age on growth performance, feed efficiency, nutrient digestibility and blood-biochemical parameters in Droughtmaster crossbred beef calves. Asian-Australas J. Anim. Sci. 2018, 31, 864–872. [Google Scholar] [CrossRef] [PubMed]
- Coma, J.; Carrion, D.; Zimmerman, D.R. Use of plasma urea nitrogen as a rapid response criterion to determine the lysine requirement of pigs. J. Anim. Sci. 1995, 73, 472–481. [Google Scholar] [CrossRef]
- Liao, P.; Shu, X.; Tang, M.; Tan, B.; Yin, Y. Effect of dietary copper source (inorganic vs. chelated) on immune response, mineral status, and fecal mineral excretion in nursery piglets. Food Agric. Immunol. 2017, 29, 548–563. [Google Scholar] [CrossRef]
- Umbreen, H.; Arshad, M.U.; Noreen, R.; Aftab, K. Ameliorative Effect of Apple Pomace and Mango Peels against Hyperlipidemia and Lipid Peroxidation Induced by Hyperlipidemic Diet. Sains Malays. 2020, 49, 1273–1282. [Google Scholar] [CrossRef]
- Ajila, C.; Sarma, S.; Brar, S.; Godbout, S.; Cote, M.; Guay, F.; Verma, M.; Valéro, J. Fermented Apple Pomace as a Feed Additive to Enhance Growth Performance of Growing Pigs and Its Effects on Emissions. Agriculture 2015, 5, 313–329. [Google Scholar] [CrossRef]
- Lu, Y.; Yeap Foo, L. Antioxidant and radical scavenging activities of polyphenols from apple pomace. Food Chem. 2000, 68, 81–85. [Google Scholar] [CrossRef]
- Herranz, B.; Fernández-Jalao, I.; Dolores Álvarez, M.; Quiles, A.; Sánchez-Moreno, C.; Hernando, I.; de Ancos, B. Phenolic compounds, microstructure and viscosity of onion and apple products subjected to in vitro gastrointestinal digestion. Innov. Food Sci. Emerg. Technol. 2019, 51, 114–125. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, Y.; Sun-Waterhouse, D.X.; Zhai, H.; Guan, H.; Rong, X.; Li, F.; Yu, J.C.; Li, D.P. MicroRNA-based regulatory mechanisms underlying the synergistic antioxidant action of quercetin and catechin in H2O2-stimulated HepG2 cells: Roles of BACH1 in Nrf2-dependent pathways. Free Radic. Biol. Med. 2020, 153, 122–131. [Google Scholar] [CrossRef]
- Pan, D.; Yu, Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microbes 2014, 5, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.E.; Engen, P.A.; Gillevet, P.M.; Shaikh, M.; Sikaroodi, M.; Forsyth, C.B.; Mutlu, E.; Keshavarzian, A. Lower Neighborhood Socioeconomic Status Associated with Reduced Diversity of the Colonic Microbiota in Healthy Adults. PLoS ONE 2016, 11, e0148952. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Yang, H.; Zhang, L.; Su, Y.; Shi, D.; Xiao, H.; Tian, Y. High-throughput sequencing technology to reveal the composition and function of cecal microbiota in Dagu chicken. BMC Microbiol. 2016, 16, 259. [Google Scholar] [CrossRef]
- Mao, S.Y.; Huo, W.J.; Zhu, W.Y. Microbiome-metabolome analysis reveals unhealthy alterations in the composition and metabolism of ruminal microbiota with increasing dietary grain in a goat model. Environ. Microbiol. 2016, 18, 525–541. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Zhu, L.; Xu, Y.; Liu, N.; Sun, X.; Hu, L.; Huang, H.; Wei, K.; Zhu, R. Dynamic Distribution of Gut Microbiota in Goats at Different Ages and Health States. Front. Microbiol. 2018, 9, 2509. [Google Scholar] [CrossRef]
- Li, H.; Ma, L.; Li, Z.; Yin, J.; Tan, B.; Chen, J.; Jiang, Q.; Ma, X. Evolution of the Gut Microbiota and Its Fermentation Characteristics of Ningxiang Pigs at the Young Stage. Animals 2021, 11, 638. [Google Scholar] [CrossRef]
- Juskiewicz, J.; Zary-Sikorska, E.; Zdunczyk, Z.; Krol, B.; Jaroslawska, J.; Jurgonski, A. Effect of dietary supplementation with unprocessed and ethanol-extracted apple pomaces on caecal fermentation, antioxidant and blood biomarkers in rats. Br. J. Nutr. 2012, 107, 1138–1146. [Google Scholar] [CrossRef]
- Sehm, J.; Lindermayer, H.; Dummer, C.; Treutter, D.; Pfaffl, M.W. The influence of polyphenol rich apple pomace or red-wine pomace diet on the gut morphology in weaning piglets. J. Anim. Physiol. Anim. Nutr. 2007, 91, 289–296. [Google Scholar] [CrossRef]


| Item | Corn Silage | Corn-AP Silage |
|---|---|---|
| Dry Matter (as fed) | 600 | 600 |
| Crude Protein | 86 | 85 |
| Crude fat | 43 | 43 |
| Ash | 15 | 15 |
| Crude fiber | 27 | 26 |
| Moisture | 400 | 400 |
| Metabolic energy 1 (MJ/kg DM) | 16.80 | 16.75 |
| Items | CON Group | AP Group |
|---|---|---|
| Ingredients/% | ||
| Silage corn-AP | 0.00 | 5.00 |
| Silage corn | 5.00 | 0.00 |
| Corn | 57.00 | 57.00 |
| Soybean meal | 23.00 | 23.00 |
| Wheat bran | 11.40 | 11.40 |
| Limestone | 0.50 | 0.50 |
| Calcium hydrophosphate | 1.20 | 1.20 |
| NaCl | 0.90 | 0.90 |
| Premix 1 | 1.00 | 1.00 |
| Nutrient level | ||
| Metabolizable Energy (MJ/kg) 2 | 10.82 | 10.69 |
| Crude protein/% | 16.92 | 16.98 |
| Calcium/% | 0.81 | 0.84 |
| Phosphorus/% | 0.62 | 0.63 |
| Lysine/% | 0.83 | 0.84 |
| Methionine/% | 0.57 | 0.58 |
| Items 1 | CON | AP | SEM | p-Value |
|---|---|---|---|---|
| IW, kg | 10.18 | 10.02 | 0.26 | 0.550 |
| FW, kg | 18.79 | 19.56 | 0.25 | 0.865 |
| ADG, g | 359.03 | 397.36 | 14.23 | 0.001 |
| ADFI, g | 697.10 | 695.60 | 29.20 | 0.972 |
| F:G | 1.89 | 1.87 | 0.15 | 0.820 |
| Diarrhea rate, % | 3.12 | 3.31 | 0.32 | 0.769 |
| Items 1 | CON | AP | SEM | p |
|---|---|---|---|---|
| TP, g/L | 62.11 | 88.44 | 8.70 | 0.054 |
| ALB, g/L | 34.58 | 43.56 | 1.23 | <0.001 |
| ALT, U/L | 47.03 | 39.42 | 3.07 | 0.088 |
| AST, U/L | 64.20 | 40.61 | 3.63 | 0.0001 |
| BUN, mmoL/L | 9.02 | 8.61 | 0.31 | 0.358 |
| TC, mmoL/L | 6.43 | 6.09 | 0.25 | 0.360 |
| T-AOC, mmoL/L | 38.66 | 42.14 | 3.65 | 0.532 |
| SOD, U/mL | 28.81 | 31.71 | 0.55 | 0.001 |
| MDA, nmoL/mL | 4.03 | 1.70 | 0.34 | 0.003 |
| IgA, mg/mL | 3.36 | 3.58 | 0.13 | 0.249 |
| IgG, mg/mL | 10.14 | 11.13 | 0.57 | 0.244 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ao, W.; Cheng, M.; Chen, Y.; Sun, J.; Zhang, C.; Zhao, X.; Liu, M.; Zhou, B. Fermented Apple Pomace Improves Plasma Biochemical and Antioxidant Indicators and Fecal Microbiota of Weaned Pigs. Agriculture 2022, 12, 1603. https://doi.org/10.3390/agriculture12101603
Ao W, Cheng M, Chen Y, Sun J, Zhang C, Zhao X, Liu M, Zhou B. Fermented Apple Pomace Improves Plasma Biochemical and Antioxidant Indicators and Fecal Microbiota of Weaned Pigs. Agriculture. 2022; 12(10):1603. https://doi.org/10.3390/agriculture12101603
Chicago/Turabian StyleAo, Weiping, Meng Cheng, Yanxu Chen, Jipeng Sun, Chunlei Zhang, Xianle Zhao, Mingzheng Liu, and Bo Zhou. 2022. "Fermented Apple Pomace Improves Plasma Biochemical and Antioxidant Indicators and Fecal Microbiota of Weaned Pigs" Agriculture 12, no. 10: 1603. https://doi.org/10.3390/agriculture12101603
APA StyleAo, W., Cheng, M., Chen, Y., Sun, J., Zhang, C., Zhao, X., Liu, M., & Zhou, B. (2022). Fermented Apple Pomace Improves Plasma Biochemical and Antioxidant Indicators and Fecal Microbiota of Weaned Pigs. Agriculture, 12(10), 1603. https://doi.org/10.3390/agriculture12101603






