Impact of Extreme Temperature and Soil Water Stress on the Growth and Yield of Soybean (Glycine max (L.) Merrill)
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Experimental Set Up
2.2. Treatment Structure
2.3. Weather Variables Measurements
2.4. Plant Development
2.5. Plant Growth and Yield
2.6. Statistical Analysis
3. Results
3.1. Weather Conditions in the Chambers and Watering Regimes
3.2. Effect of Temperature on Plant Development
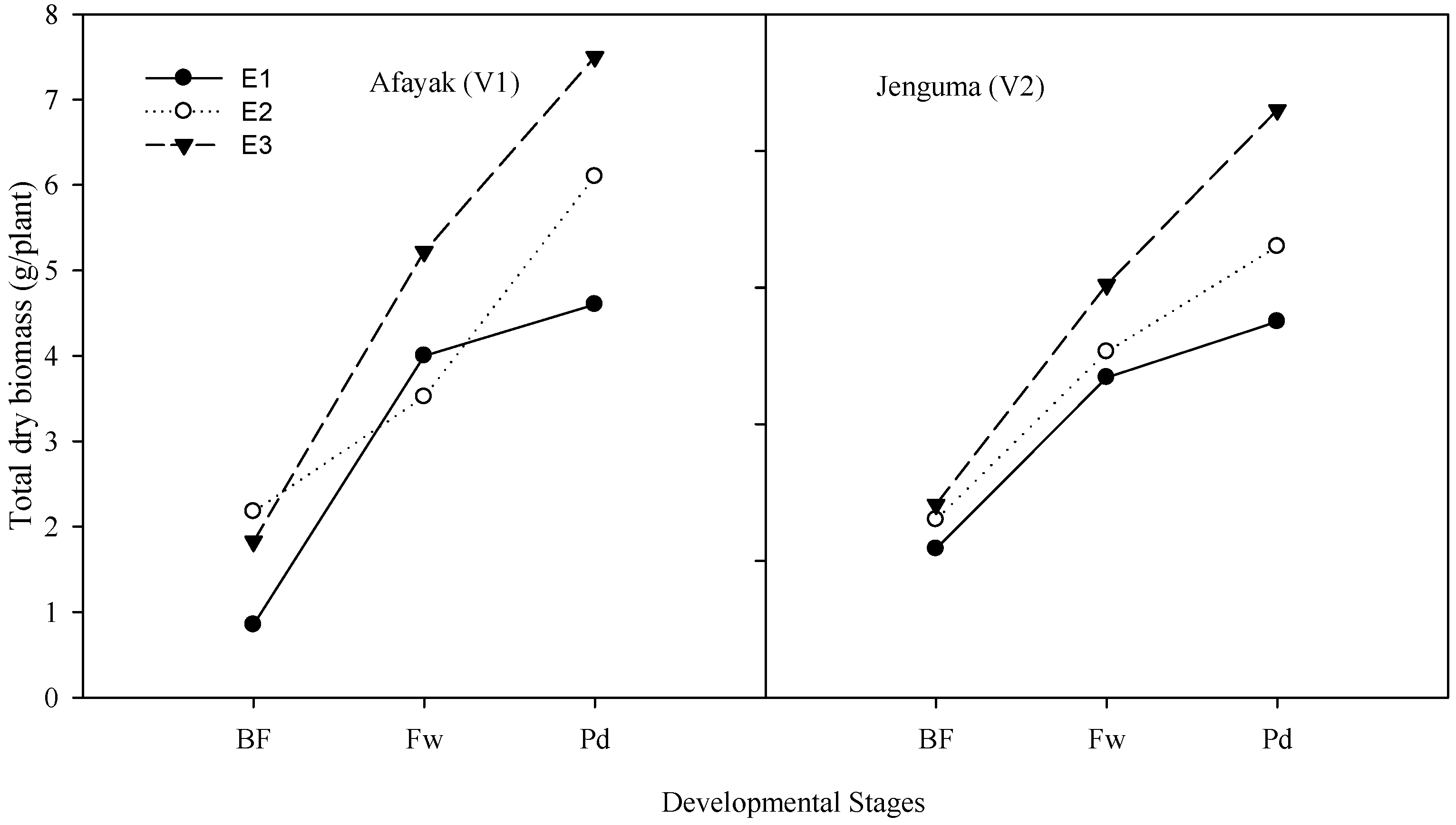
3.3. Patterns of Biomass Accumulation
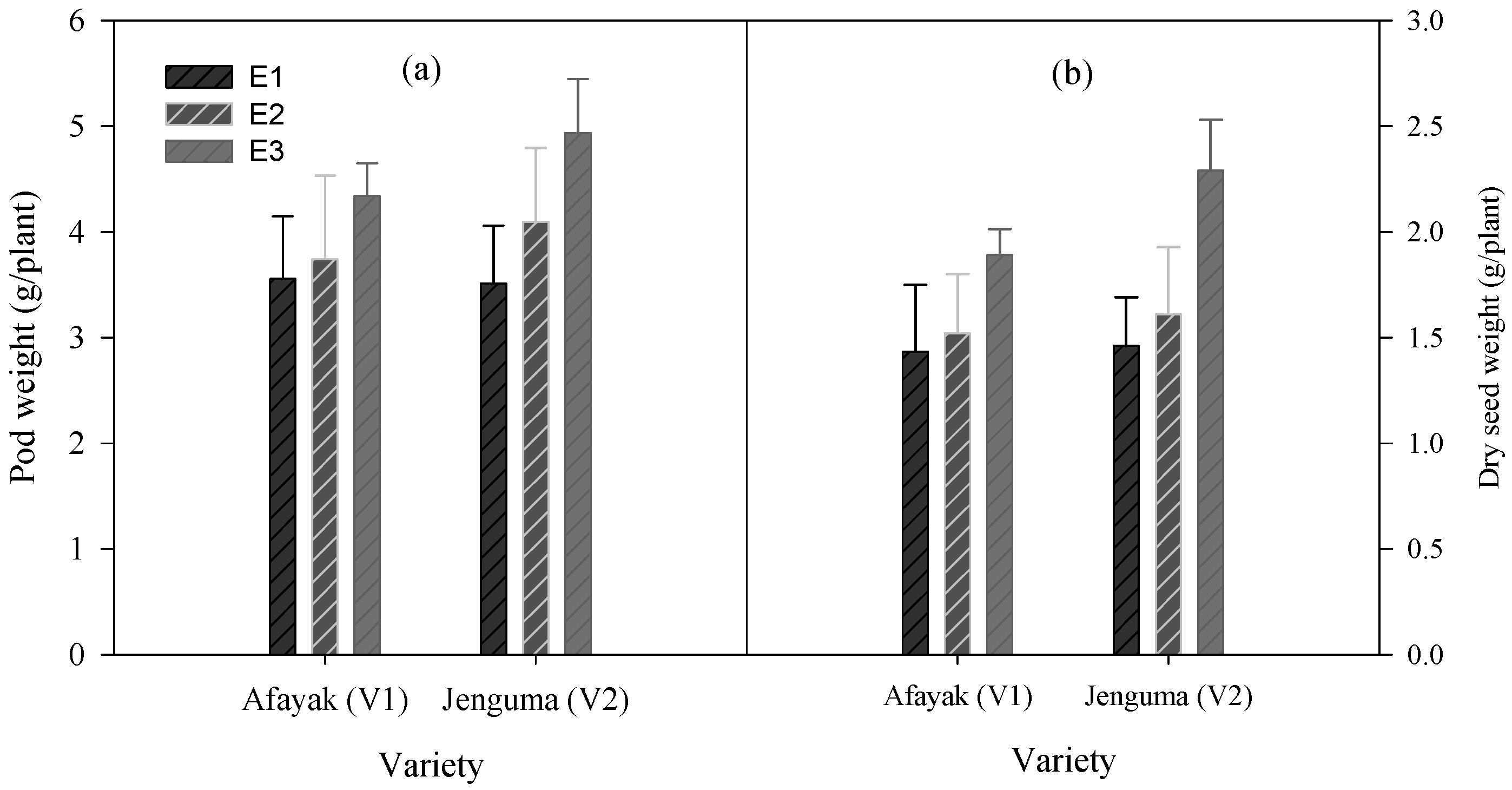
3.4. Varietal Differences in Pod and Seed Weights under Varied Temperature
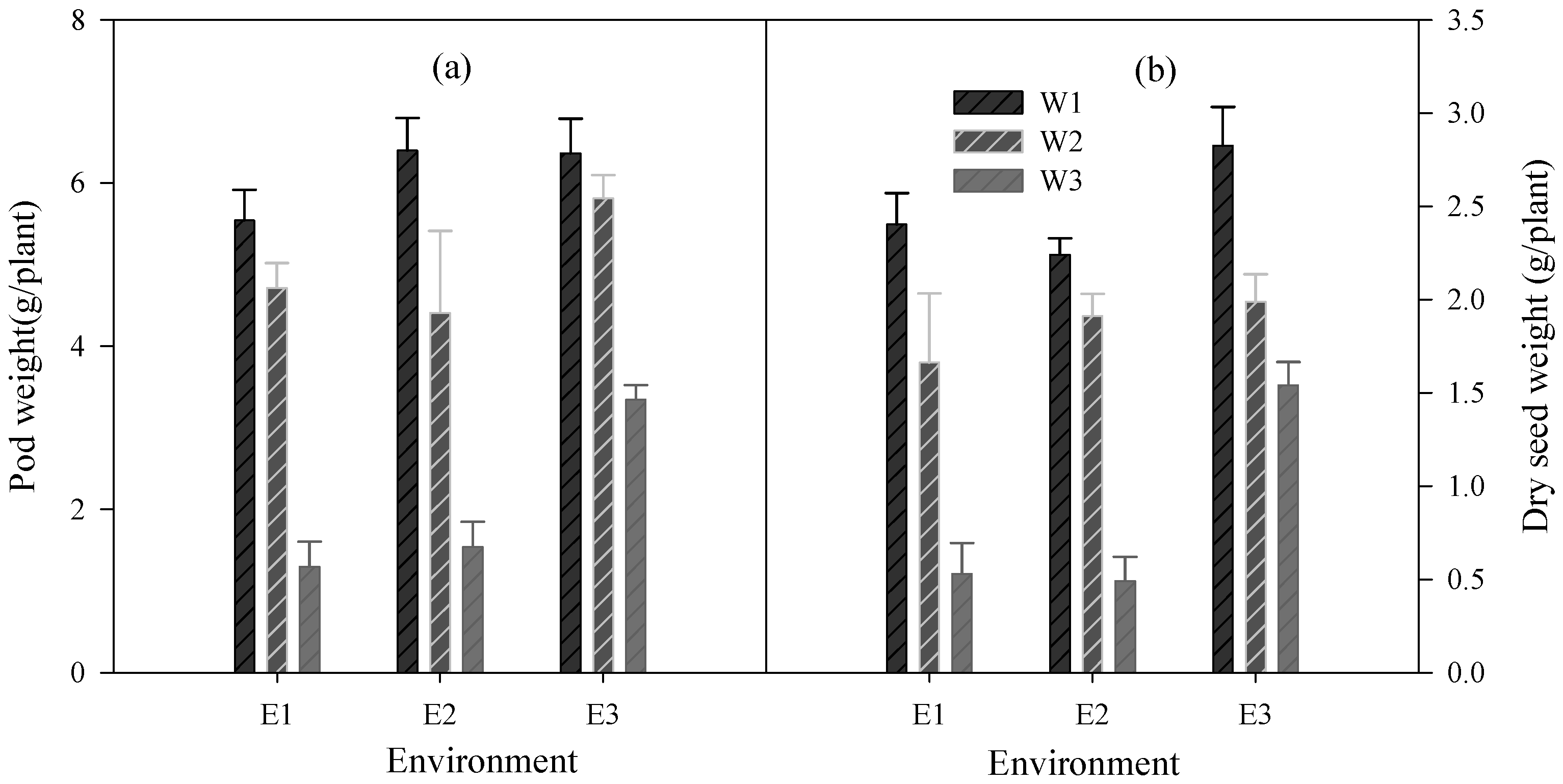
3.5. Effect of Water Treatments on Yield under Varied Temperature
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cairns, J.E.; Hellin, J.; Sonder, K.; Araus, J.L.; MacRobert, J.F.; Thierfelder, C.; Prasanna, B.M. Adapting maize production to climate change in sub-Saharan Africa. Food Secur. 2013, 5, 345–360. [Google Scholar] [CrossRef]
- Asante, F.A.; Amuakwa-Mensah, F. Climate Change and Variability in Ghana: Stocktaking. Climate 2015, 3, 78–99. [Google Scholar] [CrossRef]
- Cameron, C. Climate Change Financing and Aid Effectiveness: Ghana Case Study. 2011. Available online: http://www.agulhas.co.uk. (accessed on 2 February 2018).
- MESTI. Ghana National Climate Change Policy; Ghana Ministry of Environment, Science, Technology and Innovation (MESTI): Accra, Ghana, 2013. [Google Scholar]
- Zougmoré, R.; Partey, S.; Ouédraogo, M.; Omitoyin, B.; Thomas, T.; Ayantunde, A.; Ericksen, P.; Said, M.; Jalloh, A. Toward climate-smart agriculture in West Africa: A review of climate change impacts, adaptation strategies and policy developments for the livestock, fishery and crop production sectors. Agric. Food Secur. 2016, 5, 26. [Google Scholar] [CrossRef]
- Eze, P.N. Characterization, Classification and Pedogenesis of Soil on a Legon Catena in the Accra Plains, Ghana. Master’s Thesis, Department of Soil Science, University of Ghana, Legon, Ghana, 2008. [Google Scholar]
- Senayah, J.; Kufogbe, S.; Dedzoe, C. Land degradation in the Sudan Savanna of Ghana: A case study in the Bawku Area. West Afr. J. Appl. Ecol. 2005, 8. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Boote, K.J.; Kimball, B.A.; Ziska, L.H.; Izaurralde, R.C.; Ort, D.; Wolfe, D. Climate impacts on agriculture: Implications for crop production. Agron. J. 2011, 103, 351–370. [Google Scholar] [CrossRef]
- Hatfield, J.L.; Prueger, J.H. Temperature extremes: Effect on plant growth and development. Weather Clim. Extrem. 2015, 10, 4–10. [Google Scholar] [CrossRef]
- Paembonan, S.A.; Hagihara, A.; Hozumi, K. Long-term respiration in relation to growth and maintenance processes of the aboveground parts of a hinoki forest tree. Tree Physiol. 1992, 10, 101–110. [Google Scholar] [CrossRef]
- Jumrani, K.; Bhatia, V.S. Impact of combined stress of high temperature and water deficit on growth and seed yield of soybean. Physiol. Mol. Biol. Plants 2018, 24, 37–50. [Google Scholar] [CrossRef]
- Prasad, P.V.V.; Pisipati, S.R.; Momčilović, I.; Ristic, Z. Independent and Combined Effects of High Temperature and Drought Stress During Grain Filling on Plant Yield and Chloroplast EF-Tu Expression in Spring Wheat. J. Agron. Crop. Sci. 2011, 197, 430–441. [Google Scholar] [CrossRef]
- Amatekpor, J.K.; Oteng, J.W.; Agyiri, P. Field tour guide: Technical Center for Agricultural and Rural Co-operation (CTA). In Proceedings of the Sustaining Soil Productivity in Intensive African Agriculture, Accra, Ghana, 15–19 November 1993. [Google Scholar]
- Amatekpor, J.K.; Dowuona, G.N.N. Site Characterization. IBSRAM Vertisol Project; Department of Soil Science, University of Ghana: Legon, Ghana, 1995; p. 42. [Google Scholar]
- SARI. New Soybean Varieties Released. GNA. 2003. Available online: http://www.ghanaweb.com/GhanaHomepage/NewsArchive/artikel.php?ID=47098. (accessed on 15 February 2018).
- Asafo-Adjei, B.; Ansah, I.O.O.; Asuboah, R.A.; Dapaah, H.; Harruna, M.; Oti-Boateng, C. Soybean Production Guide; CSIR (CRI and SARI): Kumasi, Ghana, 2005; pp. 76–85. [Google Scholar]
- SARI. Soybean: A Production Guide for Northern Ghana; Alliance for Green Revolution in Africa Soil Health Project 2009 SHP 005; SARI: Nyankpala, Ghana, 2012; pp. 4–7. [Google Scholar]
- Berry, F.A.; Bolly, E.; Beers, N.R. Hand Book of Meteorology; Mcgraw-Hill Book Company, Inc.: New York, NY, USA, 1945. [Google Scholar]
- Hundal, S.S.; Singh, H.; Dhaliwal, L. Agroclimatic models for growth and yield of soybean (Glycine max). Indian J. Agric. Sci. 2003, 73, 668–670. [Google Scholar]
- Tachie-Obeng, E.; Akponikpe, P.B.I.; Adiku, S. Considering effective adaptation options to impacts of climate change for maize production in Ghana. Environ. Dev. 2013, 5, 131–145. [Google Scholar] [CrossRef]
- Freduah, B.S.; MacCarthy, D.S.; Adam, M.; Ly, M.; Ruane, A.C.; Timpong-Jones, E.C.; Traore, P.S.; Boote, K.J.; Porter, C.; Adiku, S.G.K. Sensitivity of Maize Yield in Smallholder Systems to Climate Scenarios in Semi-Arid Regions of West Africa: Accounting for Variability in Farm Management Practices. Agronomy 2019, 9, 639. [Google Scholar] [CrossRef]
- Meehl, G.A.; Meehl, G.A.; Stocker, T.F.; Collins, W.D.; Friedlingstein, P.; Gaye, T.; Noda, A. Global climate projections. In Climate Change 2007: The Physical Science Basis; Solomon, S., Qin, D., Manning, M., Chen, Z., Marquis, M., Averyt, K.B., Tignor, M., Miller, H.L., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007; pp. 747–845. [Google Scholar]
- IPCC. Contribution of Working Groups I, II and III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Reisinger, A., Eds.; Climate Change 2007: Synthesis Report; IPCC: Geneva, Switzerland, 2007; p. 104. [Google Scholar]
- Viana, J.S.; Gonçalves, E.P.; Silva, A.C.; Matos, V.P. Climatic conditions and production of soybean in northeastern Brazil. In A Comprehensive Survey of International Soybean Research-Genetics, Physiology, Agronomy and Nitrogen Relationships; InTech: London, UK, 2013; pp. 377–392. [Google Scholar]
- Hoeft, R.G.; Nafziger, E.D.; Johnson, R.R.; Aldrich, S.R. Soybean as a crop. In Modern Corn and Soybean Production; RR Donnelley and Sons: Chicago, IL, USA, 2000; pp. 31–59. [Google Scholar]
- Thornton, P.K.; Ericksen, P.J.; Herrero, M.; Challinor, A.J. Climate variability and vulnerability to climate change: A review. Glob. Chang. Biol. 2014, 20, 3313–3328. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q. Performance of agro-climate indices and wheat grain yield in a changing climate. Clim. Res. 2016, 69, 143–154. [Google Scholar] [CrossRef]
- Kumar, A.; Pndey, V.; Shekh, M.A.; Kumar, M. Growth and Yield Response of Soybean (Glycine max L.) in Relation to Temperature, Photoperiod and Sunshine Duration at Anand, Gujurat, India. Am.-Eurasian J. Sustain. Agric. 2008, 1, 45–50. [Google Scholar]
- Gibson, L.R.; Mullen, R.E. Influence of day and night temperature on soybean seed yield. Crop. Sci. 1996, 36, 98–104. [Google Scholar] [CrossRef]
- Qaderi, M.M.; Kurepin, L.V.; Reid, D.M. Growth and physiological responses of canola (Brassica napus L.) to three components of global climate change: Temperature, carbon dioxide and drought. Physiol. Plant. 2006, 128, 710–721. [Google Scholar] [CrossRef]
- Hesketh, J.D.; Myhre, D.L.; Willey, C.R. Temperature control of time intervals between vegetative and reproductive events in soybeans 1. Crop. Sci. 1973, 13, 250–254. [Google Scholar] [CrossRef]
- Tenorio, F.A.M. Temperature Control of Node Appearance and Initiation in Soybean. Master Thesis, University of Nebraska-Lincoln, Lincoln, NE, USA, 2016. [Google Scholar]
- Dreesen, P.E.; De Boeck, H.J.; Janssens, I.A.; Nijs, I. Summer heat and drought extremes trigger unexpected changes in productivity of a temperate annual/biannual plant community. Environ. Exp. Bot. 2012, 79, 21–30. [Google Scholar] [CrossRef]
- Bastidas, A.M.; Setiyono, T.D.; Dobermann, A.; Cassman, K.G.; Elmore, R.W.; Graef, G.L.; Specht, J.E. Soybean sowing date: The vegetative, reproductive, and agronomic impacts. Crop. Sci. 2008, 48, 727–740. [Google Scholar] [CrossRef]
- Qaseem, M.F.; Qureshi, R.; Shaheen, H. Effects of Pre-Anthesis Drought, Heat and Their Combination on the Growth, Yield and Physiology of diverse Wheat (Triticum aestivum L.) Genotypes Varying in Sensitivity to Heat and 0drought stress. Sci. Rep. 2019, 9, 6955. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, N.; Hussain, S.; Raza, M.A.; Yang, C.-Q.; Safdar, M.E.; Brestic, M.; Aziz, A.; Hayyat, M.S.; Asghar, M.A.; Wang, X.C.; et al. Drought Tolerance of Soybean (Glycine max L. Merr.) by Improved Photosynthetic Characteristics and an Efficient Antioxidant Enzyme Activities Under a Split-Root System. Front. Physiol. 2019, 10, 786. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Jiang, Y.; Dong, Y.; Wang, L.; Wang, W.; Ma, Z.; Yan, C.; Ma, C.; Liu, L. A study on soybean responses to drought stress and rehydration. Saudi J. Biol. Sci. 2019, 26, 2006–2017. [Google Scholar] [CrossRef]
- De Souza, P.I.; Egli, D.B.; Bruening, W.P. Water Stress during Seed Filling and Leaf Senescence in Soybean. Agron. J. 1997, 89, 807–812. [Google Scholar] [CrossRef]
- Zhu, X.G.; Long, S.P.; Ort, D.R. Improving Photosynthetic Efficiency for Greater Yield. Annu. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef]
- Long, S.P.; Ort, D.R. More than taking the heat: Crops and global change. Curr. Opin. Plant Biol. 2010, 13, 240–247. [Google Scholar] [CrossRef]

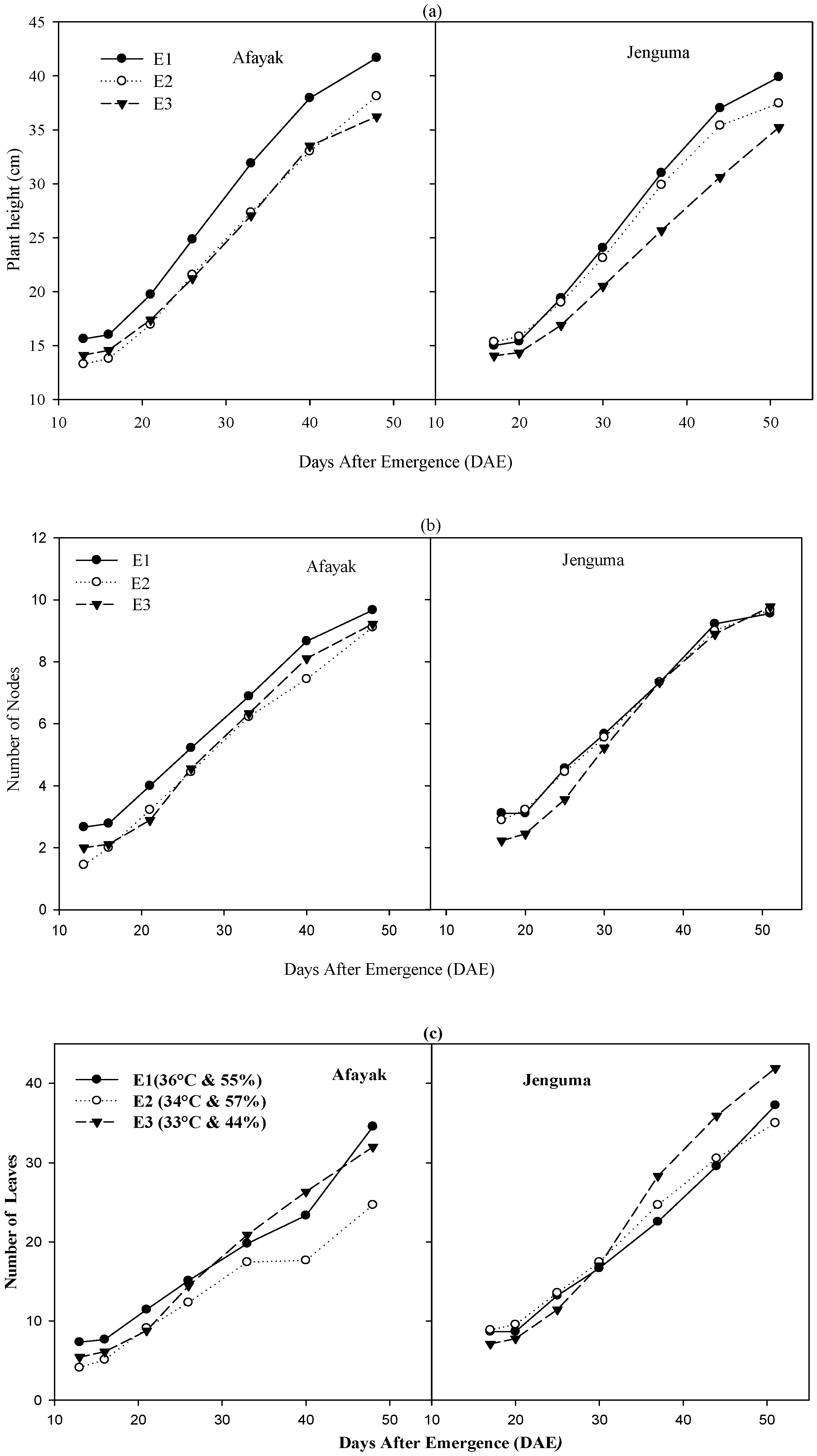
| Environment | Variety | Flowering | Podding | Maturity | |||
|---|---|---|---|---|---|---|---|
| CT (DAE) | TT (°Cd) | CT (DAE) | TT (°Cd) | CT (DAE) | TT (°Cd) | ||
| E1 (36 °C) | Afayak | 37 | 894 | 48 | 1176 | 97 | 2416 |
| E1 (36 °C) | Jenguma | 39 | 928 | 52 | 1265 | 101 | 2506 |
| E2 (34 °C) | Afayak | 38 | 895 | 50 | 1194 | 104 | 2530 |
| E2 (34 °C) | Jenguma | 40 | 935 | 52 | 1234 | 101 | 2429 |
| E3 (33 °C) | Afayak | 40 | 893 | 50 | 1134 | 97 | 2271 |
| E3 (33 °C) | Jenguma | 41 | 913 | 52 | 1174 | 101 | 2360 |
| Environment (Envt) | 0.001 | 0.123 | 0.001 | ||||
| Variety (Var) | 0.003 | 0.001 | 0.001 | ||||
| Envt × Var | 0.604 | 0.178 | 0.001 | ||||
| Environment (Envt) | Variety (Var) | Vegetative (g/Plant) | Flowering (g/Plant) | Podding (g/Plant) |
|---|---|---|---|---|
| E1 (36 °C) | Afayak | 0.85 | 4.01 | 4.57 |
| E2 (34 °C) | Afayak | 2.18 | 3.52 | 6.07 |
| E3 (33 °C) | Afayak | 1.83 | 5.22 | 7.48 |
| E1 (36 °C) | Jenguma | 2.17 | 4.69 | 5.53 |
| E2 (34 °C) | Jenguma | 2.6 | 5.06 | 6.62 |
| E3 (33 °C) | Jenguma | 2.82 | 6.04 | 8.63 |
| Envt | 0.67 | 0.95 | 0.35 | |
| lsd (0.05) | Var | 0.61 | 0.65 | 0.29 |
| Envt × Var | 0.95 | 1.18 | 0.43 |
| Environment (Envt) | Water | Variety (Var) | Dry Seed Weight (g/Plant) | Pod Weight (g/Plant) |
|---|---|---|---|---|
| E1 (36 °C) | 1 | 1 | 2.39 cd | 6.21 efg |
| 1 | 2 | 2.14 bcd | 5.09 defg | |
| 2 | 1 | 1.83 bc | 4.65 def | |
| 2 | 2 | 1.99 bcd | 4.78 defg | |
| 3 | 1 | 0.27 a | 0.88 a | |
| 3 | 2 | 0.63 a | 1.55 ab | |
| E2(34 °C) | 1 | 1 | 2.20 cd | 6.22 fg |
| 1 | 2 | 2.66 de | 6.62 fg | |
| 2 | 1 | 1.51 b | 4.61 de | |
| 2 | 2 | 1.76 bc | 4.29 de | |
| 3 | 1 | 0.68 a | 1.75 abc | |
| 3 | 2 | 0.38 a | 1.33 a | |
| E3 (33 °C) | 1 | 1 | 2.29 bcd | 5.71 efg |
| 1 | 2 | 3.15 e | 6.76 g | |
| 2 | 1 | 1.96 bcd | 4.43 de | |
| 2 | 2 | 2.03 bcd | 4.36 de | |
| 3 | 1 | 1.59 bc | 3.43 cd | |
| 3 | 2 | 1.49 b | 3.24 bcd | |
| L.S. D (0.05) | Envt | 0.28 | 0.70 | |
| Water | 0.28 | 0.70 | ||
| Var | 0.23 | 0.57 | ||
| Envt × Water | 0.48 | 1.22 | ||
| Envt × Var | 0.40 | 0.99 | ||
| Water × Var | 0.40 | 0.99 | ||
| Envt × Water × Var | 0.68 | 1.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ogunkanmi, L.; MacCarthy, D.S.; Adiku, S.G.K. Impact of Extreme Temperature and Soil Water Stress on the Growth and Yield of Soybean (Glycine max (L.) Merrill). Agriculture 2022, 12, 43. https://doi.org/10.3390/agriculture12010043
Ogunkanmi L, MacCarthy DS, Adiku SGK. Impact of Extreme Temperature and Soil Water Stress on the Growth and Yield of Soybean (Glycine max (L.) Merrill). Agriculture. 2022; 12(1):43. https://doi.org/10.3390/agriculture12010043
Chicago/Turabian StyleOgunkanmi, Labake, Dilys S. MacCarthy, and Samuel G. K. Adiku. 2022. "Impact of Extreme Temperature and Soil Water Stress on the Growth and Yield of Soybean (Glycine max (L.) Merrill)" Agriculture 12, no. 1: 43. https://doi.org/10.3390/agriculture12010043
APA StyleOgunkanmi, L., MacCarthy, D. S., & Adiku, S. G. K. (2022). Impact of Extreme Temperature and Soil Water Stress on the Growth and Yield of Soybean (Glycine max (L.) Merrill). Agriculture, 12(1), 43. https://doi.org/10.3390/agriculture12010043






