Inactivation of Salmonella enterica Serovar Enteritidis on Chicken Eggshells Using Blue Light
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Growth Conditions
2.2. In Vitro Bactericidal Test on S. Enteritidis
2.3. Preparation and Inoculation of S. Enteritidis onto Eggshells
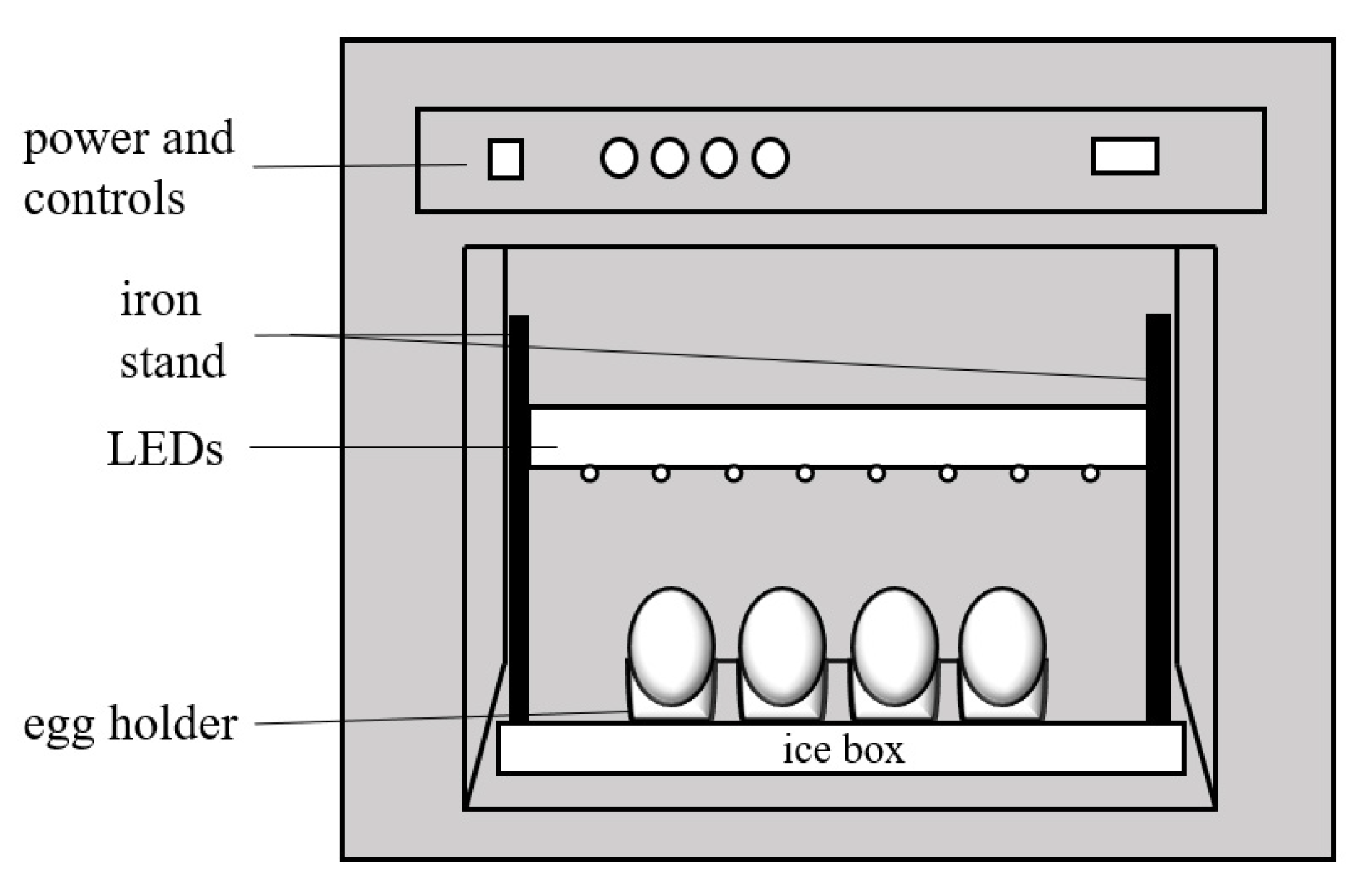
2.4. BL Irradiation on Eggshell
2.5. Enumeration of S. Enteritidis
2.6. Weight Loss
2.7. Yolk Index, HU and Albumen pH
2.8. Amino Acid Analysis of the Albumen
2.9. Statistical Analysis
3. Results and Discussion
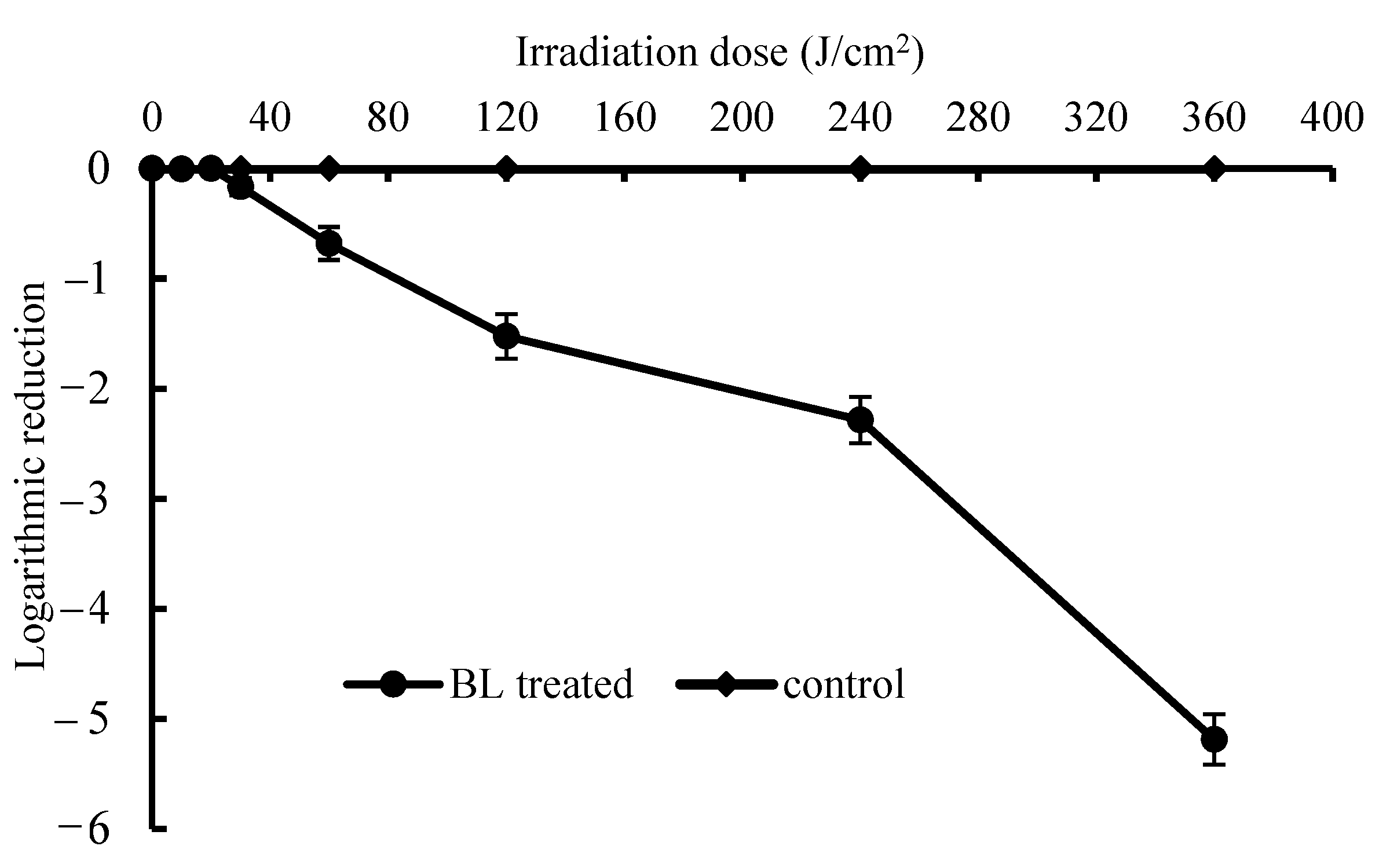
3.1. In Vitro Bactericidal Test on S. Enteritidis
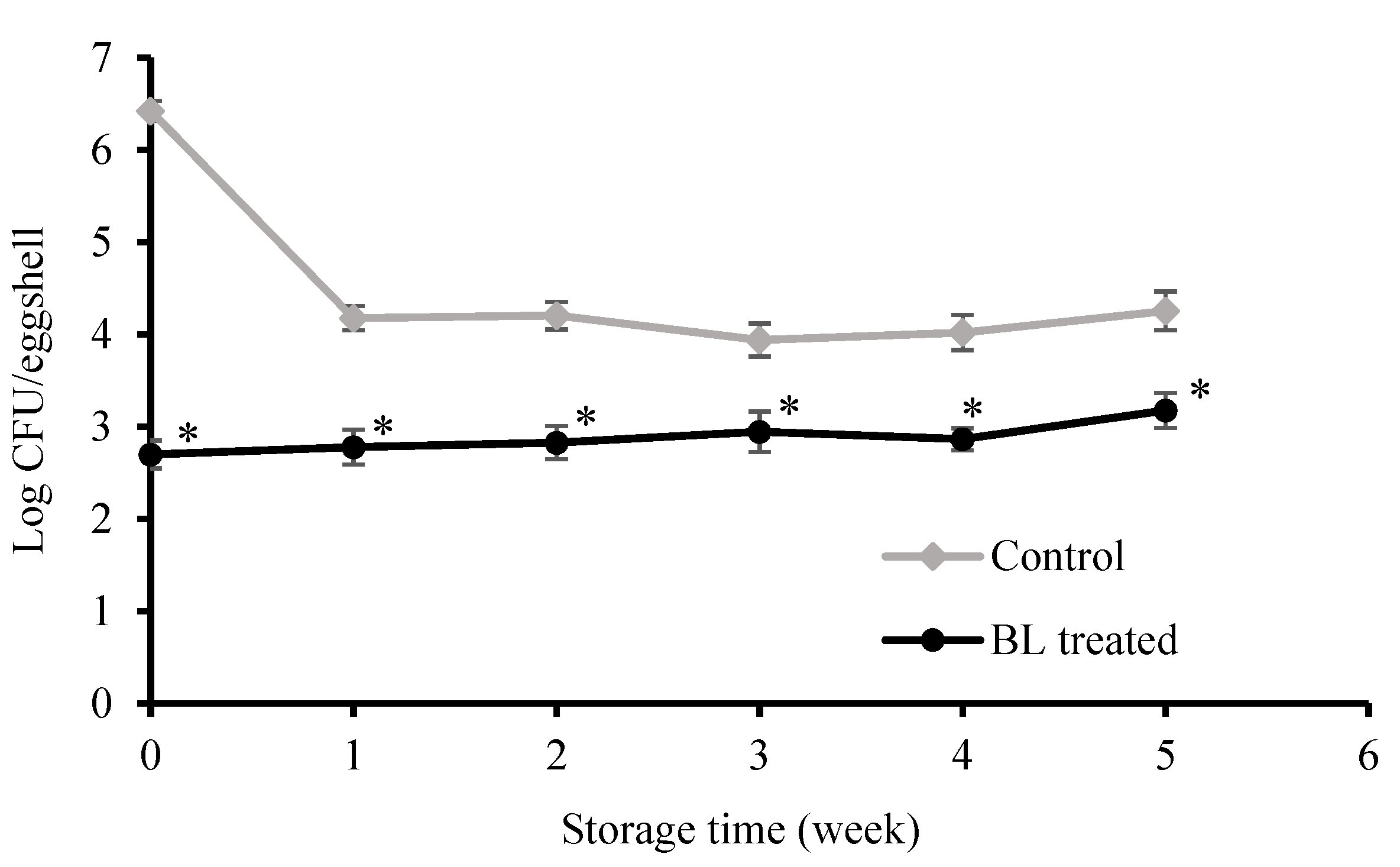
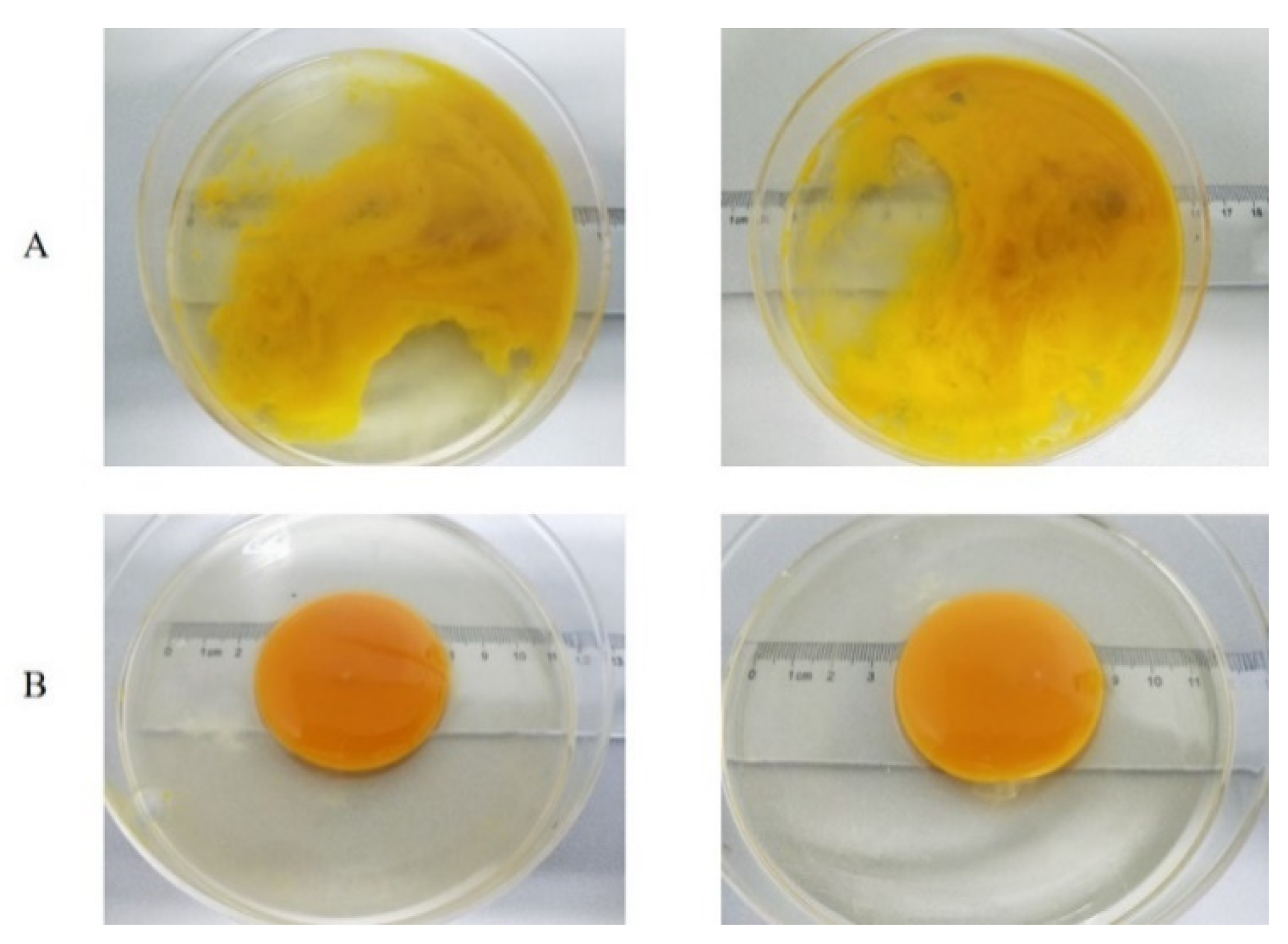
3.2. Bactericidal Effect on Eggshells
3.3. Weight Loss
3.4. Yolk Index
3.5. HU
3.6. Albumen pH
3.7. Amino Acid Profiles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adeyeye, E.I. Nutritional values of the lipid composition of the free-range chicken eggs. Agric. Biol. J. N. Am. 2012, 3, 374–384. [Google Scholar] [CrossRef]
- Magdelaine, P.; Braine, A.; Gonnier, V.; Spiess, M.P. Science et Technologie de L’oeuf et des Ovoproduits; Nau, F., Guérin-Dubiard, C., Baron, F., Eds.; Editions Tec et Doc Lavoisier: Paris, France, 2010; pp. 1–36. [Google Scholar]
- Bing, S.; Zang, Y.T.; Li, Y.J.; Shu, D.Q. The synergistic effects of slightly acidic electrolyzed water and UV-C light on the inactivation of Salmonella enteritidis on contaminated eggshells. Poult. Sci. 2019, 98, 6914–6920. [Google Scholar] [CrossRef]
- Kawasaki, T.; Musgrove, M.T.; Murata, M.; Tominaga, N.; Kawamoto, S. Comparative study of shell swab and shell crush methods for the recovery of Salmonella from shell eggs. J. Food Saf. 2008, 28, 482–498. [Google Scholar] [CrossRef]
- Painter, J.A.; Hoekstra, R.M.; Ayers, T.; Tauxe, R.V.; Braden, C.R.; Angulo, F.J.; Griffin, P.M. Attribution of foodborne illnesses, hospitalizations, and deaths to food commodities by using outbreak data, United States, 1998–2008. Emerg. Infect. Dis. 2013, 19, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Holck, A.L.; Liland, K.H.; Dromtorp, S.M.; Carlehog, M.; Mc, L.A. Comparison of UV-C and pulsed UV light treatments for reduction of Salmonella, Listeria monocytogenes, and enterohemorrhagic Escherichia coli on eggs. J. Food Prot. 2018, 81, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Keklik, N.M.; Demirci, A.; Patterson, P.H.; Puri, V.M. Pulsed UV light inactivation of Salmonella Enteritidis on eggshells and its effects on egg quality. J. Food Prot. 2010, 73, 1408–1415. [Google Scholar] [CrossRef]
- Al-Ajeeli, M.N.; Taylor, T.M.; Alvarado, C.Z.; Coufal, C.D. Comparison of eggshell surface sanitization technologies and impacts on consumer acceptability. Poult. Sci. 2016, 95, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Romo, L.A.; Yousef, A.E. Inactivation of Salmonella enterica serovar Enteritidis on shell eggs by ozone and UV radiation. J. Food Prot. 2005, 68, 711–717. [Google Scholar] [CrossRef]
- Zang, Y.T.; Bing, S.; Li, Y.J.; Shu, D.Q.; Huang, A.M.; Wu, H.X.; Lan, L.T.; Wu, H.D. Efficacy of slightly acidic electrolyzed water on the microbial safety and shelf life of shelled eggs. Poult. Sci. 2019, 98, 5932–5939. [Google Scholar] [CrossRef]
- Dai, T.; Gupta, A.; Murray, C.K.; Vrahas, M.S.; Tegos, G.P.; Hamblin, M.R. Blue light for infectious diseases: Propionibacterium acnes, Helicobacter pylori, and beyond? Drug Resist. Updates 2012, 15, 223–236. [Google Scholar] [CrossRef] [Green Version]
- Chu, Z.; Hu, X.; Wang, X.; Wu, J.; Dai, T.; Wang, X. Inactivation of Cronobacter sakazakii by blue light illumination and the resulting oxidative damage to fatty acids. Can. J. Microbiol. 2019, 65, 922–929. [Google Scholar] [CrossRef]
- Josewin, S.W.; Kim, M.J.; Yuk, H.G. Inactivation of Listeria monocytogenes and Salmonella spp. on cantaloupe rinds by blue light emitting diodes (LEDs). Food Microbiol. 2018, 76, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Bang, W.S.; Yuk, H.G. 405 ± 5 nm light emitting diode illumination causes photodynamic inactivation of Salmonella spp. on fresh-cut papaya without deterioration. Food Microbiol. 2017, 62, 124–132. [Google Scholar] [CrossRef]
- Dos Anjos, C.; Sellera, F.P.; de Freitas, L.M.; Gargano, R.G.; Telles, E.O.; Freitas, R.O.; Baptista, M.S.; Ribeiro, M.S.; Lincopan, N.; Pogliani, F.C.; et al. Inactivation of milk-borne pathogens by blue light exposure. J. Dairy Sci. 2019, 103, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Guffey, J.S.; Payne, W.C.; Motts, S.D.; Towery, P.; Hobson, T.; Harrell, G.; Meurer, L.; Lancaster, K. Inactivation of Salmonella on tainted foods: Using blue light to disinfect cucumbers and processed meat products. Food Sci. Nutr. 2016, 4, 878–887. [Google Scholar] [CrossRef] [PubMed]
- Hyun, J.E.; Lee, S.Y. Antibacterial effect and mechanisms of action of 460–470nm light-emitting diode against Listeria monocytogenes and Pseudomonas fluorescens on the surface of packaged sliced cheese. Food Microbiol. 2020, 86, 103314. [Google Scholar] [CrossRef]
- Wu, J.; Chu, Z.; Ruan, Z.; Wang, X.; Dai, T.; Hu, X. Changes of intracellular porphyrin, reactive oxygen species, and fatty acids profiles during inactivation of methicillin-resistant Staphylococcus aureus by antimicrobial blue light. Front. Physiol. 2018, 9, 1658. [Google Scholar] [CrossRef]
- Chen, J.; Thesmar, H.S.; Kerr, W.L. Outgrowth of Salmonellae and the physical property of albumen and vitelline membrane as influenced by egg storage conditions. J. Food Prot. 2005, 68, 2553–2558. [Google Scholar] [CrossRef]
- Kim, S.H.; Youn, D.K.; No, H.K.; Choi, S.W.; Prinyawiwatkul, W. Effects of chitosan coating and storage position on quality and shelf life of eggs. Int. J. Food Sci. Tech. 2009, 44, 1351–1359. [Google Scholar] [CrossRef]
- Haugh, R.R. The Haugh unit for measuring egg quality. US. Egg Poult. Mag. 1937, 43, 552–555. [Google Scholar]
- Koros, A.; Varga, Z.; Molnar-Perl, I. Simultaneous analysis of amino acids and amines as their o-phthalaldehyde-ethanethiol-9-fluorenylmethyl chloroformate derivatives in cheese by high-performance liquid chromatography. J. Chromatogr. A 2008, 1203, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Yuk, H.G. Antibacterial mechanism of 405-nanometer light-emitting diode against Salmonella at refrigeration temperature. Appl. Environ. Microbiol. 2017, 83, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottselig, S.M.; Dunn-Horrocks, S.L.; Woodring, K.S.; Coufal, C.D.; Duong, T. Advanced oxidation process sanitization of eggshell surfaces. Poult. Sci. 2016, 95, 1356–1362. [Google Scholar] [CrossRef]
- Allende, A.; Tomas-Barberan, F.A.; Gil, M.I. Minimal processing for healthy traditional foods. Trends Food Sci. Technol. 2006, 17, 513–519. [Google Scholar] [CrossRef]
- Trinetta, V.; Vaidya, N.; Linton, R.; Morgan, M. Evaluation of chlorine dioxide gas residues on selected food produce. J. Food Sci. 2011, 76, T11–T15. [Google Scholar] [CrossRef]
- De Reu, K.; Grijspeerdt, K.; Messens, W.; Heyndrickx, M.; Uyttendaele, M.; Debevere, J.; Herman, L. Eggshell factors influencing eggshell penetration and whole egg contamination by different bacteria, including Salmonella enteritidis. Int. J. Food Microbiol. 2006, 112, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Pires, P.G.S.; Machado, G.S.; Franceschi, C.H.; Kindlein, L.; Andretta, I. Rice protein coating in extending the shelf-life of conventional eggs. Poult. Sci. 2019, 98, 1918–1924. [Google Scholar] [CrossRef] [PubMed]
- Bhale, S.D.; No, H.K.; Prinyawiwatkul, W.; Farr, A.J.; Nadarajah, K.; Meyers, S.P. Chitosan coating improves shelf life of eggs. J. Food Sci. 2003, 68, 2378–2383. [Google Scholar] [CrossRef]
- Caner, C.; Yuceer, M. Efficacy of various protein-based coating on enhancing the shelf life of fresh eggs during storage. Poult. Sci. 2015, 94, 1665–1677. [Google Scholar] [CrossRef]
- Yuceer, M.; Aday, M.S.; Caner, C. Ozone treatment of shell eggs to preserve functional quality and enhance shelf life during storage. J. Sci. Food Agric. 2016, 96, 2755–2763. [Google Scholar] [CrossRef]
- Gast, R.K.; Holt, P.S.; Murase, T. Penetration of Salmonella enteritidis and Salmonella heidelberg into egg yolks in an in vitro contamination model. Poult. Sci. 2005, 84, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Nongtaodum, S.; Jangchud, A.; Jangchud, K.; Dhamvithee, P.; No, H.K.; Prinyawiwatkul, W. Oil coating affects internal quality and sensory acceptance of selected attributes of raw eggs during storage. J. Food Sci. 2013, 78, S329–S335. [Google Scholar] [CrossRef] [PubMed]
- Lechevalier, V.; Jeantet, R.; Arhaliass, A.; Legrand, J.; Nau, F. Egg white drying: Influence of industrial processing steps on protein structure and functionalities. J. Food Eng. 2007, 83, 404–413. [Google Scholar] [CrossRef]
- Mishanina, T.V.; Libiad, M.; Banerjee, R. Biogenesis of reactive sulfur species for signaling by hydrogen sulfide oxidation pathways. Nat. Chem. Biol. 2015, 11, 457–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Catalogue | Samples | Week 0 | Week 1 | Week 2 | Week 3 1 | Week 4 1 | Week 5 2 |
|---|---|---|---|---|---|---|---|
| Weight loss | Control | NA | 0.84 ± 0.09eA | 1.29 ± 0.09dA | 1.88 ± 0.12cA | 2.32 ± 0.34bA | 3.88 ± 0.13aA |
| BL-treated | NA | 0.87 ± 0.08dA | 1.26 ± 0.16dA | 1.75 ± 0.19cA | 2.24 ± 0.26bA | 3.09 ± 0.23aB | |
| Yolk index | Control | 0.36 ± 0.04aA | 0.26 ± 0.01bA | 0.20 ± 0.01cA | 0.21 ± 0.01bcA | 0.19 ± 0.03cA | ND |
| BL-treated | 0.36 ± 0.04aA | 0.26 ± 0.01bA | 0.19 ± 0.01cA | 0.21 ± 0.02cA | 0.20 ± 0.02cA | 0.19 ± 0.01c | |
| Haugh unit | Control | 86.27 ± 5.89aA | 74.22 ± 1.43bB | 52.13 ± 0.51cB | 47.72 ± 1.14cB | 33.54 ± 2.82dB | ND |
| BL-treated | 86.27 ± 5.89aA | 79.00 ± 0.16aA | 58.57 ± 1.59bA | 55.21 ± 1.17bcA | 49.28 ± 3.28cdA | 45.66 ± 2.64d | |
| Albumen pH | Control | 8.92 ± 0.04cA | 9.54 ± 0.02aA | 9.34 ± 0.02bA | 9.04 ± 0.77cA | 9.28 ± 0.04bA | 8.95 ± 0.17cA |
| BL-treated | 8.92 ± 0.04eA | 9.48 ± 0.05aA | 9.32 ± 0.22abA | 9.04 ± 0.16deA | 9.26 ± 0.02bcA | 9.11 ± 0.01cdA |
| Amino Acid | Week 0 | Week 1 | Week 2 | Week 3 | Week 4 | Week 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BL-Treated | Control | BL-Treated | Control | BL-Treated | Control | BL-Treated | Control | BL-Treated | Control | ||
| Aspartic acid | 8.68 ± 0.28a | 8.61 ± 0.07a | 8.07 ± 0.02cd | 8.36 ± 0.03b | 8.01 ± 0.02cde | 8.16 ± 0.01bc | 7.95 ± 0.02cde | 8.09 ± 0.01c | 7.82 ± 0.02e | 7.87 ± 0.02de | 7.44 ± 0.03f |
| Glutamine | 12.35 ± 0.43a | 12.09 ± 0.09ab | 11.38 ± 0.02de | 11.83 ± 0.02bc | 11.18 ± 0.01def | 11.51 ± 0.02cd | 11.19 ± 0.01def | 11.29 ± 0.01de | 11.04 ± 0.01ef | 11.10 ± 0.15ef | 10.84 ± 0.04f |
| Serine | 4.57 ± 0.25a | 4.64 ± 0.06a | 4.24 ± 0.02de | 4.52 ± 0.01ab | 4.29 ± 0.02cde | 4.46 ± 0.02abc | 4.15 ± 0.01e | 4.36 ± 0.01bcd | 4.18 ± 0.02de | 4.29 ± 0.03bcde | 4.16 ± 0.03e |
| Histidine | 2.00 ± 0.09b | 2.13 ± 0.01a | 2.00 ± 0.01b | 1.94 ± 0.01bc | 1.89 ± 0.02cd | 1.88 ± 0.04cd | 1.94 ± 0.01bc | 1.87 ± 0.02cd | 1.89 ± 0.01cd | 1.91 ± 0.03c | 1.82 ± 0.02d |
| Glycine | 3.08 ± 0.22a | 3.02 ± 0.04ab | 2.84 ± 0.02cde | 2.96 ± 0.01abc | 2.71 ± 0.02e | 2.89 ± 0.01bcd | 2.78 ± 0.03de | 2.86 ± 0.01bcde | 2.69 ± 0.01e | 2.77 ± 0.05de | 2.76 ± 0.04de |
| Threonine | 3.51 ± 0.09a | 3.56 ± 0.05a | 3.28 ± 0.01a | 3.51 ± 0.02a | 3.19 ± 0.01a | 3.39 ± 0.02a | 3.21 ± 0.02a | 3.28 ± 0.04a | 3.17 ± 0.01a | 3.26 ± 0.04a | 3.19 ± 0.03a |
| Arginine | 4.97 ± 0.06a | 4.86 ± 0.04ab | 4.58 ± 0.02cd | 4.86 ± 0.02ab | 4.51 ± 0.02cde | 4.62 ± 0.01bc | 4.42 ± 0.02cde | 4.55 ± 0.02cd | 4.26 ± 0.01e | 4.44 ± 0.04cde | 4.33 ± 0.04de |
| Alanine | 5.21 ± 0.09a | 5.11 ± 0.03b | 4.76 ± 0.03ef | 4.93 ± 0.02c | 4.73 ± 0.01ef | 4.87 ± 0.02cd | 4.67 ± 0.01f | 4.81 ± 0.02de | 4.52 ± 0.01f | 4.69 ± 0.03f | 4.49 ± 0.03f |
| Tyrosine | 2.82 ± 0.08bc | 3.04 ± 0.04a | 2.87 ± 0.02bc | 2.93 ± 0.02ab | 2.57 ± 0.01f | 2.77 ± 0.11cd | 2.79 ± 0.01cd | 2.69 ± 0.01de | 2.58 ± 0.02ef | 2.78 ± 0.06cd | 2.54 ± 0.03f |
| Cysteine | 1.05 ± 0.07a | 0.98 ± 0.01ab | 0.86 ± 0.04c | 0.98 ± 0.01ab | 0.87 ± 0.01c | 0.97 ± 0.01b | 0.85 ± 0.01c | 0.95 ± 0.01b | 0.83 ± 0.01c | 0.87 ± 0.02c | 0.85 ± 0.04c |
| Valine | 6.26 ± 0.05a | 6.10 ± 0.02b | 5.75 ± 0.02de | 5.95 ± 0.01c | 5.69 ± 0.01f | 5.82 ± 0.02d | 5.61 ± 0.07f | 5.77 ± 0.02de | 5.53 ± 0.01f | 5.60 ± 0.05f | 5.44 ± 0.04f |
| Methionine | 3.38 ± 0.04a | 3.29 ± 0.01ab | 3.09 ± 0.08c | 3.22 ± 0.01b | 3.02 ± 0.01cd | 3.11 ± 0.01c | 2.98 ± 0.07de | 3.09 ± 0.01c | 2.88 ± 0.02e | 2.96 ± 0.04de | 2.56 ± 0.05f |
| Phenylalanine | 5.20 ± 0.05a | 5.05 ± 0.02b | 4.74 ± 0.01de | 4.85 ± 0.02c | 4.69 ± 0.01ef | 4.81 ± 0.04cd | 4.66 ± 0.11ef | 4.83 ± 0.02cd | 4.52 ± 0.01f | 4.61 ± 0.03f | 4.32 ± 0.02f |
| Isoleucine | 4.91 ± 0.04a | 4.76 ± 0.01b | 4.49 ± 0.04d | 4.67 ± 0.01c | 4.38 ± 0.02e | 4.53 ± 0.02d | 4.39 ± 0.02e | 4.42 ± 0.01e | 4.23 ± 0.02f | 4.38 ± 0.02e | 4.14 ± 0.03f |
| Leucine | 7.25 ± 0.07a | 7.09 ± 0.02b | 6.66 ± 0.06e | 6.91 ± 0.02c | 6.55 ± 0.0f | 6.77 ± 0.02d | 6.50 ± 0.03f | 6.63 ± 0.02ef | 6.35 ± 0.01f | 6.49 ± 0.04f | 6.33 ± 0.03f |
| Lysine | 5.92 ± 0.06a | 5.53 ± 0.01b | 5.45 ± 0.03b | 5.48 ± 0.02c | 5.21 ± 0.02f | 5.39 ± 0.01c | 5.06 ± 0.04f | 5.29 ± 0.0d | 5.01 ± 0.01f | 5.26 ± 0.06de | 5.14 ± 0.03f |
| Proline | 3.39 ± 0.07a | 3.26 ± 0.02b | 2.89 ± 0.01d | 3.22 ± 0.01b | 3.11 ± 0.02c | 3.18 ± 0.02bc | 2.61 ± 0.04e | 3.10 ± 0.01c | 2.59 ± 0.01ef | 3.07 ± 0.06c | 2.51 ± 0.02f |
| Total amino acid | 84.58 ± 0.35a | 82.19 ± 0.16b | 77.71 ± 0.1de | 81.12 ± 0.1b | 76.61 ± 0.13def | 79.13 ± 0.08c | 75.76 ± 0.31f | 77.88 ± 0.06cd | 74.09 ± 0.08f | 76.40 ± 0.32ef | 72.90 ± 0.27f |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, X.; Sun, X.; Luo, S.; Wu, S.; Chu, Z.; Zhang, X.; Liu, Z.; Wu, J.; Wang, X.; Liu, C.; et al. Inactivation of Salmonella enterica Serovar Enteritidis on Chicken Eggshells Using Blue Light. Agriculture 2021, 11, 762. https://doi.org/10.3390/agriculture11080762
Hu X, Sun X, Luo S, Wu S, Chu Z, Zhang X, Liu Z, Wu J, Wang X, Liu C, et al. Inactivation of Salmonella enterica Serovar Enteritidis on Chicken Eggshells Using Blue Light. Agriculture. 2021; 11(8):762. https://doi.org/10.3390/agriculture11080762
Chicago/Turabian StyleHu, Xiaoqing, Xiaoying Sun, Shuanghua Luo, Shuyan Wu, Zhaojuan Chu, Xiujuan Zhang, Zhaojun Liu, Jiaxin Wu, Xiaohong Wang, Chang Liu, and et al. 2021. "Inactivation of Salmonella enterica Serovar Enteritidis on Chicken Eggshells Using Blue Light" Agriculture 11, no. 8: 762. https://doi.org/10.3390/agriculture11080762
APA StyleHu, X., Sun, X., Luo, S., Wu, S., Chu, Z., Zhang, X., Liu, Z., Wu, J., Wang, X., Liu, C., & Wang, X. (2021). Inactivation of Salmonella enterica Serovar Enteritidis on Chicken Eggshells Using Blue Light. Agriculture, 11(8), 762. https://doi.org/10.3390/agriculture11080762








