Genetic Protection of Soft Wheat from Diseases in the Southern Ural of Russia and Virulence Variability of Foliar Pathogens
Abstract
:1. Introduction
2. Cultivars and Promising Lines of Soft Spring Wheat Produced in ChRIA
3. Variability of Pathogens’ Population Structure in Response to Genetically Protected Cultivar Production
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sysoev, A.D. Essays on the Physical Geography of the Chelyabinsk Region; Chelyabinskoie Knizhnoe Izd-Vo: Chelyabinsk, Russia, 1959; 205p. (In Russian) [Google Scholar]
- Levit, L.I. Southern Urals: Geography, Environmental Management; Yuzh.Ural.kn.izd-vo: Chelyabinsk, Russia, 2001; 245p. (In Russian) [Google Scholar]
- Ryub, V.K. Results and Prospects of Work on the Selection of Spring Wheat; Collection of Scientific Papers; Yuzh.Ural.kn.izd-vo: Chelyabinsk, Russia, 1970; Volume 3, pp. 150–170. (In Russian) [Google Scholar]
- Ryub, V.K. Some Results of Breeding Work with Spring Wheat as a Result of the Use of Samples from the World Collection of VIR in Hybridization; Proceeding of Scientific Papers; Chelyab. Experimental Station: Chelyabinsk, Russia, 1973; Volume 4, pp. 6–15. (In Russian) [Google Scholar]
- Expert and Analytical Center for Agribusiness. Available online: https://ab-centre.ru/page/selskoe-hozyaystvo-chelyabinskoy-oblasti (accessed on 10 May 2021).
- Agrovestnik. Available online: https://agrovesti.net/lib/industries/cereals/posevnye-ploshchadi-valovye-sbory-i-urozhajnost-pshenitsy-v-rossii-itogi-2018-goda.html (accessed on 10 May 2021).
- Tyunin, V.A. Ecological substantiation of requirements to breeds of soft spring wheat for Southern Ural. Orenbg. State Univ. Bull. 2005, 5, 122–124. (In Russian) [Google Scholar]
- Tyunin, V.A.; Shreyder, E.R. Features of the Technology of Selection of Soft Spring Wheat for Resistance to Carbohydrate-Protein Depletion of Seeds and Other Stresses in the Conditions of the Southern Urals; Russian Academy of Agricultural Sciences, State Scientific Institution Chelyabinsk Scientific Research, Institute of Agriculture: Chelyabinsk, Russia, 2010; 119p. (In Russian) [Google Scholar]
- Meshkova, L.V.; Rosseeva, L.P.; Shreyder, E.R.; Sidorov, A.V. Virulence of pathotypes of wheat leaf rust pathogen to TcLR 9 in the regions of Siberia and the Urals. In Proceedings of the the Second All-Russian Conference “Modern Problems of Plant Immunity to Harmful Organisms”, St. Petersburg, Russia, 29 September–2 October 2008; pp. 70–73. (In Russian). [Google Scholar]
- Gultyaeva, E.; Tyunin, V.; Shreyder, E.; Kushnirenko, I.; Shaydayuk, E.; Kovalenko, N.; Bondarenko, N.; Kolesova, M. Breeding of spring bread wheat for resistance to foliar diseases in the southern Ural. Russ. Agric. Sci. 2021, 8–12. [Google Scholar] [CrossRef]
- Gultyaeva, E.I.; Baranova, O.A.; Dmitriev, A.P. Virulence and population structure of Puccinia triticina in Russian Federation in 2007. Plant Prot. News 2009, 4, 33–38. (In Russian) [Google Scholar]
- Odintsova, I.G.; Agafonova, N.A.; Boguslavsky, R.L. Introgressive lines of common wheat with resistantance to leaf rust transferred from Aegilops speltoides, in Source material and problems of wheat and triticale breeding. Proc. Appl. Botany. Genet. Breed. 1991, 142, 106–110. (In Russian) [Google Scholar]
- Ibraimova, Z.K.I.; Tankimanova, M.K.; Bersimbaev, R.I. Chromosomal localization of the gamete-killing gene in the wheat line. Tsitol Genet. 2001, 35, 15–19. (In Russian) [Google Scholar]
- Tyunin, V.A.; Shreyder, E.R.; Gultyaeva, E.I.; Shaydayuk, E.L. Characteristics of virulence of Puccinia triticina populations and the potential of the Lr24, Lr25, LrSp genes for spring common wheat breeding in the Southern Ural. Vavilov J. Genet. Breed. 2017, 21, 523–529. [Google Scholar] [CrossRef]
- Adonina, I.G.; Leonova, I.N.; Badaeva, E.D.; Salina, E.A. Genotyping of hexaploid wheat varieties from different Russian regions. Russ. J. Genet. Appl. Res. 2017, 7, 6–13. [Google Scholar] [CrossRef]
- Gultyaeva, E.I.; Orina, A.S.; Gannibal, P.B.; Mitrofanova, O.P.; Odintsova, I.G.; Laikova, L.I. The effectiveness of molecular markers for the identification of Lr28, Lr35, and Lr47 genes in common wheat. Genetika 2014, 50, 147–156. [Google Scholar] [CrossRef]
- Marais, G.F.; Bekker, T.A.; Eksteen, A.; McCallum, B.D.; Fetch, T.G.; Marais, A. Attempts to remove gametocidal genes co-transferred to common wheat with rust resistance from Aegilops speltoides. Euphytica 2009, 171, 71–85. [Google Scholar] [CrossRef]
- Morgounov, A.; Pozherukova, V.; Kolmer, J.; Gultyaeva, E.; Abugalieva, A.; Chudinov, V.; Kuzmin, O.; Rasheed, A.; Rsymbetov, A.; Shepelev, S.; et al. Genetic basis of spring wheat resistance to leaf rust (Puccinia triticina) in Kazakhstan and Russia. Euphytica 2020, 216, 1–15. [Google Scholar] [CrossRef]
- Gultyaeva, E.I.; Shreyder, E.R.; Kushnirenko, I.Y.; Shaydayuk, E.L.; Kovalenko, N.M. Evaluation of advanced bread spring wheat lines for field and seedling resistance to foliar pathogens. In BIO Web of Conferences; EDP Sciences: Les Ulis, France, 2020. [Google Scholar] [CrossRef]
- Zhang, Z.; Friesen, T.L.; Simons, K.J.; Xu, S.S.; Faris, J.D. Development, identification, and validation of markers for marker-assisted selection against the Stagonospora nodorum toxin sensitivity genes Tsn1 and Snn2 in wheat. Mol. Breed. 2008, 23, 35–49. [Google Scholar] [CrossRef]
- Sibikeev, S.N.; Badaeva, E.D.; Gultyaeva, E.I.; Druzhin, A.E.; Shishkina, A.A.; Dragovich, A.Y.; Kroupin, P.Y.; Karlov, G.I.; Khuat, T.M.; Divashuk, M.G. Comparative analysis of Agropyron intermedium (Host) Beauv 6Ag i and 6Ag i 2 chromosomes in bread wheat cultivars and lines with wheat–wheatgrass substitutions. Russ. J. Genet. 2017, 53, 314–324. [Google Scholar] [CrossRef]
- Tyunin, V.; Shreyder, E.; Gultyaeva, E.; Shaydayuk, E. Virulence of leaf rust pathogen of wheat in South Ural. Plant Prot. News 2018, 1, 16–20. [Google Scholar] [CrossRef]
- Gultyaeva, E.I.; Shreyder, E.; Shaydayuk, E.; Bondarenko, N. Monitoring of virulence and phenotypes composition of Puccinia triticina population in southern ural in 2018. Plant Prot. News 2019, 2, 28–33. [Google Scholar] [CrossRef] [Green Version]
- Gultyaeva, E.I.; Kovalenko, N.M.; Shamanin, V.P.; Tyunin, V.A.; Shreyder, E.R.; Shaydayuk, E.L.; Morgunov, A.I. Population structure of leaf pathogens of common spring wheat in the west Asian regions of Russia and north Kazakhstan in 2017. Vavilov J. Genet. Breed. 2018, 22, 363–369. [Google Scholar] [CrossRef]
- Gultyaeva, E.I.; Shaydayuk, E.L.; Kosman, E.G. Regional and temporal differentiation of virulence phenotypes of Puccinia triticina from common wheat in Russia during the period 2001–2018. Plant Pathol. 2020, 69, 860–871. [Google Scholar] [CrossRef]
- Markelova, T.S. Study of population structure and variability of wheat leaf rust population in the Volga region. Agro XXI 2007, 4–6. Available online: https://www.agroxxi.ru/journal/20070406/20070406018.pdf (accessed on 26 July 2021). (In Russian).
- Zhemchuzhina, A.; Kiseleva, M.; Zhemchuzhina, N.; Belyakova, S. Virulence of Puccinia triticina Erikss. Population in non-chernozem area of Russia. Agrar. Sci. 2019, 326, 137–141. (In Russian) [Google Scholar] [CrossRef]
- Long, D.L.; Kolmer, J.A. A North American system of nomenclature for Puccinia recondita f. sp. tritici. Phytopathology 1989, 79, 525–529. [Google Scholar] [CrossRef]
- Skolotneva, E.S.; Kosman, E.; Patpour, M.; Kelbin, V.N.; Morgounov, A.I.; Shamanin, V.P.; Salina, E.A. Virulence phenotypes of siberian wheat stem rust population in 2017–2018. Front. Agron. 2020, 2, 6. [Google Scholar] [CrossRef]
- Lamari, L.; Gilbert, J.; Tekauz, A. Race differentiation in Perynophora tritici-repentis and survey of physiologic variation in western Canada. Can. J. Plant Pathol. 1998, 20, 396–400. [Google Scholar] [CrossRef]
- Mikhailova, L.A.; Kokorina, N.M.; Kopahnke, D. Genetics of tan spot (Pyrenophora tritici-repentis) resistance in spring wheat lines 181-5, Vicam“S”70, 292. Russ. Agric. Sci. 2003, 1, 20–22. (In Russian) [Google Scholar]
- Friesen, T.L.; Ali, S.; Klein, K.K.; Rasmussen, J.B. Population genetic analysis of a global collection of Pyrenophora tritici-repentis, causal agent of tan spot of wheat. Phytopathology 2005, 95, 1144–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.R.-C. Agropyron and Psathyrostachys. In Wild Crop Relatives: Genomic and Breeding Resources; Springer: Berlin, Germany, 2011; pp. 77–108. [Google Scholar] [CrossRef]
- Mikhailova, L.A.; Ternuk, I.G.; Mironenko, N.V. Characteristic of Pyrenophora tritici-repentis populations by their virulence. Mikol. Phytopathol. 2010, 44, 262–272. (In Russian) [Google Scholar]
- Manning, V.A.; Pandelova, I.; Dhillon, B.; Wilhelm, L.J.; Goodwin, S.; Berlin, A.M.; Figueroa, M.; Freitag, M.; Hane, J.; Henrissat, B.; et al. Comparative Genomics of a Plant-Pathogenic Fungus, Pyrenophora tritici-repentis, Reveals Transduplication and the Impact of Repeat Elements on Pathogenicity and Population Divergence. G3: Genes Genomes Genet. 2013, 3, 41–63. [Google Scholar] [CrossRef] [Green Version]
- Aboukhaddour, R.; Cloutier, S.; Ballance, G.M.; Lamari, L. Genome characterization ofPyrenophora tritici-repentisisolates reveals high plasticity and independent chromosomal location of ToxA and ToxB. Mol. Plant Pathol. 2009, 10, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Hafez, M.; Gourlie, R.; Despins, T.; Turkington, T.K.; Friesen, T.L.; Aboukhaddour, R. Parastagonospora nodorumand Related Species in Western Canada: Genetic Variability and Effector Genes. Phytopathology 2020, 110, 1946–1958. [Google Scholar] [CrossRef] [PubMed]
- Friesen, T.L.; Meinhardt, S.W.; Faris, J. The Stagonospora nodorum-wheat pathosystem involves multiple proteinaceous host-selective toxins and corresponding host sensitivity genes that interact in an inverse gene-for-gene manner. Plant J. 2007, 51, 681–692. [Google Scholar] [CrossRef]
- Gao, Y.; Faris, J.; Liu, Z.; Kim, Y.M.; Syme, R.; Oliver, R.P.; Xu, S.S.; Friesen, T.L. Identification and characterization of the SnTox6-Snn6 interaction in the Parastagonospora nodorum–wheat pathosystem. Mol. Plant-Microbe Interact. 2015, 28, 615–625. [Google Scholar] [CrossRef] [Green Version]
- Friesen, T.L.; Stukenbrock, E.H.; Liu, Z.; Meinhardt, S.; Ling, H.; Faris, J.; Rasmussen, J.B.; Solomon, P.; McDonald, B.; Oliver, R.P. Emergence of a new disease as a result of interspecific virulence gene transfer. Nat. Genet. 2006, 38, 953–956. [Google Scholar] [CrossRef]
- Andrie, R.M.; Pandelova, I.; Ciuffetti, L.M. A combination of phenotypic and genotypic characterization strengthens Pyrenophora tritici-repentis race identification. Phytopathology 2007, 97, 694–701. [Google Scholar] [CrossRef] [Green Version]
- Brown, S.P.; Hochberg, M.E.; Grenfell, B.T. Does multiple infection select for raised virulence? Trends Microbiol. 2002, 10, 401–405. [Google Scholar] [CrossRef]
- Van Baalen, M.; Sabelis, M.W. The scope for virulence management: A comment on Ewald’s view on the evolution of virulence. Trends Microbiol. 1995, 3, 414–416. [Google Scholar] [CrossRef] [Green Version]
- Abdullah, A.S.; Moffat, C.S.; Lopez-Ruiz, F.J.; Gibberd, M.R.; Hamblin, J.; Zerihun, A. Host-multi-pathogen warfare: Pathogen interactions in co-infected plants. Front. Plant. Sci. 2017, 8, 1806. [Google Scholar] [CrossRef] [Green Version]
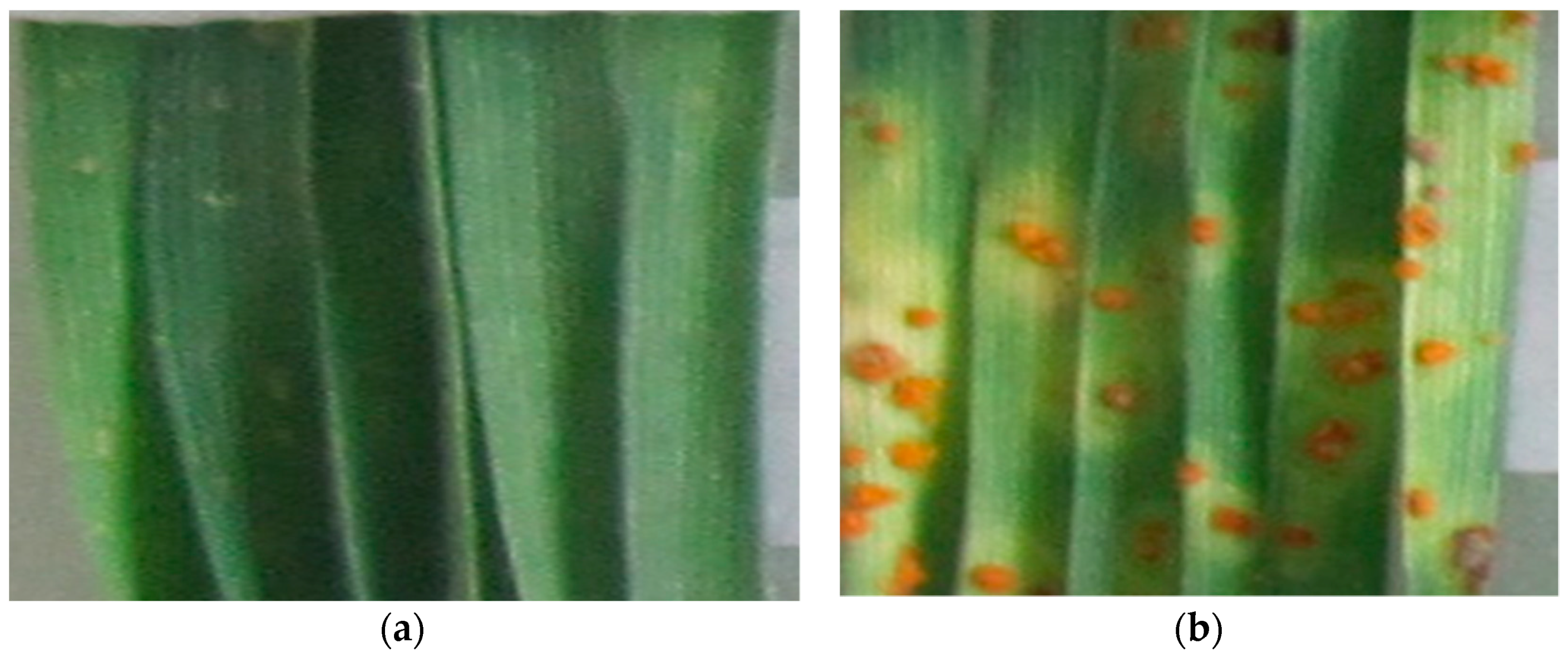
| Cultivar | Pedigree | Year of Involvement in the SRBA/ Transfer to SCT | Ripening Time | Grain Quality | Resistance Genes |
|---|---|---|---|---|---|
| Iskra | Milturum 321 × Kitchener | 1949 | late-ripe | weak | |
| Vesna | - | 1961 | mid-ripe | weak | |
| Ural’skaya 52 | Cesium 111 × Lutescens 324 | 1974 | mid-ripe | strong | |
| Rossiyanka | Saunders × Svenno | 1981 | mid-ripe | strong | |
| Uralochka | Svenno × (Lee × Kenya Farmer) | 1987 | mid-ripe | strong | |
| Eritrospermum 59 | Chayka × Irtyshanka 10 | 1994 | mid-ripe | strong | Lr10 |
| Izumrudnaya | Waldron × Ural’skaya 52 | 1996 | mid-ripe | filler | Lr26/Sr31/Pm8/Yr9 |
| Niva 2 | Ps 360/76 × Irtyshanka 10 | 1997 | mid-ripe | strong | |
| Duet | Eritrospermum 59 × (Tselinnaya 20 × ANK-02) | 2003 | mid-ripe | valuable | Lr9 Lr10 |
| Chelyaba 2 | {(Tezpishar × Irtyshanka 10) × Irtushanka 10} × Tselinnaya 20 × ANK-102) | 2005 | mid-early | valuable | Lr9 Lr10 |
| Pamyati Ryuba | Tertsiya × Eritrospermum 19542 | 2006 | mid-ripe | valuable | Lr9 Lr10 |
| Chelyaba yubileynaya | Eritrospermum 59 × Tertsiya | 2010 | mid-late | filler | Lr9 Lr10 |
| Chelyaba stepnaya | Eritrospermum 59 × Tertsiya | 2011 | mid-early | valuable | Lr9 Lr10 |
| Chelyaba 75 | Chernyava 13 × Eritrospermum 21338 | 2012 | mid-ripe | valuable | Lr1 Lr10 LrSp |
| Ural’skaya kukushka | Lutescens 4 × Tulunskaya10 × Lutescens 22178 | 2016 | mid-early | filler | |
| Chelyaba rannyaya | Chelyaba 2 × ANK-104 | 2016 | early-ripe | filler | Lr9 Lr10 |
| Silach | Lutescens 210/99-10 × Eritrospermum 23090 | 2020 | mid-late | filler | Lr10 Lr9 Lr26/Sr31/Pm8/Yr9 |
| Chelyaba 80 | Cuckoo line 210 × Rossiyanka × Novosibirskaya 15 | SCT 2017 | mid-late | valuable | LrSp |
| Il’menskaya 2 | Chelyaba 75 × (Chelyaba 2 × Fori 7) | SCT 2018 | mid-early | valuable | LrSp |
| Odintsovskaya | Chelyaba 75 × ANK-17B | SCT 2018 | early-ripe | valuable | LrSp |
| Chelyabinka | Vatan × Duet | SCT 2021 | mid-ripe | valuable | Lr9 Lr26 |
| Wheat Line/ Cultivar | Idenified Resistance Genes | Reaction Type to Foliar Wheat Pathogens at the Seedling Stage | Disease Severity in the Field (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Puccinia triticina | Puccinia graminis | Parastagonospora nodorum | Parastagonospora avenae f. sp. tritici | Pyrenophora tritici-repentis | Stem Rust | Leaf Rust | Septoria Leaf Blotch | Tan Spot | ||||||
| PtK1 | PtK2 | PtK3 | Pg1 | Pn | Pa | Ptr ToxA+ | Ptr ToxA− | Ptr ToxB+ | ||||||
| Lut. 26534 | 3 | 0–1 | 3 | 3–4 | 5 | 3–4 | 2–3/2–3 | 2–3/2–3 | 1–2/1–2 | 1–5 MS | 0 | 5 | 1 | |
| Er. 26596 | Lr10 LrSp/SrSp Lr34/Yr18/Sr57/Pm38 | 0 | 0 | 0 | 1–2 | 2–3 | 2–3 | 2–3/2–3 | 2–3/2–3 | 2–3/2–3 | 01 MR | 0 | 5 | 1 |
| Lut. 26708 | Lr3 Lr10 Lr6Agi2 | 0–1 | 0 | 0–1 | 0–1 | 3–4 | 3 | 3/3–4 | 2–3/2–3 | 2–3/2–3 | 0 | 0 | 0 | 0 |
| Lut. 26720 | Lr1 Lr3 Lr10 Tsn1 | 3 | 3 | 3 | 0–1 | 5 | 2–3/2–3 | 2/2 | 1/1 | 0 | 0 | 5 | 0 | |
| Er. 26725 | Lr24/Sr24 | 0 | 0 | 0 | 0 | 5 | 4 | 1–2/1–2 | 3/3 | 1–2/1 | 0 | 0 | 5 | 10 |
| Ferr. 26727 | Lr10 Lr24/Sr24 | 0 | 0 | 0 | 0–1 | 5 | 4 | 1–2/1–2 | 1–2/1–2 | 1–2/1–2 | 0 | 0 | 15 | 1 |
| Er. 26762 | Lr26/Sr31/Pm8/Yr9 Sr35 | 0–1 | 0 | 0 | 0–1 | 5 | 3 | 1–2/1–2 | 1–2/1–2 | 1–2/1–2 | 0 | 0 | 1 | 1 |
| Er. 26775 | Lr10 | 1–2 | 3 | 3 | 0 | 3–4 | 3 | 2–3/2–3 | 2–3/2–3 | 2–3/2–3 | 0 | 0 | 5 | 0 |
| M. 26690 | Lr1 Lr3 Lr10 | 0 | 0–1 | 0–1 | 0 | 3–4 | 1–2 | 1/1 | 1/1 | 1/1 | 0 | 0 | 20 | 1 |
| Ferr. 26757 | Lr1 Lr3 Lr10 LrSp/SrSp | 0 | 0 | 0 | 1–2 | 5 | 3 | 2–3/2–3 | 2–3/2–3 | 2–3/2–3 | 0 | 0 | 10 | 10 |
| Ferr. 26774 | Lr10 Lr21 Lr34/Yr18/Sr57/Pm38 | 0–1 | 0 | 0 | 0 | 2–3 | 1–2 | 2–3/2–3 | 2–3/2–3 | 1–2/2 | 0 | 0 | 5 | 0 |
| Chelyaba 75 | Lr1 Lr10 LrSp/SrSp | 0 | 0 | 0 | 1–2 | 3–4 | 2–3 | 2–3/2–3 | 2–3/2–3 | 2–3/2–3 | 1 MR | 0 | 10 | 1 |
| Chelyaba yubileynaya | Lr9 Lr10 | 3 | 0 | 0 | 3 | 4 | 3 | 3/3 | 3/3 | 2–3/2–3 | 20 S | 10–20 S | 5 | 5 |
| Er. 59 | 3–4 | 3–4 | 3–4 | 3–4 | 5 | 3 | 3/3 | 3/3 | 3/3 | 30 S | 70 S | 20 | 10 | |
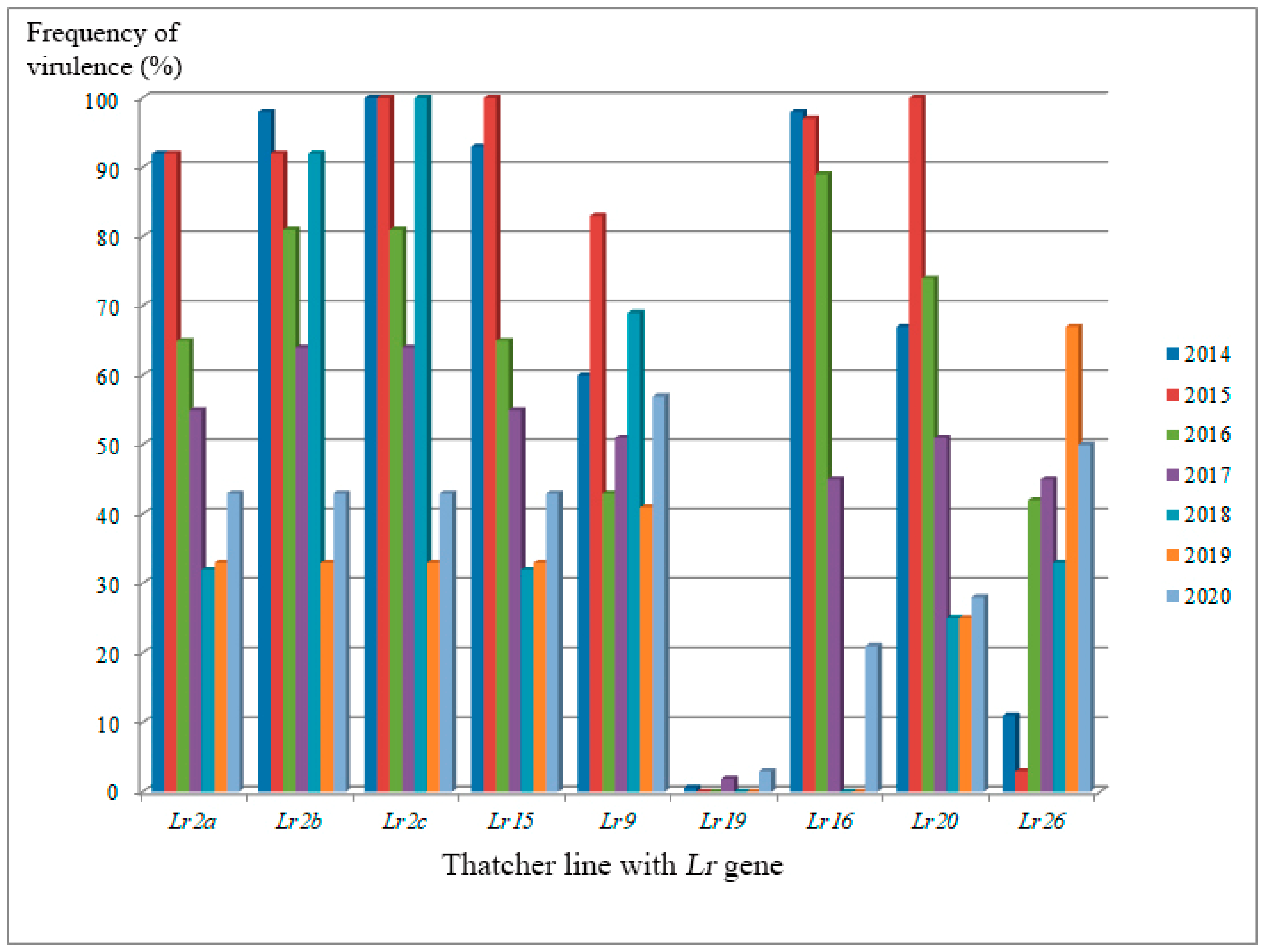
| Wheat Cultivar | P. triticina races | ||||||
|---|---|---|---|---|---|---|---|
| 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | |
| Duet | TQTTQ TQTTR | TQTTR | PQTTG | MQPKH | PLPTG | TLTTR | TLTTR |
| Chelyaba 2 | TQTTQ TQTTR | TLTTR TQTTR | TQPTR | MQPKG | PLPTG | MCTKG | TLTTR |
| Chelyaba yubileynaya | TQTTR | TQTTR | TQTTR | MLTKG | PLTTG | MCTKG | - |
| Chelyaba rannyaya | TQTTQ | TQTTR | - | TQTTQ | PLTKG | MCTKG | TLTTR |
| Eritrospermum 59 | TGTTR TQTTQ | PGTKR TQTTR | TGTTR | MLTKH THTTR | PLTTG | MCTKG | TCTTQ |
| Race | Ptr Toxins | 2017 | 2019 | 2020 |
|---|---|---|---|---|
| 1 | PtrToxA, ToxC | 26 | 2 | 32 |
| 2 | ToxA | 53 | 8 | 11 |
| 3 | ToxC | 0 | 3 | 8 |
| 4 | No toxins | 5 | 29 | 27 |
| 5 | PtrToxB | 0 | 14 | 0 |
| 6 | ToxB + Tox | 0 | 12 | 8 |
| 7 | ToxA + ToxB | 5 | 16 | 0 |
| 8 | ToxA + ToxB + ToxC | 7 | 16 | 11 |
| Number of isolates | 19 | 86 | 37 |
| Line | Pedigree (Alien Genetic Material) | Race Number | |
|---|---|---|---|
| 2019 | 2020 | ||
| Ferr. 26758 | Chelyaba 75 × Ferr. 24205 (Ae. speltoides) | 4 (Tox−), 7 (ToxA + ToxB) | 6 (ToxB + ToxC) |
| Ferr. 26754 | Chelyaba 75 × Ferr. 24205 (Ae. speltoides) | 3 (ToxC), 4 (Tox−) | |
| Ferr. 26680 | Er.59 × TcLr22a × Iren’ × Chelyaba 75 (Ae. tauschii, Ae. speltoides) | 4 (Tox−) | 4 (Tox−) |
| Lut. 26509 | Er. 23315 × Ecada 45 (Ae. speltoides) | 3 (ToxC), 8 (ToxA + ToxB + ToxC) | 2 (ToxA), 3 (ToxC), 4 (Tox−) |
| Ferr. 26635 | Sharada × Ferr. 23736 | 4 (Tox−), 7 (ToxA + ToxB) | 1 (ToxA + ToxC), 2 (ToxA) |
| Er. 26751 | Chelyaba 75 × Ferr. 24205 (Ae. speltoides) | 4 (Tox−), 5 (ToxB) | 1 (ToxA + ToxC) |
| Er. 26726 | Ferr. 23736 × Lut. 23814 (Ag. elongatum, Ae. umbellulaya) | 7 (ToxA + ToxB) | 1 (ToxA + ToxC), 4 (Tox−) |
| Er.26752 | Chelyaba 75 × Ferr. 24205 (Ae. speltoides) | 5 (ToxB), 6 (ToxB + ToxC) | 1 (ToxA + ToxC), 3 (ToxC), 4 (Tox−), 8 (ToxA + ToxB + ToxC) |
| Er. 26677 | Er.59 × TcLr22a × Iren’ × Chelyaba 75 (Ae. tauschii, Ae. speltoides) | 5 (ToxB), 8 (ToxA + ToxB + ToxC) | 1 (ToxA + ToxC), 2 (ToxA), 8 (ToxA + ToxB + ToxC), |
| Er. 26740 | Chelyaba 75 × Apasovka (Ae. speltoides, Ae. umbellulaya) | 6 (ToxB + ToxC), 7 (ToxA + ToxB) | 4 (Tox−), 6 (ToxB + ToxC) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kushnirenko, I.; Shreyder, E.; Bondarenko, N.; Shaydayuk, E.; Kovalenko, N.; Titova, J.; Gultyaeva, E. Genetic Protection of Soft Wheat from Diseases in the Southern Ural of Russia and Virulence Variability of Foliar Pathogens. Agriculture 2021, 11, 703. https://doi.org/10.3390/agriculture11080703
Kushnirenko I, Shreyder E, Bondarenko N, Shaydayuk E, Kovalenko N, Titova J, Gultyaeva E. Genetic Protection of Soft Wheat from Diseases in the Southern Ural of Russia and Virulence Variability of Foliar Pathogens. Agriculture. 2021; 11(8):703. https://doi.org/10.3390/agriculture11080703
Chicago/Turabian StyleKushnirenko, Igor, Ekaterina Shreyder, Nadezhda Bondarenko, Ekaterina Shaydayuk, Nadezhda Kovalenko, Julia Titova, and Elena Gultyaeva. 2021. "Genetic Protection of Soft Wheat from Diseases in the Southern Ural of Russia and Virulence Variability of Foliar Pathogens" Agriculture 11, no. 8: 703. https://doi.org/10.3390/agriculture11080703
APA StyleKushnirenko, I., Shreyder, E., Bondarenko, N., Shaydayuk, E., Kovalenko, N., Titova, J., & Gultyaeva, E. (2021). Genetic Protection of Soft Wheat from Diseases in the Southern Ural of Russia and Virulence Variability of Foliar Pathogens. Agriculture, 11(8), 703. https://doi.org/10.3390/agriculture11080703







