Mycorrhizal Inoculation Improves Mineral Content of Organic Potatoes Grown under Calcareous Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Site, Soil, and Climate
2.2. Field Experimental Design, Plant Material and Management Practices
2.3. Sampling and Determination of Tuber Minerals Content
2.4. Soil Sampling, DNA Extraction, and Real-Time Quantitative PCR Assay
2.5. Statistical Analysis
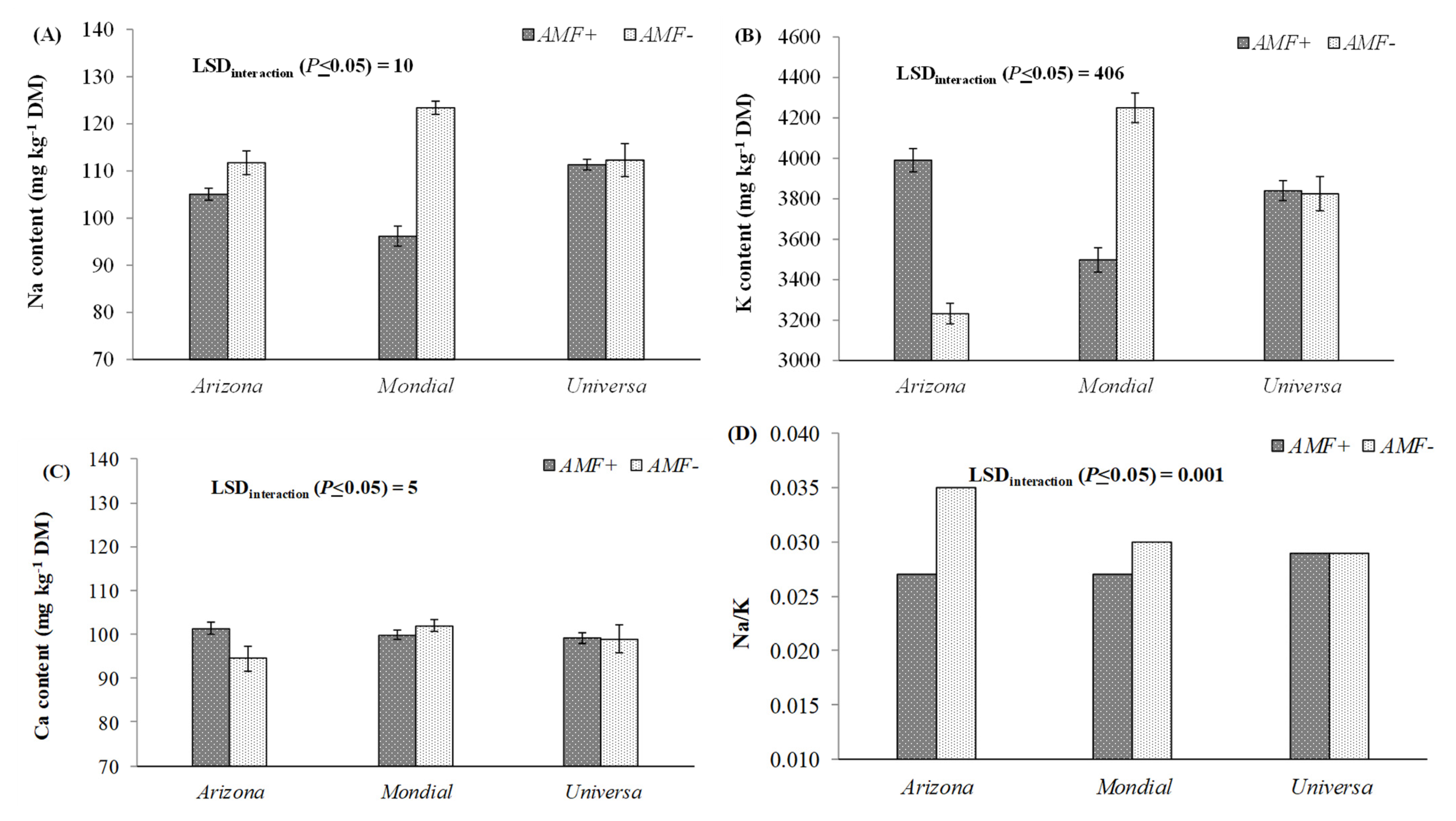
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lairon, D. Nutritional quality and safety of organic food. A review. Agron. Sustain. Dev. 2009, 30, 33–41. [Google Scholar] [CrossRef]
- Scavo, A.; Mauromicale, G. Integrated weed management in herbaceous field crops. Agronomy 2020, 10, 466. [Google Scholar] [CrossRef]
- Altieri, M.A.; Nicholls, C.I.; Montalba, R. Technological approaches to sustainable agriculture at a crossroads: An agroecological perspective. Sustainability 2017, 9, 349. [Google Scholar] [CrossRef]
- Willer, H.; Lernoud, J.; Kemper, L. The world of organic agriculture 2019: Summary. In The World of Organic Agriculture Statistics and Emerging Trends; Willer, H., Lernoud, J., Eds.; Research Institute of Organic Agriculture FiBL and IFOAM—Organics International: Nuremberg, Germany, 2020. [Google Scholar]
- Bacchi, M.A.; De Nadai Fernandes, E.A.; Tsai, S.M.; Santos, L.G.C. Conventional and organic potatoes: Assessment of elemental composition using k0-INAA. J. Radioanal. Nucl. Chem. 2004, 259, 421–424. [Google Scholar] [CrossRef]
- Warman, P.R.; Havard, K.A. Yield, vitamin and mineral contents of organically and conventionally grown potatoes and sweet corn. Agric. Ecosyst. Environ. 1998, 68, 207–216. [Google Scholar] [CrossRef]
- Maggio, A.; Carillo, P.; Bulmetti, G.S.; Fuggi, A.; Barbieri, G.; De Pascale, S. Potato yield and metabolic profiling under conventional and organic farming. Eur. J. Agron. 2008, 28, 343–350. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G. Nutritional and sensory characteristics of early potato cultivars under organic and conventional cultivation systems. Food Chem. 2012, 133, 1249–1254. [Google Scholar] [CrossRef]
- Lombardo, S.; Lo Monaco, A.; Pandino, G.; Parisi, B.; Mauromicale, G. The phenology: Yield and tuber composition of ‘early’ crop potatoes: A comparison between organic and conventional cultivation systems. Renew. Agric. Food Syst. 2013, 28, 50–58. [Google Scholar] [CrossRef]
- Mauromicale, G.; Signorelli, P.; Ierna, A.; Foti, S. Effects of intraspecific competition on yield of early potato grown in mediterranean environment. Am. J. Potato Res. 2003, 80, 281–288. [Google Scholar] [CrossRef]
- De Cicco, A.; Jeanty, J.C. The EU Potato Sector—Statistics on Production, Prices and Trade. Available online: https://ec.europa.eu/eurostat/statistics-explained/pdfscache/49931.pdf (accessed on 30 December 2020).
- Lynch, D.H.; Sharifi, M.; Hammermeister, A.; Burton, D. Nitrogen management in organic potato production. In Sustainable Potato Production: Global Case Studies; He, Z., Larkin, R., Honeycutt, W., Eds.; Springer: Dordrecht, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Van Delden, A.; Schroder, J.J.; Kropff, M.J.; Grashoff, C.; Booij, R. Simulated potato yield and crop and soil nitrogen dynamics under different organic nitrogen management strategies in the Netherlands. Agric. Ecosyst. Environ. 2003, 96, 77–95. [Google Scholar] [CrossRef]
- Ierna, A.; Mauromicale, G. Potato growth, yield and water productivity response to different irrigation and fertilization regimes. Agric. Water Manag. 2018, 201, 21–26. [Google Scholar] [CrossRef]
- Taalab, A.S.; Ageeb, G.W.; Siam, H.S.; Mahmoud, S.A. Some characteristics of calcareous soils. A review. Middle East J. Agric. Res. 2019, 8, 96–105. [Google Scholar]
- Koch, M.; Naumann, M.; Pawelzik, E.; Gransee, A.; Thiel, H. The importance of nutrient management for potato production Part I: Plant nutrition and yield. Potato Res. 2019, 63, 97–119. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G. Optimizing nitrogen fertilization to improve qualitative performances and physiological and yield responses of potato (Solanum tuberosum L.). Agronomy 2020, 10, 352. [Google Scholar] [CrossRef]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, UK, 2008. [Google Scholar]
- Scavo, A.; Abbate, C.; Mauromicale, G. Plant allelochemicals: Agronomic, nutritional and ecological relevance in the soil system. Plant Soil 2019, 442, 23–48. [Google Scholar] [CrossRef]
- Pandino, G.; Lombardo, S.; Lo Monaco, A.; Ruta, C.; Mauromicale, G. In vitro micropropagation and mycorrhizal treatment influences the polyphenols content profile of globe artichoke under field conditions. Food Res. Int. 2017, 99, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, S.; Uchida, T.; Matsunami, H.; Kobayashi, H. Effect of winter wheat cover cropping with no-till cultivation on the community structure of arbuscular mycorrhizal fungi colonizing the subsequent soybean. Soil Sci. Plant Nutr. 2018, 64, 545–553. [Google Scholar] [CrossRef]
- Higo, M.; Takahashi, Y.; Gunji, K.; Isobe, K. How are arbuscular mycorrhizal associations related to maize growth performance during short-term cover crop rotation? J. Sci. Food Agric. 2018, 98, 1388–1396. [Google Scholar] [CrossRef] [PubMed]
- Higo, M.; Sato, R.; Serizawa, A.; Takahashi, Y.; Gunji, K.; Tatewaki, Y.; Isobe, K. Can phosphorus application and cover cropping alter arbuscular mycorrhizal fungal communities and soybean performance after a five-year phosphorus-unfertilized crop rotational system? PeerJ 2018, 6, e4606. [Google Scholar] [CrossRef] [PubMed]
- Higo, M.; Azuma, M.; Kamiyoshiihara, Y.; Kanda, A.; Tatewaki, Y. Impact of phosphorus fertilization on tomato growth and arbuscular mycorrhizal fungal communities. Microorganisms 2020, 8, 178. [Google Scholar] [CrossRef] [PubMed]
- Brito, I.; Goss, M.J.; de Carvalho, M.; Chatagnier, O.; van Tuinen, D. Impact of tillage system on arbuscular mycorrhiza fungal communities in the soil under Mediterranean conditions. Soil Till. Res. 2012, 121, 63–67. [Google Scholar] [CrossRef]
- Karasawa, T.; Takahashi, S. Introduction of various cover crop species to improve soil biological P parameters and P uptake of the following crops. Nutr. Cycl. Agroecosyst. 2015, 103, 15–28. [Google Scholar] [CrossRef]
- Higo, M.; Tatewaki, Y.; Gunji, K.; Kaseda, A.; Isobe, K. Cover cropping can be a stronger determinant than host crop identity for arbuscular mycorrhizal fungal communities colonizing maize and soybean. PeerJ 2019, 7, e6403. [Google Scholar] [CrossRef] [PubMed]
- Gosling, P.; Hodge, A.; Goodlass, G.; Bending, G.D. Arbuscular mycorrhizal fungi and organic farming. Agric. Ecosyst. Environ. 2006, 113, 17–35. [Google Scholar] [CrossRef]
- Wu, F.; Wang, W.; Ma, Y.; Liu, Y.; Ma, X.; An, L.; Feng, H. Prospect of beneficial microorganisms applied in potato cultivation for sustainable agriculture. Afr. J. Microbiol. Res. 2013, 7, 2150–2158. [Google Scholar] [CrossRef][Green Version]
- Rytel, E. The effect of industrial potato processing on the concentrations of glycoalkaloids and nitrates in potato granules. Food Control 2012, 28, 380–384. [Google Scholar] [CrossRef]
- White, P.J.; Bradshaw, J.E.; Dale, M.F.B.; Ramsay, G.; Hammond, J.P.; Broadley, M.R. Relationships between yield and mineral concentrations in potato tubers. HortScience 2009, 44, 6–11. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G. The mineral profile in organically and conventionally grown early crop potato tubers. Sci. Hortic. 2014, 167, 169–173. [Google Scholar] [CrossRef]
- Lombardo, S.; Pandino, G.; Mauromicale, G. The effect on tuber quality of an organic versus a conventional cultivation system in the early crop potato. J. Food Compos. Anal. 2017, 62, 189–196. [Google Scholar] [CrossRef]
- Duffy, E.M.; Cassells, A.C. The effect of inoculation of potato (Solanum tuberosum L.) microplants with arbuscular mycorrhizal fungi on tuber yield and tuber size distribution. Appl. Soil Ecol. 2000, 15, 137–144. [Google Scholar] [CrossRef]
- Johnstone, P.D.; Lowther, W.L.; Keoghan, J.M. Design and analysis of multi-site agronomic evaluation trials. N. Z. J. Agric. Res. 1993, 36, 323–326. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; US Government Print Office: Washington, DC, USA, 1999. [Google Scholar]
- Italian Society of Soil Science. Metodi Normalizzati di Analisi del Suolo; Edagricole: Bologna, Italy, 1985. [Google Scholar]
- Lombardo, S.; Abbate, C.; Pandino, G.; Parisi, B.; Scavo, A.; Mauromicale, G. Productive and physiological response of organic potato grown under highly calcareous soils to fertilization and mycorrhization management. Agronomy 2020, 10, 1200. [Google Scholar] [CrossRef]
- Dierauer, H.; Siegrist, F.; Weidmann, G. Commercial Organic Fertiliser as Supplementary Fertilisers in Potato Crop Production. Research Institute of Organic Agriculture-FiBL, Practice Abstract. 2017. Available online: https://www.orgprints.org/31027/ (accessed on 30 September 2020).
- AOAC International. Official Methods of Analysis, 16th ed.; AOAC: Washington, DC, USA, 1995. [Google Scholar]
- Lombardo, S.; Pandino, G.; Mauromicale, G. The influence of growing environment on the antioxidant and mineral content of early crop potato. J. Food Compos. Anal. 2013, 32, 28–35. [Google Scholar] [CrossRef]
- Scavo, A.; Restuccia, A.; Abbate, C.; Mauromicale, G. Seeming field allelopathic activity of Cynara cardunculus L. reduces the soil weed seed bank. Agron. Sustain. Dev. 2019, 39, 41. [Google Scholar] [CrossRef]
- Shuab, R.; Lone, R.; Koul, K.K. Influence of arbuscular mycorrhizal fungi on storage metabolites, mineral nutrition, and nitrogen-assimilating enzymes in potato (Solanum tuberosum L.) plant. J. Plant Nutr. 2017, 40, 1386–1396. [Google Scholar] [CrossRef]
- Dechassa, N.; Schenk, M.K.; Claassen, N.; Steingrobe, B. Phosphorus efficiency of cabbage (Brassica oleraceae L. var. capitata), carrot (Daucus carota L.), and potato (Solanum tuberosum L.). Plant Soil 2003, 250, 215–224. [Google Scholar] [CrossRef]
- Lone, R.; Alaklabi, A.; Malik, J.A.; Koul, K.K. Mycorrhizal influence on storage metabolites and mineral nutrition in seed propagated potato (Solanum tuberosum L.) plant. J. Plant Nutr. 2020, 43, 2164–2175. [Google Scholar] [CrossRef]
- Tandon, H.L.S. Micronutrients in Soils, Crops, and Fertilizers; Fertilizer Development and Consultation Organization: New Delhi, India, 1995. [Google Scholar]
- Gupta, U.C.; Gupta, S.C. Sources and deficiency diseases of mineral nutrients in human health and nutrition: A review. Pedosphere 2014, 24, 13–38. [Google Scholar] [CrossRef]
- Douds, D.D.; Nagahashi, G.; Reider, C.; Drinkwater, L.E. Inoculation with arbuscular mycorrhizal fungi increases the yield of potatoes in a high P soil. Biol. Agric. Hortic. 2007, 25, 67–78. [Google Scholar] [CrossRef]
- Kothari, S.K.; Marschner, H.; Römheld, V. Directand indirect effects of VA mycorrhizal fungi and rhizosphere microorganisms on acquisition of mineral nutrients by maize (Zea mays L.) in a calcareous soil. New Phytol. 1990, 116, 637–645. [Google Scholar] [CrossRef]
- George, E.; Haussler, K.; Vetterlein, G.; Gorgus, E.; Marschner, H. Water and nutrient translocation by hyphaeof Glomus mosseae. Can. J. Bot. 1992, 70, 2130–2137. [Google Scholar] [CrossRef]
- Pinochet, J.; Fernandez, C.; Jaizme, M.; Tenoury, P. Micropropagated banana infected with Meloidogyne javani-caresponds to Glomus intraradices and phosphorus. HortScience 1997, 32, 101–103. [Google Scholar] [CrossRef]
- Rodríguez, L.H.; Morales, D.A.; Rodríguez, E.; Romero, C.D. Minerals and trace elements in a collection of wheat landraces from the Canary Islands. J. Food Compos. Anal. 2011, 24, 1081–1090. [Google Scholar] [CrossRef]
- Pandino, G.; Mattiolo, E.; Lombardo, S.; Lombardo, G.M.; Mauromicale, G.M. organic cropping system affects grain chemical composition, rheological and agronomic performance of durum wheat. Agriculture 2020, 10, 46. [Google Scholar] [CrossRef]
- Senés-Guerrero, C.; Torres-Cortés, G.; Pfeiffer, S.; Rojas, M.; Schüßler, A. Potato-associated arbuscular mycorrhizal fungal communities in the Peruvian Andes. Mycorrhiza 2014, 24, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.C.; Hernández, P.S.; Rodríguez, E.M.R.; Martín, J.D.; Díaz Romero, C. Mineral concentrations in cultivars of potatoes. Food Chem. 2003, 83, 247–253. [Google Scholar] [CrossRef]
- Andrè, C.M.; Ghislain, M.; Bertin, P.; Oufir, M.; del Rosario Herrera, M.; Hoffmann, L.; Hausman, J.F.; Larondelle, Y.; Evers, D. Andean potato cultivars (Solanum tuberosum L.) as a source of antioxidant and mineral micronutrients. J. Agric. Food Chem. 2007, 55, 366–378. [Google Scholar] [CrossRef]
- Tekalign, T.; Hammes, P.S. Growth and productivity of potato as influenced by cultivar and reproductive growth. II. Growth analysis, tuber yield and quality. Sci. Hort. 2005, 105, 29–44. [Google Scholar] [CrossRef]
- Trehan, S.P.; Sharma, R.C. External phosphorus requirement of different potato (Solanum tuberosum) cultivars resulting from different internal requirements and uptake efficiencies. Indian J. Agric. Sci. 2003, 73, 54–56. [Google Scholar]
- Sarkar, D.; Pandey, S.K.; Sud, K.C.; Chanemougasoundharam, A. In vitro characterization of manganese toxicity in relation to phosphorus nutrition in potato (Solanum tuberosum L.). Plant Sci. 2004, 167, 977–986. [Google Scholar] [CrossRef]
- Gransee, A.; Führs, H. Magnesium mobility in soils as a challenge for soil and plant analysis, magnesium fertilization and root uptake under adverse growth conditions. Plant Soil 2012, 368, 5–21. [Google Scholar] [CrossRef]
- Karley, A.J.; White, P.J. Moving cationic minerals to edible tissues: Potassium, magnesium, calcium. Curr. Opin. Plant Biol. 2009, 12, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Heijden van der, G.; Legout, A.; Midwood, A.J.; Craig, C.A.; Pollier, B.; Ranger, J.; Dambrine, E. Mg and Ca root uptake and vertical transfer in soils assessed by an in situ ecosystem scale multi-isotopic (26Mg & 44Ca) tracing experiment in a beech stand (Breuil-chenue, France). Plant Soil 2013, 369, 33–45. [Google Scholar] [CrossRef]
- Yan, B.; Hou, Y. Effect of soil magnesium on plants: A review. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Banda Aceh, Indonesia, 26–27 September 2018; Volume 170, p. 022168. [Google Scholar] [CrossRef]
- Ding, Y.; Luo, W.; Xu, G. Characterisation of magnesium nutrition and interaction of magnesium and potassium in rice. Ann. Appl. Biol. 2006, 149, 111–123. [Google Scholar] [CrossRef]
- Li, H.; Chen, Z.; Zhou, T.; Liu, Y.; Zhou, J. High potassium to magnesium ratio affected the growth and magnesium uptake of three tomato (Solanum lycopersicum L.) cultivars. J. Integr. Agric. 2018, 17, 2813–2821. [Google Scholar] [CrossRef]
- Wekesa, M.N.; Okoth, M.W.; O. Abong’, G.; Muthoni, J.; Kabira, J.N. Effect of soil characteristics on potato tuber minerals composition of selected Kenyan varieties. J. Agric. Sci. 2014, 6, 163–171. [Google Scholar] [CrossRef][Green Version]
- Kabatha-Pendias, A. Trace Elements in Soils and Plants, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2001; p. 403. [Google Scholar] [CrossRef][Green Version]
- Sharma, P.N.; Tripathi, A.; Bisht, S.S. Zinc requirement for stomatal opening in cauliflower. Plant Physiol. 1995, 107, 751–756. [Google Scholar] [CrossRef]
- Chen, W.; Yang, X.; He, Z.; Feng, Y.; Hu, F. Differential changes in photosynthetic capacity, 77K chlorophyll fluorescence and chloroplast ultrastructure between Zn-efficienct and Zn-inefficient rice genotypes (Oryza sativa L.) under low Zn stress. Plant Physiol. 2007, 132, 89–101. [Google Scholar] [CrossRef]
- Zvezdanović, J.; Marković, D. Copper, iron, and zinc interactions with chlorophyll in extracts of photosynthetic pigments studied by VIS spectroscopy. Russ. J. Phys. Chem. A 2009, 83, 1542–1546. [Google Scholar] [CrossRef]
- Di Giacomo, F.; Del Signore, A.; Giaccio, M. Determining the geographic origin of potatoes using mineral and trace element content. J. Agric. Food Chem. 2007, 55, 860–866. [Google Scholar] [CrossRef]
- Nassar, A.M.K.; Sabally, K.; Kubow, S.; Leclerc, Y.N.; Donnelly, D.J. Some Canadian grown potato cultivars contribute to a substantial content of essential dietary minerals. J. Agric. Food Chem. 2012, 60, 4688–4696. [Google Scholar] [CrossRef] [PubMed]


| Soil Characteristic | Location I | Location II |
|---|---|---|
| Sand (%) | 54.1 | 51.8 |
| Silt (%) | 24.8 | 22.0 |
| Clay (%) | 21.1 | 26.2 |
| Total limestone (%) | 44.2 | 65.6 |
| Active limestone (%) | 15.5 | 18.0 |
| Organic matter (%) | 1.7 | 2.6 |
| C/N ratio | 7.5 | 7.5 |
| pH | 7.8 | 7.5 |
| Total N (g kg−1) | 1.3 | 2.0 |
| Assimilable P2O5 (mg kg−1) | 66 | 135 |
| Exchangeable K2O (mg kg−1) | 455 | 612 |
| Fe (mg kg−1) | 5.58 | 10.45 |
| Zn (mg kg−1) | 0.99 | 2.03 |
| Mn (mg kg−1) | 11.48 | 25.11 |
| Cu (mg kg−1) | 2.21 | 3.92 |
| Electrical conductivity (dS m−1) | 1.32 | 1.14 |
| Cation exchange capacity (meq 100 g−1) | 22.8 | 26.0 |
| Ca (%) | 83.34 | 83.27 |
| Mg (%) | 9.95 | 10.67 |
| K (%) | 4.23 | 5.17 |
| Na (%) | 2.48 | 0.90 |
| Phenological Stage of Application | Commercial Product | Applications (n.) | Dose Per Application | Active Ingredient | Source | Manufacturer |
|---|---|---|---|---|---|---|
| At sowing | Ricin-Xed® | 1 | 1.2 t ha−1 | 4% of N | Castor seeds | XEDA Italia s.r.l., Forlì, Italy |
| At sowing | Xedaneem Pel® | 1 | 1.2 t ha−1 | 3% of N | Neem seeds after oil extraction | “ |
| At sowing | Kalisop® | 1 | 0.6 t ha−1 | 50% of K2O; 45% of SO3 | Commercial granular product | K+S KALI GmbH, Verona, Italy |
| At sowing | Fosfonature 26® | 1 | 0.4 t ha−1 | 26% of P2O5; 41% of CaO | ‘Pheoflore’ algal origin | Fosfonature 26®, TIMAC Agro, Milan, Italy |
| At sowing | Xedaopen® a | 40 kg ha−1 | 7 active propagules g−1 of the genus Glomus spp. and Gigaspora spp. | Commercial inoculant | XEDA Italia s.r.l., Forlì, Italy | |
| After emergence | Biosin® | 3 | 150 cc hL | 7.7% of N | Commercial liquid product | “ |
| Source of Variation | Df | Mineral Element | Na/K | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| K | P | Mg | N | Na | Ca | Fe | Mn | Cu | Zn | |||
| Soil mycorrhization (M) | 1 | 0.0 NS | 49.6 *** | 11.5 ** | 0.0 NS | 39.1 ** | 10.7 NS | 0.0 NS | 40.1 *** | 50.3 *** | 1.6 NS | 27.6 ** |
| Location (L) | 1 | 0.5 NS | 22.5 *** | 60.4 *** | 41.1 NS | 26.7 ** | 4.0 NS | 59.2 *** | 13.6 *** | 27.1 *** | 92.3 *** | 10.3 * |
| Cultivar (C) | 2 | 5.7 NS | 22.2 *** | 20.6 NS | 0.1 NS | 1.1 NS | 12.8 NS | 0.6 NS | 12.8 *** | 4.8 NS | 1.2 NS | 3.4 NS |
| (M) × (L) | 1 | 2.7 NS | 1.1 NS | 1.2 NS | 0.1 NS | 8.7 NS | 18.7 NS | 0.5 NS | 14.6 *** | 13.6 *** | 1.5 NS | 13.8 NS |
| (M) × (C) | 2 | 40.7 *** | 2.8 NS | 3.7 NS | 3.5 NS | 18.4 ** | 30.7 * | 13.8 * | 8.6 *** | 1.6 NS | 2.9 ** | 10.3 ** |
| (L) × (C) | 2 | 6.8 NS | 1.6 NS | 0.6 NS | 41.5 NS | 0.5 NS | 7.1 NS | 2.7 NS | 5.0 NS | 1.6 NS | 0.1 NS | 6.9 NS |
| Total mean square | - | 2,804,890 | 2,165,154 | 39,276 | 1523 | 2073 | 142 | 28 | 5.01 | 1.25 | 4.11 | 0.003 |
| Main Factor | Mineral Element | Na/K | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| K | P | Mg | N | Na | Ca | Fe | Mn | Cu | Zn | ||
| Soil mycorrhization | |||||||||||
| AMF+ | 3776 ± 50 | 2116 ± 55 a | 180 ± 3 b | 122 ± 2 | 104 ± 4 b | 100 ± 3 | 20.8 ± 0.5 | 2.48 ± 0.17 a | 2.11 ± 0.10 a | 1.26 ± 0.12 a | 0.028 b |
| AMF− | 3769 ± 33 | 1693 ± 70 b | 207 ± 6 a | 122 ± 2 | 115 ± 2 a | 98 ± 1 | 20.9 ± 0.7 | 1.90 ± 0.11 b | 1.79 ± 0.20 b | 0.93 ± 0.10 b | 0.031 a |
| Cultivar | |||||||||||
| Arizona | 3612 ± 137 | 1760 ± 31 b | 174 ± 3 b | 123 ± 3 | 108 ± 3 | 98 ± 2 | 20.7 ± 0.1 | 2.32 ± 0.21 a | 1.91 ± 0.20 | 1.16 ± 0.10 | 0.031 |
| Mondial | 3874 ± 101 | 2188 ± 60 a | 177 ± 2 b | 122 ± 1 | 110 ± 5 | 101 ± 3 | 20.9 ± 0.3 | 1.87 ± 0.71 b | 2.05 ± 0.10 | 1.01 ± 0.10 | 0.028 |
| Universa | 3833 ± 132 | 1766 ± 26 b | 230 ± 6 a | 122 ± 2 | 112 ± 2 | 99 ± 2 | 21.0 ± 0.4 | 2.39 ± 0.10 a | 1.89 ± 0.10 | 1.11 ± 0.10 | 0.029 |
| Location | |||||||||||
| I | 3749 ± 39 | 1762 ± 81 b | 225 ± 10 a | 117 ± 9 | 115 ± 1 a | 99 ± 2 | 21.7 ± 0.5 a | 2.36 ± 0.11 a | 2.07 ± 0.50 a | 0.69 ± 0.31 b | 0.031 a |
| II | 3797 ± 61 | 2074 ± 43 a | 162 ± 9 b | 127 ± 8 | 105 ± 4 b | 100 ± 2 | 20.0 ± 0.7 b | 2.02 ± 0.13 b | 1.83 ± 0.30 b | 1.49 ± 0.43 a | 0.028 b |
| Cultivar | Origin | Tuber Maturity | Plant Vigor | Skin Colour | Flesh Colour | Cooking Type a |
|---|---|---|---|---|---|---|
| Mondial (Spunta × VE66-295) | Dutch | late | medium-high | yellow | yellow | B |
| Arizona (UK 150-19D22 × Mascotte) | Dutch | medium-late | high | “ | “ | AB |
| Universa (Agata × 88F164.1) | French | early-medium | medium | “ | “ | AB |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lombardo, S.; Scavo, A.; Abbate, C.; Pandino, G.; Parisi, B.; Mauromicale, G. Mycorrhizal Inoculation Improves Mineral Content of Organic Potatoes Grown under Calcareous Soil. Agriculture 2021, 11, 333. https://doi.org/10.3390/agriculture11040333
Lombardo S, Scavo A, Abbate C, Pandino G, Parisi B, Mauromicale G. Mycorrhizal Inoculation Improves Mineral Content of Organic Potatoes Grown under Calcareous Soil. Agriculture. 2021; 11(4):333. https://doi.org/10.3390/agriculture11040333
Chicago/Turabian StyleLombardo, Sara, Aurelio Scavo, Cristina Abbate, Gaetano Pandino, Bruno Parisi, and Giovanni Mauromicale. 2021. "Mycorrhizal Inoculation Improves Mineral Content of Organic Potatoes Grown under Calcareous Soil" Agriculture 11, no. 4: 333. https://doi.org/10.3390/agriculture11040333
APA StyleLombardo, S., Scavo, A., Abbate, C., Pandino, G., Parisi, B., & Mauromicale, G. (2021). Mycorrhizal Inoculation Improves Mineral Content of Organic Potatoes Grown under Calcareous Soil. Agriculture, 11(4), 333. https://doi.org/10.3390/agriculture11040333











