Evaluation of Leaf Rust Resistance in the Spanish Core Collection of Tetraploid Wheat Landraces and Association with Ecogeographical Variables
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Fungal Material
- -
- CDJ13: Lr10, Lr14a, Lr14b, Lr20, LrB / Lr1, Lr2a, Lr2c, Lr3, Lr3bg, Lr3ka, Lr9, Lr11, Lr16, Lr17, Lr18, Lr24, Lr26, Lr28, Lr30.
- -
- JDJ15: Lr1, Lr10, Lr14b, Lr18, Lr20, LrB / Lr2a, Lr2c, Lr3, Lr3bg, Lr3ka, Lr9, Lr11, Lr14a, Lr16, Lr17, Lr18, Lr24, Lr26, Lr28, Lr30.
- -
- PG14: Lr1, Lr3, Lr3bg, Lr10, Lr14a, Lr14b, Lr16, Lr17, Lr18, Lr20, Lr26, Lr30, LrB / Lr2a, Lr2c, Lr3ka, Lr9, Lr11, Lr18, Lr24, Lr28.
2.3. Experiment of Hypersensitive Resistance at Seedling Stage in the Greenhouse
2.4. Experiment of Partial Resistance at Seedling Stage in the Greenhouse
2.5. Leaf Rust Evaluation at Adult Plant Stage in the Field Experiments
2.6. Agronomic Characterisation
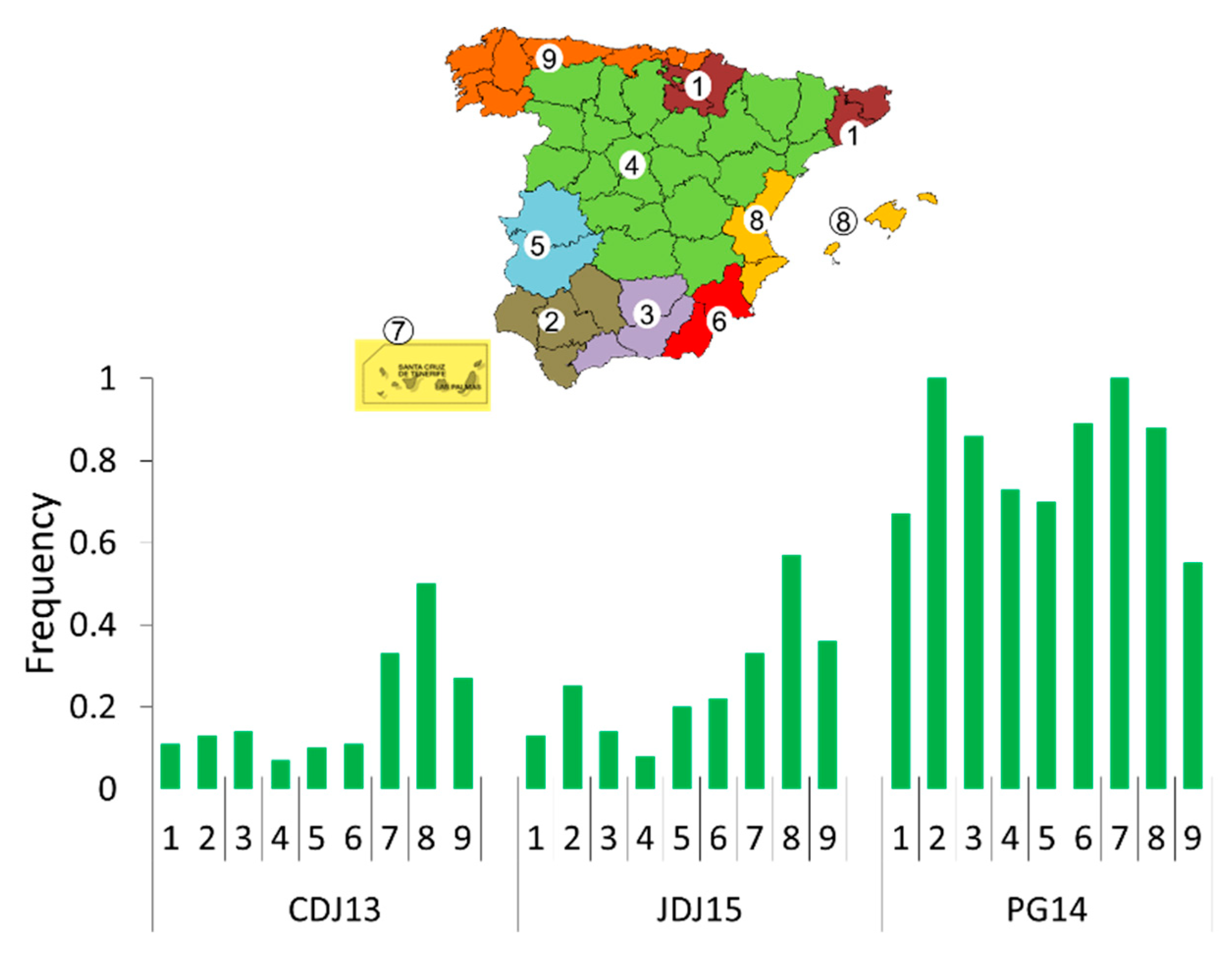
2.7. Ecogeographic Characterisation
2.8. Data Analyses
3. Results
3.1. Leaf Rust Resistance of the Core Collection at Seedling Stage in the Greenhouse
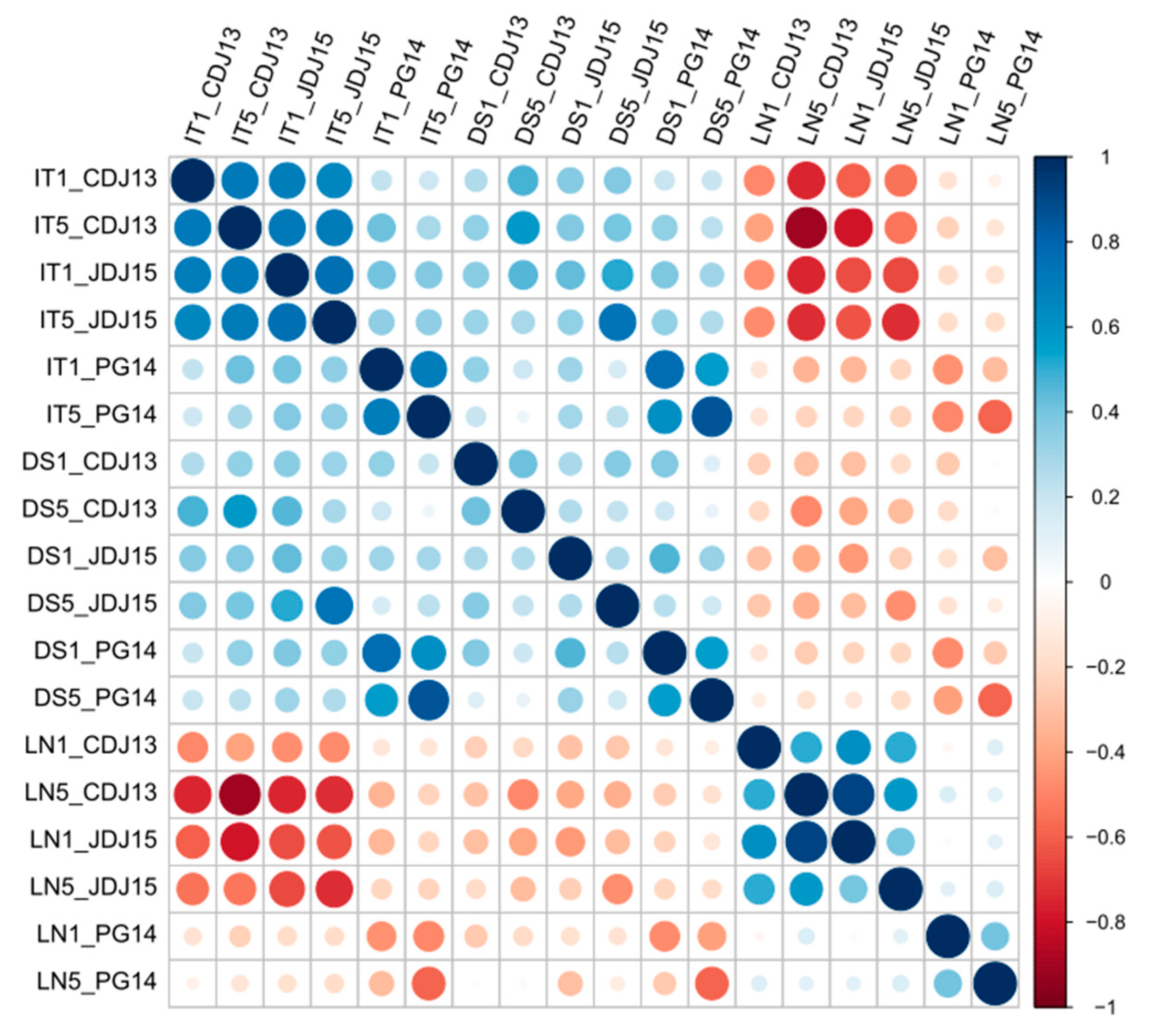
3.1.1. Correlations between Disease Parameters
3.1.2. Identification of Resistant Accessions to Leaf Rust
3.1.3. Relations between Seedling Resistance and Agronomic Traits
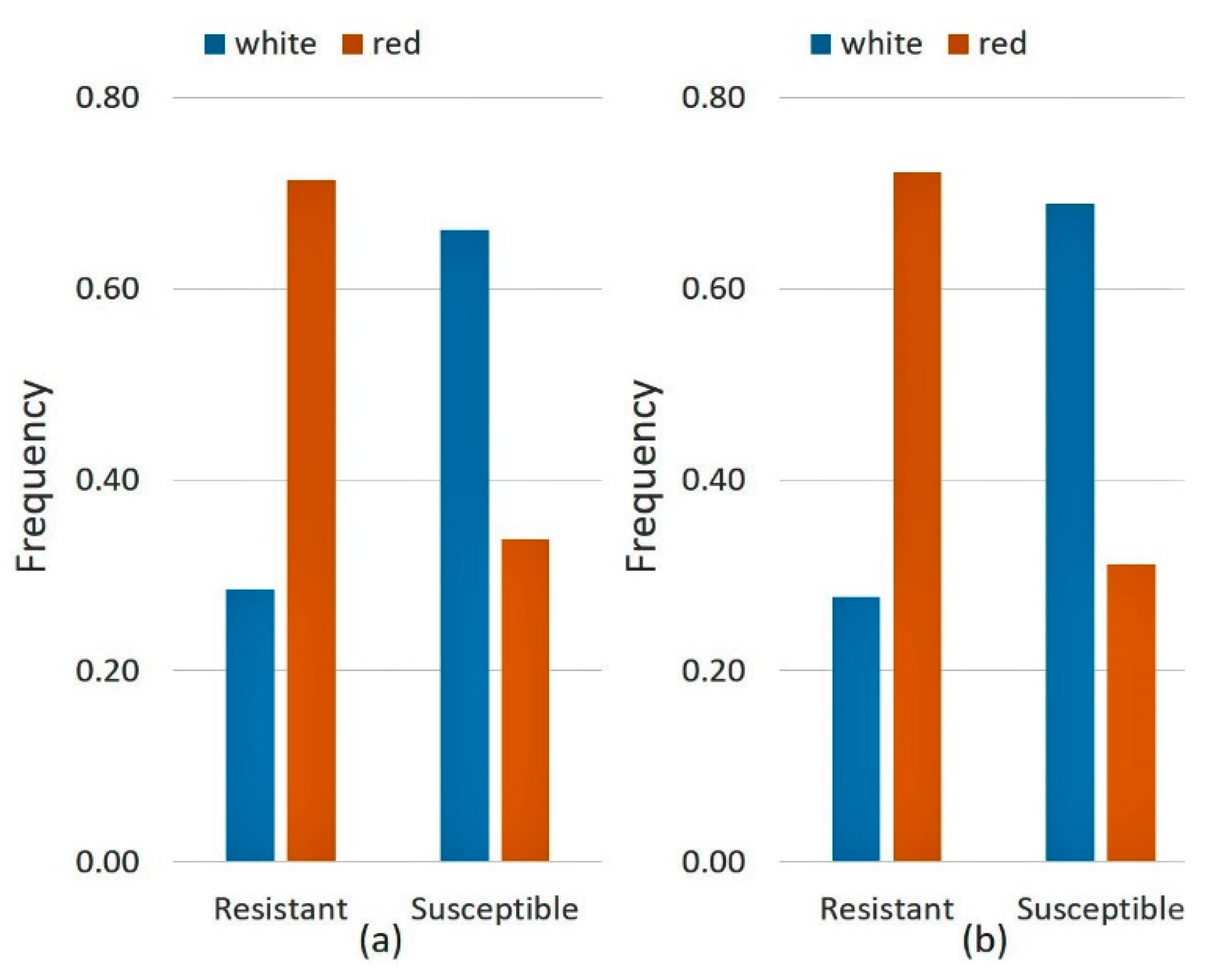
3.1.4. Relations between Seedling Resistance and Ecogeographic Variables of the Collection Site
3.2. Leaf rust Resistance at Adult Plant Stage in the Field Experiments
3.3. Partial Resistance to Leaf Rust at Seedling Stage in the Greenhouse
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kabbaj, H.; Sall, A.T.; Al-Abdallat, A.; Geleta, M.; Amri, A.; Filali-Maltouf, A.; Belkadi, B.; Ortiz, R.; Bassi, F.M. Genetic diversity within a global panel of durum wheat (Triticum durum) landraces and modern germplasm reveals the history of alleles exchange. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Zaharieva, M.; Ayana, N.G.; Hakimi, A.A.; Misra, S.C.; Monneveux, P. Cultivated emmer wheat (Triticum dicoccon Schrank), an old crop with promising future: A review. Genet. Resour. Crop Evol. 2010, 57, 937–962. [Google Scholar] [CrossRef]
- Roelfs, A.P.; Singh, R.P.; Saari, E.E.; International Maize and Wheat Improvement Center. Rust Diseases of Wheat: Concepts and Methods of Disease Management; CIMMYT: Mexico D.F., Mexico, 1992; ISBN 968612747X. [Google Scholar]
- Martínez-Moreno, F.; Solís, I. Wheat rust evolution in Spain: An historical review. Phytopathol. Mediterr. 2019, 58, 3–16. [Google Scholar] [CrossRef]
- Herrera-Foessel, S.A.; Huerta-Espino, J.; Calvo-Salazar, V.; Lan, C.X.; Singh, R.P. Lr72 Confers Resistance to Leaf Rust in Durum Wheat Cultivar Atil C2000. Plant Dis. 2014, 98, 631–635. [Google Scholar] [CrossRef] [PubMed]
- Maccaferri, M.; Mantovani, P.; Tuberosa, R.; DeAmbrogio, E.; Giuliani, S.; Demontis, A.; Massi, A.; Sanguineti, M.C. A major QTL for durable leaf rust resistance widely exploited in durum wheat breeding programs maps on the distal region of chromosome arm 7BL. Theor. Appl. Genet. 2008, 117, 1225–1240. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Rodrigue, N.; Kolmer, J. Population divergence in the wheat leaf rust fungus Puccinia triticina is correlated with wheat evolution. Heredity 2014, 112, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Ordoñez, M.E.; Kolmer, J.A. Virulence phenotypes of a worldwide collection of Puccinia triticina from durum wheat. Phytopathology 2007, 97, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Anikster, Y.; Bushnell, W.R.; Eilam, T.; Manisterski, J.; Roelfs, A.P. Puccinia recondita causing leaf rust on cultivated wheats, wild wheats, and rye. Can. J. Bot. Can. Bot. 1997, 75, 2082–2096. [Google Scholar] [CrossRef]
- Johnson, R. A critical analysis of durable resistance. Annu. Rev. Phytopathol. 1984, 22, 309–330. [Google Scholar] [CrossRef]
- Singh, R.P.; Rajaram, S. Genetics of Adult-Plant Resistance of Leaf Rust in Frontana and 3 CIMMYT Wheats. Genome 1992, 35, 24–31. [Google Scholar] [CrossRef]
- Broers, L. Partial resistance to wheat leaf rust in 18 spring wheat cultivars. Euphytica 1989, 44, 247–258. [Google Scholar] [CrossRef]
- Ledesma-Ramírez, L.; Solis-Moya, E.; Ramírez-Pimentel, J.G.; Dreisigacker, S.; Huerta-Espino, J.; Aguirre-Mancilla, C.L.; Mariscal-Amaro, L.A. Relationship between the number of partial resistance genes and the response to leaf rust in wheat genotypes. Chil. J. Agric. Res. 2018, 78, 400–408. [Google Scholar] [CrossRef]
- Parlevliet, J.E. Partial resistance of barley to leaf rust, Puccinia hordei. I. Effect of cultivar and development stage on latent period. Euphytica 1975, 24, 21–27. [Google Scholar] [CrossRef]
- Herrera-Foessel, S.A.; Singh, R.P.; Huerta-Espino, J.; Crossa, J.; Djurle, A.; Yuen, J. Evaluation of slow rusting resistance components to leaf rust in CIMMYT durum wheats. Euphytica 2007, 155, 361–369. [Google Scholar] [CrossRef]
- Herrera-Foessel, S.A.; Lagudah, E.S.; Huerta-Espino, J.; Hayden, M.J.; Bariana, H.S.; Singh, D.; Singh, R.P. New slow-rusting leaf rust and stripe rust resistance genes Lr67 and Yr46 in wheat are pleiotropic or closely linked. Theor. Appl. Genet. 2011, 122, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Negri, V.; Maxted, N.; Veteläinen, M. European Landraces Conservation: An Introduction. In European Landraces: On-Farm Conservation, Management and Use; Veteläinen, M., Negri, V., Maxted, N., Eds.; Bioversity International: Rome, Italy, 2009; pp. 1–22. [Google Scholar]
- Loladze, A.; Kthiri, D.; Pozniak, C.; Ammar, K. Genetic analysis of leaf rust resistance in six durum wheat genotypes. Phytopathology 2014, 104, 1322–1328. [Google Scholar] [CrossRef]
- Qureshi, N.; Bariana, H.; Kolmer, J.A.; Miah, H.; Bansal, U. Genetic and molecular characterization of leaf rust resistance in two durum wheat landraces. Phytopathology 2017, 107, 1381–1387. [Google Scholar] [CrossRef]
- Huerta-Espino, J.; Contreras, M.E.R.; Garcia, M.F.R.; Mir, H.E.V.; Mir, S.G.L.; Rangel, E.E. Genetic variation of resistance against Puccinia triticina E. in durum wheats from Oaxaca, Mexico. Rev. Fitotec. Mex. 2011, 34, 35–41. [Google Scholar]
- Broers, L.H.M.; Dehaan, A.A. Relationship between the origin of European landraces and the level of partial resistance to wheat leaf rust. Plant Breed. Z. Pflanzenzucht. 1994, 113, 75–78. [Google Scholar] [CrossRef]
- Martinez, F.; Niks, R.E.; Moral, A.; Urbano, J.M.; Rubiales, D. Search for partial resistance to leaf rust in a collection of ancient Spanish wheats. Hereditas 2001, 135, 193–197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ruiz, M.; Giraldo, P.; Royo, C.; Carrillo, J.M. Creation and validation of the spanish durum wheat core collection. Crop Sci. 2013, 53, 2530–2537. [Google Scholar] [CrossRef]
- Ruiz, M.; Giraldo, P.; González, J.M. Phenotypic variation in root architecture traits and their relationship with eco-geographical and agronomic features in a core collection of tetraploid wheat landraces (Triticum turgidum L.). Euphytica 2018, 214, 54. [Google Scholar] [CrossRef]
- Ruiz, M.; Bernal, G.; Giraldo, P. An update of low molecular weight glutenin subunits in durum wheat relevant to breeding for quality. J. Cereal Sci. 2018, 83, 236–244. [Google Scholar] [CrossRef]
- Pascual, L.; Fernández, M.; Aparicio, N.; López-Fernández, M.; Fité, R.; Giraldo, P.; Ruiz, M. Development of a multipurpose core collection of bread wheat based on high-throughput genotyping data. Agronomy 2020, 10, 534. [Google Scholar] [CrossRef]
- Paillard, S.; Goldringer, I.; Enjalbert, J.; Trottet, M.; David, J.; De Vallavieille-Pope, C.; Brabant, P. Evolution of resistance against powdery mildew in winter wheat populations conducted under dynamic management. II. Adult plant resistance. Theor. Appl. Genet. 2000, 101, 457–462. [Google Scholar] [CrossRef]
- Endresen, D.T.F.; Street, K.; Mackay, M.; Bari, A.; de Pauw, E. Predictive association between biotic stress traits and eco-geographic data for wheat and barley landraces. Crop Sci. 2011, 51, 2036–2055. [Google Scholar] [CrossRef]
- Endresen, D.T.F.; Street, K.; Mackay, M.; Bari, A.; Amri, A.; de Pauw, E.; Nazari, K.; Yahyaoui, A. Sources of resistance to stem rust (Ug99) in bread wheat and durum wheat identified using focused identification of germplasm strategy. Crop Sci. 2012, 52, 764–773. [Google Scholar] [CrossRef]
- Bari, A.; Street, K.; Mackay, M.; Endresen, D.T.F.; de Pauw, E.; Amri, A. Focused identification of germplasm strategy (FIGS) detects wheat stem rust resistance linked to environmental variables. Genet. Resour. Crop Evol. 2012, 59, 1465–1481. [Google Scholar] [CrossRef]
- Bari, A.; Amri, A.; Street, K.; Mackay, M.; De Pauw, E.; Sanders, R.; Nazari, K.; Humeid, B.; Konopka, J.; Alo, F. Predicting resistance to stripe (yellow) rust (Puccinia striiformis) in wheat genetic resources using focused identification of germplasm strategy. J. Agric. Sci. 2014, 152, 906–916. [Google Scholar] [CrossRef]
- Ruiz, M.; Giraldo, P.; Royo, C.; Villegas, D.; Aranzana, M.J.; Carrillo, J.M. Diversity and genetic structure of a collection of Spanish durum wheat landraces. Crop Sci. 2012, 52, 2262–2275. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Chang, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- McNeal, F.H.; Konzak, C.F.; Smith, E.P.; Tate, W.S.; Russell, T.S. A Uniform System for Recording and Processing Cereal Research Data; USDA Agricultural Research Service: Washington, DC, USA, 1971; pp. 34–121. [Google Scholar]
- Petersen, R.G. Augmented designs for preliminary yield trials (revised). Rachis 1985, 4, 27–32. [Google Scholar]
- Peterson, R.F.; Campbell, A.B.; Hannan, A.E. A diagrammatic scale for estimating rust intensity of leaves and stem of cereals. Can. J. Res. Sect. 1948, 26, 496–500. [Google Scholar] [CrossRef]
- Parra-Quijano, M.; Torres, E.; Iriondo, J.M.; López, F. Capfitogen Tools. User Manual Version 2.0. International Treaty on Plant Genetic Resources for Food and Agriculture; FAO Books: Rome, Italy, 2015; ISBN 9789253082551. [Google Scholar]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Scott, J.M.; Heglund, P.; Morrison, M.L.; Wall, W.A.; Haufler, J. Predicting Species Occurrences: Issues of Accuracy and Scale; Island Press: Washington, DC, USA, 2002; ISBN 1-55963-787-0. [Google Scholar]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Park, R.F.; McIntosh, R.A. Adult plant resistances to Puccinia recondita f. sp. tritici in wheat. N. Zeal. J. Crop Hortic. Sci. 1994, 22, 151–158. [Google Scholar] [CrossRef][Green Version]
- Aoun, M.; Breiland, M.; Kathryn Turner, M.; Loladze, A.; Chao, S.; Xu, S.S.; Ammar, K.; Anderson, J.A.; Kolmer, J.A.; Acevedo, M. Genome-Wide Association Mapping of Leaf Rust Response in a Durum Wheat Worldwide Germplasm Collection. Plant Genome 2016, 9. [Google Scholar] [CrossRef]
- Soleiman, N.H.; Solis, I.; Soleiman, M.H.; Sillero, J.C.; Villegas, D.; Alvaro, F.; Royo, C.; Serra, J.; Ammar, K.; Martinez-Moreno, F. Emergence of a new race of leaf rust with combined virulence to Lr14a and Lr72 genes on durum wheat. Span. J. Agric. Res. 2016, 14, 1–4. [Google Scholar] [CrossRef]
- Ordoñez, M.E.; Kolmer, J. Simple sequence repeat diversity of a worldwide collection of Puccinia triticina from durum wheat. Phytopathology 2007, 97, 574–583. [Google Scholar] [CrossRef][Green Version]
- Herrera-Foessel, S.A.; Singh, R.P.; Huerta-Espino, J.; Yuen, J.; Djurle, A. New genes for leaf rust resistance in CIMMYT durum wheats. Plant Dis. 2005, 89, 809–814. [Google Scholar] [CrossRef]
- Mantovani, P.; Maccaferri, M.; Tuberosa, R.; Kolmer, J. Virulence Phenotypes and Molecular Genotypes in Collections of Puccinia triticina from Italy. Plant Dis. 2010, 94, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R. Past, present and future opportunities in breeding for disease resistance, with examples from wheat. Euphytica 1992, 63, 3–22. [Google Scholar] [CrossRef]
- Kolmer, J. Leaf Rust of Wheat: Pathogen Biology, Variation and Host Resistance. Forests 2013, 4, 70–84. [Google Scholar] [CrossRef]
- Rubiales, D.; Niks, R.E. Characterization of Lr34, a major gene conferring nonhypersensitive resistance to wheat leaf rust. Plant Dis. 1995, 79, 1208–1212. [Google Scholar] [CrossRef]
- Piarulli, L.; Gadaleta, A.; Mangini, G.; Signorile, M.A.; Pasquini, M.; Blanco, A.; Simeone, R. Molecular identification of a new powdery mildew resistance gene on chromosome 2BS from Triticum turgidum ssp. dicoccum. Plant Sci. 2012, 196, 101–106. [Google Scholar] [CrossRef]
- Liu, X.M.; Brown-Guedira, G.L.; Hatchett, J.H.; Owuoche, J.O.; Chen, M.S. Genetic characterization and molecular mapping of a Hessian fly-resistance gene transferred from T. turgidum ssp. dicoccum to common wheat. Theor. Appl. Genet. 2005, 111, 1308–1315. [Google Scholar] [CrossRef]
- McIntosh, R.; Wellings, C.R.; Park, R.F. Wheat Rusts, An Atlas of Resistance Genes; CSIRO Publications: Melbourne, Australia, 1995. [Google Scholar]
- Hussein, S.; Spies, J.J.; Pretorius, Z.A.; Labuschagne, M.T. Chromosome locations of leaf rust resistance genes in selected tetraploid wheats through substitution lines. Euphytica 2005, 141, 209–216. [Google Scholar] [CrossRef]
- Oliveira, H.R.; Campana, M.G.; Jones, H.; Hunt, H.V.; Leigh, F.; Redhouse, D.I.; Lister, D.L.; Jones, M.K. Tetraploid wheat landraces in the Mediterranean basin: Taxonomy, evolution and genetic diversity. PLoS ONE 2012, 7, e37063. [Google Scholar] [CrossRef]
- Giraldo, P.; Royo, C.; González, M.; Carrillo, J.M.; Ruiz, M. Genetic diversity and association mapping for agromorphological and grain quality traits of a structured collection of durum wheat landraces including subsp. durum, turgidum and diccocon. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Kolmer, J.A.; Garvin, D.F.; Hayden, M.; Spielmeyer, W. Adult plant leaf rust resistance derived from the wheat landrace cultivar Americano 44d is conditioned by interaction of three QTL. Euphytica 2018, 214. [Google Scholar] [CrossRef]
- Qureshi, N.; Bariana, H.; Kumran, V.V.; Muruga, S.; Forrest, K.L.; Hayden, M.J.; Bansal, U. A new leaf rust resistance gene Lr79 mapped in chromosome 3BL from the durum wheat landrace Aus26582. Theor. Appl. Genet. 2018, 131, 1091–1098. [Google Scholar] [CrossRef]
- Bonman, J.M.; Bockelman, H.E.; Jin, Y.; Hijmans, R.J.; Gironella, A.I.N. Geographic distribution of stem rust resistance in wheat landraces. Crop Sci. 2007, 47, 1955–1963. [Google Scholar] [CrossRef]
- Urríes, M.J. Las royas de los cereales. Bol. Inst. Nac. Inv. Agron. 1950, 23, 397–476. [Google Scholar]
- Daamen, R.A.; Stubbs, R.W.; Stol, W. Surveys of Cereal Diseases and Pests in the Netherlands. 4. Occurrence of Powdery Mildew and Rusts in Winter-Wheat. Neth. J. Plant Pathol. 1992, 98, 301–312. [Google Scholar] [CrossRef]
- Moschini, R.C.; Pérez, B.A. Predicting Wheat Leaf Rust Severity Using Planting Date, Genetic Resistance, and Weather Variables. Plant Dis. 1999, 83, 381–384. [Google Scholar] [CrossRef]
- Barkley, A.; Tack, J.; Nalley, L.L.; Bergtold, J.; Bowden, R.; Fritz, A. Weather, disease, and wheat breeding effects on Kansas wheat varietal yields, 1985 to 2011. Agron. J. 2014, 106, 227–235. [Google Scholar] [CrossRef]
- Eversmeyer, M.G.; Kramer, C.L. Models of early spring survival of wheat leaf rust in the central Great Plains. Plant Dis. 1998, 82, 987–991. [Google Scholar] [CrossRef]
- Savary, S.; Jouanin, C.; Félix, I.; Gourdain, E.; Piraux, F.; Willocquet, L.; Brun, F. Assessing plant health in a network of experiments on hardy winter wheat varieties in France: Multivariate and risk factor analyses. Eur. J. Plant Pathol. 2016, 146, 757–778. [Google Scholar] [CrossRef]
- Hýsek, J.; Vavera, R.; Růžek, P. Influence of temperature, precipitation, and cultivar characteristics on changes in the spectrum of pathogenic fungi in winter wheat. Int. J. Biometeorol. 2017, 61, 967–975. [Google Scholar] [CrossRef]
- El Bouhssini, M.; Street, K.; Joubi, A.; Ibrahim, Z.; Rihawi, F. Sources of wheat resistance to Sunn pest, Eurygaster integriceps Puton, in Syria. Genet. Resour. Crop Evol. 2009, 56, 1065–1069. [Google Scholar] [CrossRef]
- El Bouhssini, M.; Street, K.; Amri, A.; Mackay, M.; Ogbonnaya, F.C.; Omran, A.; Abdalla, O.; Baum, M.; Dabbous, A.; Rihawi, F. Sources of resistance in bread wheat to Russian wheat aphid (Diuraphis noxia) in Syria identified using the Focused Identification of Germplasm Strategy (FIGS). Plant Breed. 2011, 130, 96–97. [Google Scholar] [CrossRef]
- Bhullar, N.K.; Zhang, Z.; Wicker, T.; Keller, B. Wheat gene bank accessions as a source of new alleles of the powdery mildew resistance gene Pm3: A large scale allele mining project. BMC Plant Biol. 2010, 10, 88. [Google Scholar] [CrossRef]

| Observed | |||
|---|---|---|---|
| Resistant | Susceptible | ||
| Predicted | Resistant | a | b |
| Susceptible | c | d | |
| Subspecies/Isolate | No. | CDJ13 | JDJ15 | PG14 | |||
|---|---|---|---|---|---|---|---|
| No. | % | No. | % | No. | % | ||
| Domesticated emmer wheat | 10 | 6 | 67 | 6 | 75 | 10 | 100 |
| Rivet wheat | 32 | 7 | 23 | 6 | 20 | 18 | 56 |
| Durum wheat | 52 | 7 | 14 | 12 | 24 | 47 | 90 |
| Total | 94 | 20 | 22 | 24 | 28 | 75 | 80 |
| Isolate | Bioclimatic (Thermal Variables) | Bioclimatic (Hydric Variables) |
|---|---|---|
| CDJ13 | Isothermality (+) 1 | - |
| JDJ15 | Temperature Seasonality (−) | October precipitation (+) |
| Annual temperature range (−) | November precipitation (+) | |
| Mean daily temperature range (−) | Precipitation of wettest month (+) | |
| July maximum temperature (−) | Precipitation of wettest quarter (+) | |
| Maximum temperature of hottest month (−) | December precipitation (+) | |
| August maximum temperature (−) | Annual precipitation (+) | |
| July mean temperature (−) | January precipitation (+) | |
| Precipitation of coldest quarter (+) | ||
| February precipitation (+) |
| Isolate | Bioclimatic (Thermal Variables) | Bioclimatic (Hydric Variables) |
|---|---|---|
| CDJ13 | Annual temperature range | |
| April maximum temperature | ||
| Isothermality | ||
| JDJ15 | February maximum temperature | November precipitation |
| Annual temperature range | ||
| Isothermality |
| Correct Classification (Frequency) | Kappa Coefficient | Sensitivity (Frequency) | Specificity (Frequency) | ||
|---|---|---|---|---|---|
| CDJ13 | set 1 1 | 0.93 | 0.58 | 0.80 | 0.94 |
| set 2 | 1.00 | na | na | 1.00 | |
| set 3 | 0.97 | 0.87 | 0.80 | 1.00 | |
| JDJ15 | set 1 | 0.89 | 0.45 | 0.80 | 0.90 |
| set 2 | 1.00 | na | na | 1.00 | |
| set 3 | 0.97 | 0.87 | 0.80 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez-Moreno, F.; Giraldo, P.; Cátedra, M.d.M.; Ruiz, M. Evaluation of Leaf Rust Resistance in the Spanish Core Collection of Tetraploid Wheat Landraces and Association with Ecogeographical Variables. Agriculture 2021, 11, 277. https://doi.org/10.3390/agriculture11040277
Martínez-Moreno F, Giraldo P, Cátedra MdM, Ruiz M. Evaluation of Leaf Rust Resistance in the Spanish Core Collection of Tetraploid Wheat Landraces and Association with Ecogeographical Variables. Agriculture. 2021; 11(4):277. https://doi.org/10.3390/agriculture11040277
Chicago/Turabian StyleMartínez-Moreno, Fernando, Patricia Giraldo, María del Mar Cátedra, and Magdalena Ruiz. 2021. "Evaluation of Leaf Rust Resistance in the Spanish Core Collection of Tetraploid Wheat Landraces and Association with Ecogeographical Variables" Agriculture 11, no. 4: 277. https://doi.org/10.3390/agriculture11040277
APA StyleMartínez-Moreno, F., Giraldo, P., Cátedra, M. d. M., & Ruiz, M. (2021). Evaluation of Leaf Rust Resistance in the Spanish Core Collection of Tetraploid Wheat Landraces and Association with Ecogeographical Variables. Agriculture, 11(4), 277. https://doi.org/10.3390/agriculture11040277