Abstract
Malaysia has begun to locally mass-cultivate grain corn to reduce import dependency for animal feed industries. Since the Malaysian tropical climate constantly exposes grain corn to fungal colonization and mycotoxin production by mycotoxigenic species, it is, therefore, important to investigate the presence of fungal species, especially the mycotoxigenic strains in the Malaysian grain corn agroecosystem. In the present work, corn kernel, tassel, plant debris, and soil were collected from two pioneer grain corn farms (Kampong Dadong, KD; Rhu Tapai, RT), and morphological and molecular identifications were conducted. A total of 131 fungal isolates from 30 fungal species were recovered. Both KD and RT yielded log 4.7–6.7 CFU/g total fungal loads. Fusarium verticillioides was predominant in both farms, followed by the phytopathogenic Lasiodiplodia theobromae and the mycotoxigenic Aspergillus flavus, A. niger, F. incarnatum, and F. proliferatum. Mycotoxin analyses by high-performance liquid chromatography revealed that among 30 mycotoxigenic isolates tested for aflatoxins, deoxynivalenol, fumonisins, HT-2, T-2, ochratoxins A, and zearalenone, approximately 25 of the isolates could produce at least one mycotoxin in vitro. The present work serves as a baseline for more comprehensive research to better predict and control fungal contamination and the subsequent mycotoxin accumulation in Malaysian grain corn agroecosystems.
1. Introduction
Grain corn (also known as field corn) is typically utilized as a key ingredient in animal feed destined for ruminant, poultry, and swine due to it being an excellent source of energy. In Malaysia, however, over the past decades, grain corn supply for the local animal feed industries has been entirely dependent on importation, mainly from Argentina and Brazil [1]. The huge increase in the grain corn import bill (MYR 3.09 billion in 2016; Malaysian Department of Statistics) [2] has substantially contributed to the country’s economic burden, and if left unchecked, may eventually jeopardize the local food security. To reduce the country’s dependency on grain corn importation, Malaysia has taken the initiative to locally mass-cultivate grain corn. To further strengthen this effort, grain corn also has been recently gazetted by the Malaysian Ministry of Agriculture and Food Industries as one of the five new wealth-generating crop commodities alongside coconut, durian, dairy, and culinary industries, as announced in the Malaysian 2018 budget [3]. Following this, two pioneer grain corn plantations have been established in Kampong Dadong (13 hectares cultivated area) and Rhu Tapai (3.8 hectares cultivated area) in Terengganu, Malaysia, to maximize land use and increase farmers’ income by intercropping grain corn with rice paddy.
Like other cereals, grain corn is naturally susceptible to fungal infestation preharvest. The tropical climate of Malaysia, which is hot and humid all year round, further provides favorable conditions for fungal proliferation, following which the grain corn yields and quality would be severely affected. Furthermore, certain fungal contaminants could also be mycotoxigenic, which increases the risk of mycotoxin contamination of the grain corn. Mycotoxins are low molecular weight and thermal-stable secondary metabolites of fungi capable of causing diseases and death in vertebrates [4]. Although the primary role of grain corn is to provide carbohydrate sources in animal feed, mycotoxin-contaminated feed consumed by poultry or ruminants can also indirectly contaminate humans as the end consumers, thus posing a serious issue in food safety. To humans and animals, mycotoxins have been shown to be carcinogenic, hepatotoxic, nephrotoxic, mutagenic, and teratogenic [4].
Fungi from the genera Aspergillus, Fusarium, and Penicillium, have often been associated with corn pre- and post-harvest and can produce some of the most relevant mycotoxins in corn, such as aflatoxins, fumonisins, ochratoxins, trichothecenes, and zearalenone [5,6]. Since mycotoxins are thermal-stable, they are likely to persist in the animal feed even after grain corn processing and storage. Upon ingestion of mycotoxin-contaminated feed, different animals will respond differently and develop different symptoms/syndromes, as reviewed by Zain [7]. Aflatoxins (AFB1, AFB2, AFG1, AFG2) produced by Aspergillus section Flavi have been linked to hepatocellular carcinoma in animals. Ochratoxin A (OTA) produced by Aspergillus spp. and Penicillium spp. have been shown to be a potent teratogen and nephrotoxin in poultry and pigs. Fumonisins (FB1, FB2) produced by F. verticillioides and F. proliferatum have been linked with neural tube defects and acute toxicity in pigs and horses. Other mycotoxins produced by Fusarium spp. in corn are trichothecene type A (e.g., HT-2, T-2, neosolaniol), type B (e.g., deoxynivalenol; DON, nivalenol; NIV), and zearalenone (ZEN). Trichothecenes have been associated with mycotoxicoses in animals with symptoms such as hemorrhage, edema, and reduced milk and egg production. Deoxynivalenol has been shown to induce feed refusal and vomiting in pigs, while zearalenone has been implicated with hyperestrogenism in pigs [6,7].
Most previous research on fungal species and the potential mycotoxin accumulation by mycotoxigenic strains in Malaysian corn preharvest (sweet corn) rarely discussed the colonization of fungal species other than Fusarium spp. [8,9,10,11]. Although that is understandable since members of Fusarium genera are notorious contaminants of corn, the tropical climate of Malaysia is also a suitable breeding ground for many other fungal species that may thrive in this weather. Further, this tropical environment may as well stimulate greater proliferation and mycotoxin production of other mycotoxigenic species, such as Aspergillus section Flavi, important mycotoxigenic fungi that produce deleterious aflatoxins [12]. Therefore, it is important to establish more thorough information on the fungal community structure that exists within the Malaysian corn agroecosystem, specifically in the pioneer grain corn farms. Such information may assist in better prediction of possible fungal contamination and the subsequent mycotoxin production within the said agroecosystem. This, in turn, may facilitate the development and establishment of proper management and control strategies in order to minimize fungal-related diseases and their mycotoxin formation in the farms [13]. Thus, the present work was aimed (1) to investigate the fungal community structure present in the grain corn agroecosystem from two pioneer grain corn farms in Terengganu, with special emphasis on the mycotoxigenic species; and (2) to evaluate the mycotoxigenic potential of the mycotoxigenic fungal isolates from both grain corn farms. This is the first work that provides information on fungal community structure and the occurrence of mycotoxigenic species, as well as their mycotoxigenic potentials in the agroecosystems of pioneer grain corn plantations in Malaysia.
2. Materials and Methods
2.1. Sampling
The present work was conducted during the cropping season of March–August 2017 at two grain corn farms in Terengganu. Both grain corn farms, namely Kampong Dadong (latitude: 4°16′47.2512′′ N; longitude: 103°13′44.238′′ E) and Rhu Tapai (latitude: 5°30′48.15′′ N; longitude: 102°58′48.018′′ E) are lowlands located at the southern and north-east region of Terengganu, respectively. Both farms were gazetted by the Malaysian government as pioneer farms intended for mass-cultivation and commercialization. The soil at Kampong Dadong is riverine alluvium soil (i.e., USDA taxonomy: ultisol, texture class: fine sandy clay), while that of Rhu Tapai is beach ridges interspersed with swales soil (BRIS; USDA taxonomy: spodosol, texture class: sandy). Both farms practiced a conventional farming system. Soil tillage was conducted twice before sowing. Fertilizers and chemical pesticides (herbicides, fungicides, and insecticides) were applied before sowing and during plantation. In Kampong Dadong, corn plantation was rotated with rice paddy. After harvesting of paddy and before corn sowing, a microbial decomposer was applied on the field to decompose the rice straw [14]. The data on monthly temperature (°C), relative humidity (%) and rainfall (mm) during the cropping season were provided by the Malaysian Meteorological Department, Ministry of Energy, Science, Technology, Environment, and Climate Change, and obtained from the closest meteorological station to the grain corn farms.
A total of 24 samples of cobs (n = 6), tassels (n = 6), debris (n = 6), and soil (n = 6) were aseptically collected at the late maturation stage of the grain corn nearing the harvesting period; 2 farms × 4 sample types × 3 subsamples. Subsamples were collected from three sampling points in a diagonal section across a plot. The soil samples were collected from the upper layer (≈13 cm depth) of the plot upon which microbial activity primarily occurs [15]. All samples were placed in sterile polyethylene sampling bags before immediately transported to the laboratory and refrigerated at 4 °C. The grain corn kernels were threshed from the cobs, placed in another sterile polyethylene bag, and also refrigerated at 4 °C. Samples were analyzed within one to two days after sampling.
2.2. Isolation and Enumeration of Indigenous Fungi
Quantitative enumeration of fungal load by dilution plating of each debris, kernel, soil, and tassel sample was performed on dichloran rose bengal chloramphenicol (DRBC; Oxoid, Basingstoke, UK) agar. Prior to homogenization, grain corn kernels were left to soak with sterile 0.1% peptone water for 60 min. For homogenization, 10 g of each sample were mixed with 90 mL of sterile 0.1% peptone (Oxoid, Basingstoke, UK) water and homogenized for 2 min using a stomacher (Seward Ltd., Worthing, UK). Then, serial dilutions of 10−2 to 10−4 were performed, and 0.1 mL aliquot from each dilution were spread-plated on DRBC in triplicate [16,17]. Inoculated plates were incubated in the dark at 30 °C for 7 d. Following incubation, plates with 10–100 colony-forming units (CFU) were used to calculate the fungal populations, and results were expressed as CFU per gram of sample (CFU/g). Next, fungal colonies of different morphology were subcultured onto fresh potato dextrose agar (PDA; Oxoid, Basingstoke, UK) and incubated at 30 °C for 7 d to obtain axenic culture for further identification.
Direct plating of grain corn kernels and tassels was performed on dichloran-glycerol 18 (DG-18; Oxoid, Basingstoke, UK) agar and PDA supplemented with 0.0001% (w/v) chloramphenicol (Merck, Darmstadt, Germany). DG-18 was used to isolate moderately xerophilic fungi. Prior to inoculation, the tassel was cut to 0.5–1.0 cm length. Next, both samples were immersed in 5% (v/v) sodium hypochlorite separately (RandM Chemicals, London, UK) for 5 min, followed by rinsing twice with sterile distilled water. Five of each kernel and tassel were aseptically inoculated at equal distance on both growth media. All plates were incubated in the dark at 30 °C for 7 d. Following incubation, the isolation frequency (%) of a species recovered from the kernel and tassel samples was calculated by dividing the number of infected kernel/tassel by the total number of kernel/tassel inoculated on a plate [18]. Next, fungal colonies of different morphology were subcultured onto fresh PDA and incubated at 30 °C for 7 d to obtain axenic culture for further identification.
2.3. Morphological and Molecular Identification of Fungal Isolates
Fungal isolates were distinguished macromorphologically by their colony color (reverse and obverse), texture and size, and pigmentation. The micromorphological identification was done using an optical microscope (Olympus, Tokyo, Japan) to distinguish fungal colonies based on their mycelial characteristic and/or spore formation and morphology. Aspergillus spp. were morphologically identified based on keys provided by Pitt and Hocking [18]. Penicillium spp. were morphologically identified based on keys provided by Pitt and Hocking [18], Samson et al. [19], and Visagie et al. [20]. Fusarium spp. were morphologically identified based on keys provided by Leslie and Summerell [21]. The remaining fungal isolates were morphologically identified based on keys provided by Pitt and Hocking [18] and Watanabe [22]. Thereafter, all fungal isolates were grouped according to similar morphology.
The representative isolates of distinct morphology were molecularly identified/validated using polymerase chain reaction (PCR). Briefly, fungal mycelia/spores from axenic solid cultures were suspended in 10 mL of potato dextrose broth (PDB; Oxoid, Basingstoke, UK) and incubated with shaking at 100 rpm and 30 °C for 1–5 d, depending on the fungal growth characteristic of either fast or slow. Then, fungal cultures were harvested by filtration using sterile filter paper No. 1 (Whatman, Kent, UK), followed by washing with sterile distilled water before left to dry under a laminar flow. Subsequently, the dried mycelia were grounded to a fine powder in liquid nitrogen with a mortar and pestle before transferred into a 2 mL Eppendorf tube. The genomic DNA extraction was performed using the DNeasy plant mini kit (Qiagen, Germantown, MD, USA) following the manufacturer’s instructions, and the purified DNA was stored at −20 °C until further analyses.
PCR reaction for internal transcribed spacer (ITS) region was performed using a primer pair ITS1: 5′ TCC GTA GGT GAA CCT GCG GCG G 3′ and ITS 4: 5′ TCC TCC GCT TAT TGA TAT GC 3′. The PCR reaction mixture was prepared in a total volume of 25 µL, which contained 12.5 µL of 1× HotStarTaq Plus Master Mix (Qiagen, Maryland, USA), 2.5 µL of 1× CoralLoad concentrate, which contained orange and red gel-tracking dye (Qiagen, Maryland, USA), 1.25 µL (0.5 µM) of each primer, 2.0 µL of DNA template, and 6.75 µL of RNase-free water (Qiagen, Maryland, USA). The HotStarTaq Plus Master Mix consisted of HotStarTaq Plus DNA polymerase, PCR buffer with 3 mM magnesium chloride (MgCl2), and 400 µM of each dNTP. The amplification program was carried out using SuperCycler SC-200 (Kyratec, Mansfield, QLD, Australia) under the following condition: an initial activation step at 95 °C for 2 min; 30 cycles of denaturation at 95 °C for 45 s, annealing at 52 °C for 30 s, and extension at 72 °C for 1 min 20 s; followed by a final extension at 72 °C for 10 min. Next, the amplicons were separated by gel electrophoresis using 1% agarose gel (Promega, Madison, WI, USA) stained with 3 µL of ethidium bromide (Promega, Wisconsin, USA) and run in 1× Tris-acetate-ethylene diamine tetraacetic acid. The electrophoresis was conducted for 45 min at 90 V and 400 mA. The amplified genes were visualized under ultraviolet light using gel documentation (Syngene, Bangalore, India), and their size was estimated using Gel Pilot® 100 bp Plus DNA ladder (Qiagen, Maryland, USA). The amplicons were then submitted to a local service provider (MyTACG Bioscience Enterprise, Kuala Lumpur, Malaysia) for DNA purification and sequencing. The sequences obtained were edited and aligned using BioEdit version 7.2 before compared with other sequences previously deposited in the National Centre for Biotechnology Information (NCBI) database using the basic local alignment search tool (BLAST) tool (https://blast.ncbi.nlm.nih.gov, accessed on 29 January 2021). BLAST search results showing ≥99% similarity with the isolates’ ITS sequences were considered for assigning fungal isolates to the species name. The fungal species names were then validated with databases from Index Fungorum (http://www.indexfungorum.org, accessed on 29 January 2021) and Mycobank (http://www.mycobank.org, accessed on 29 January 2021), and fungal isolates were then assigned to the names used in the present work. To deposit the sequences onto the GenBank database, the sequences were first transformed into FASTA format. The FASTA format line (also known as FASTA definition line) includes the “>” symbol, followed by sequence ID (SeqID), sequence source information (known as Source Modifier), and feature annotation (biological features, e.g., genes, structural RNA). In the present work, the FASTA definition line was “>SeqID [organism = XXX] [strain = XXX] [isolation_source = XXX] [country = XXX] 18 S small subunit ribosomal RNA, partial sequence; internal transcribed spacer 1, 5.8 S ribosomal RNA, internal transcribed spacer 2, complete sequence; 28 S large subunit ribosomal RNA, partial sequence”. Sequence was added after “hard return” (ENTER button). All sequences were kept in one file and directly submitted to GenBank via their web-based submission tool, BankIt (https://www.ncbi.nlm.nih.gov/WebSub/, accessed on 29 January 2021). Following successful sequence deposition, the GenBank would release accession numbers that give the scientific community a unique label for each species/strain with which they may retrieve the species/strain data from the GenBank online servers.
2.4. Preparation of Grain Corn Agar as Semi-Synthetic Growth Medium
Grain corn agar (GCA) with grain corn as natural substrate was used as a semi-synthetic growth medium to simulate the mycotoxin-producing ability of fungal isolates on natural grain corn [23]. Briefly, grain corn kernels were ground to a fine powder using a Waring blender (Waring, Torrington, CT, USA). Next, approximately 30 g of fine grain corn powder and 15 g of technical agar (Oxoid, Basingstoke, UK) as a solidifying agent were added into 1 L distilled water [24]. The medium was autoclaved at 121 °C and 15 psi for 15 min, before poured into 90 mm Ø Petri plates and left to solidify. Solidified GCA plates were refrigerated at 4 °C prior to fungal inoculation.
2.5. Mycotoxigenic Potentials of Fungal Isolates
The mycotoxigenic potentials of A. flavus (i.e., AFB1, AFB2, AFG1, AFG2), A. niger (i.e., OTA), and Fusarium spp. (i.e., DON, FB1, FB2, HT-2, T-2, ZEN) were determined by reverse-phase high-performance liquid chromatography (HPLC; Waters 600, Haverhill, MA, USA) using a C18 column (Gemini®; 5 µm, 250 × 4.6 mm; Phenomenex, Washington, DC, USA). For mycotoxin extraction, 10 µL spore suspension of each isolate was inoculated centrally on GCA and incubated in the dark at 30 °C for 10 d. Next, five mycelial plugs were transferred into preweighed Eppendorf (Hamburg, Germany) tubes before the final weight was recorded. Prior to mycotoxin quantification, the mobile phases were filtered using a nylon membrane filter (0.45 µm; Merck, Darmstadt, Germany) and sonicated using an ultrasonic bath (Power-Sonic 420, Seoul, Korea) for 30 min to degas. Ultrapure water for the mobile phase was obtained from Elga Purelab Classic UV MK2 (Lane End, UK). Following quantification, data were processed using Empower 2 chromatography data software (Waters, MA, USA).
2.5.1. Extraction and Quantification of Aflatoxins
The extraction and quantification of aflatoxins were performed following the method described by Bragulat et al. [25] with slight modification. Briefly, 1000 μL of absolute methanol (Merck, Darmstadt, Germany) was added to the Eppendorf (Hamburg, Germany) tube containing mycelial plugs, and the mixture was vortexed using a vortex mixer (LMS Co. Ltd., Tokyo, Japan). Then, the tube was incubated for 30 min at room temperature before centrifuged (Sartorius, Göttingen, Germany) at 10,000× g for 5 min. The resulting extract was filtered through a nylon syringe filter (0.22 µm; Macherey-Nagel, Düren, Germany) into HPLC vials (Thermo Scientific, Waltham, MA, USA). The aflatoxin separation and detection were done with a mobile phase of methanol:acetonitrile:water (35:55:10, v/v/v) at 40 °C and a flow rate of 0.6 mL/min. The injection volume was 20 µL. A post-column (i.e., photochemical reactor for enhanced detection, PHRED; Aura Industries, New York, NY, USA) was employed for aflatoxin derivatization. A fluorescence detector (Waters 2475, Watertown, MA, USA) with the excitation and emission wavelengths of 365 nm and 435 nm, respectively [26] was employed.
2.5.2. Extraction and Quantification of Ochratoxin A
The extraction and quantification of OTA were performed following the method described by Mohale et al. [27]. Briefly, 1000 µL of absolute methanol was added to the Eppendorf (Hamburg, Germany) tube containing mycelial plugs, and the mixture was shaken for 60 min. The resulting supernatant was filtered using a 0.22 µm nylon syringe filter into HPLC vials. Next, OTA was separated with a mobile phase of acetonitrile:water:glacial acetic acid (57:41:2, v/v/v) and a flow rate of 1.0 mL/min. The injection volume was 20 µL. A fluorescence detector was employed for OTA detection with the excitation and emission wavelengths of 360 nm and 440 nm, respectively. Acetonitrile and glacial acetic acid used in the present work were purchased from Merck (Darmstadt, Germany) and RandM Chemicals (London, UK), respectively.
2.5.3. Extraction, Derivatization, and Quantification of Fumonisins
The extraction, derivatization, and quantification of fumonisins were performed following the method described by Visconti et al. [28]. Briefly, 1000 µL of methanol:acetonitrile:water (25:25:50, v/v) was added to the Eppendorf (Hamburg, Germany) tube containing mycelial plugs, vigorously vortexed before shaken for 60 min, and filtered with nylon syringe filter (0.22 µm) into HPLC vials. Then, the filtrate was evaporated to dryness in a stream of nitrogen before redissolved in the mobile phase for HPLC analysis. Prior to analysis, the fumonisin standards and samples (50 µL) were derivatized with 100 µL ortho-phthalaldehyde (OPA; Sigma-Aldrich, St. Louis, MO, USA) for 1 min. The derivatization reagent was prepared by dissolving 40 mg OPA in 1 mL absolute methanol before adding 5 mL of 0.1 M disodium tetraborate (Chemiz, Selangor, Malaysia) and 50 μL of 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO, USA) in a capped amber vial. Fumonisin separation was done with a flow rate of 1.0 mL/min in a mobile phase of methanol: 0.1 M sodium dihydrogen phosphate (77:23, v/v) adjusted to pH 3.35 using phosphoric acid. Sodium dihydrogen phosphate was obtained from Chemiz (Selangor, Malaysia). The injection volume was 30 µL. Fumonisins were detected using a fluorescence detector with the excitation and emission wavelengths of 335 nm and 440 nm, respectively.
2.5.4. Extraction and Quantification of Trichothecenes
The extraction and quantification of DON were performed following the method described by Moazami and Jinap [29] with slight modification. Briefly, 1000 µL of acetonitrile:water (17:83, v/v) was added to the Eppendorf (Hamburg, Germany) tube containing mycelial plugs and vortexed for 30 s. Next, the tube was agitated using an orbital shaker (Scilogex, Rocky Hill, CT, USA) at 180 rpm for 60 min before centrifuged at 4200× g for 15 min. The resulting supernatant was filtered through a 0.22 µm nylon syringe filter into HPLC vials. Then, the filtrate was evaporated to dryness in a stream of nitrogen before redissolved in 500 µL of absolute methanol. The separation was performed with a mobile phase of acetonitrile:water (17:83, v/v) and a flow rate of 1.0 mL/min. The injection volume was 20 µL. A photodiode array detector (Waters 2996, Milford, MA, USA) with a wavelength set at 218 nm was used to detect DON.
The extraction and quantification of HT-2 and T-2 toxins were performed following the method described by Medina and Magan [30]. Briefly, 1000 µL of methanol:water (80:20 v/v) was added to the Eppendorf (Hamburg, Germany) tube containing mycelial plugs. Next, the tube was agitated in the dark for 90 min at 150 rpm before centrifuged at 8000× g for 15 min. Following filtration using a nylon syringe filter (0.22 µm), the extract was transferred into HPLC vials and evaporated to dryness in a stream of nitrogen. The dried extracts were redissolved in 300 µL of acetonitrile:water (50:50 v/v). The separation was done in gradient mode with the mobile phase of A (water) and B (acetonitrile). The gradient concentrations were initiated by holding for the first 3 min with 30% B, after which the condition was changed linearly to 55% B for 18 min. The condition was then changed to 99% B for 1 min and maintained for 5 min as a cleaning step to improve the results. After cleaning, the condition was returned to the initial 30% B. The flow rate was 1 mL/min. The injection volume was 50 µL. A photodiode array detector with a wavelength of 200 nm was employed to detect HT-2 and T-2 toxins.
2.5.5. Extraction and Quantification of Zearalenone
The extraction and quantification of ZEN were performed following the method described by Iqbal et al. [31] with slight modification. Briefly, 1000 µL of acetonitrile:water (90:10, v/v) was added to the Eppendorf (Hamburg, Germany) tube containing mycelial plugs and vortexed for 30 s. Next, the tube was agitated at 180 rpm for 60 min before centrifuged at 4200 × g for 15 min. Next, the supernatant was filtered through a 0.22 µm nylon syringe filter into HPLC vials. The filtrate was evaporated to dryness in a stream of nitrogen before redissolved in 250 µL of HPLC mobile phase. The separation was done with a mobile phase of acetonitrile:water:methanol (48:50:2, v/v/v) and a flow rate of 1.0 mL/min. The injection volume was 20 µL. ZEN was detected using a fluorescence detector with the excitation and emission wavelengths of 274 nm and 450 nm, respectively.
2.5.6. Linearity, Limit of Detection (LOD) and Limit of Quantification (LOQ)
Following mycotoxin detection, linear calibration curves were constructed by plotting the peak area (response) obtained from HPLC analysis against the concentrations of each standard calibrant solution, after which the actual mycotoxin production of fungal isolates was calculated. The standard calibrant solutions were prepared and diluted in different solvents as follows (i.e., AFB1, AFB2, AFG1, AFG2 and OTA in methanol; HT-2 and T-2 in acetonitrile; FB1, FB2, DON and ZEN in acetonitrile:water). The mycotoxin standards of AFB1, AFB2, AFG1, AFG2, DON, FB1, FB2, OTA, and ZEN used in the present work were obtained from Sigma-Aldrich (St. Louis, MO, USA), while those of HT-2 and T-2 were obtained from Trilogy (Washington, MO, USA).
Subsequently, linear regression was fitted to establish the correlation coefficient (R2). The limit of detection (LOD) and limit of quantification (LOQ) of each chromatographic analysis were estimated following the method prescribed by the International Conference on Harmonization (ICH) by using the formula; LOD = 3σ/s and LOQ = 10σ/s, in which σ was the standard deviation of blank responses, and s was the slope of the calibration curve [32]. The linearity, LOD, and LOQ of all analyses are shown in Table S1 Overall, the calibration curves yielded a strong linear response (R2 > 0.99) for all the mycotoxins analyzed.
2.6. Statistical Analysis
All experiments were conducted in three replicates (n = 3). Measurements were then averaged and presented as mean ± SE (standard error). The CFU of fungal isolates were tested for normality using Kolmogorov–Smirnov test before log-transformed to obtain variance homogeneity. Next, statistical analysis for the total fungal load was carried out using one-way analysis of variance (ANOVA) with a 95% confidence interval, and p < 0.05 was accepted as a significant difference. To compare the total fungal loads between farms, data on each farm were pooled. Grain corn farms and sample types served as independent variables. A post hoc test (i.e., Tukey’s honest significant difference; Tukey’s HSD) with α = 0.05 was applied to compare the significant difference between means of treatments. A statistical software Minitab® version 17 (Minitab Inc.; State College, PA, USA) was used to perform the analysis.
3. Results
3.1. Temperature, Relative Humidity, and Rainfall of Kampong Dadong and Rhu Tapai Grain Corn Farms during 2017 Cropping Season
Table 1 depicts the monthly mean temperature (°C), rainfall (mm), and relative humidity (%) during the 2017 cropping season of grain corn from Kampong Dadong and Rhu Tapai grain corn farms in Terengganu. The monthly temperatures of both grain corn farms during the cropping season were uniform with very little variation. High relative humidity and rainfall were also recorded at both grain corn farms. This climatic trend corresponds to the Af climate (i.e., tropical, fully humid) as categorized by the Köppen–Geiger climate classification [33].

Table 1.
Monthly mean temperature (°C), rainfall (mm), and relative humidity (%) during the 2017 cropping season of grain corn from Kampong Dadong and Rhu Tapai grain corn farms in Terengganu. Data were supplied by the Malaysian Meteorological Department, Ministry of Energy, Science, Technology, Environment, and Climate Change, and obtained from the closest meteorological station to the grain corn farms.
3.2. Density and Diversity of Mycobiota Isolated from Kampong Dadong and Rhu Tapai Grain Corn Farms
Figure 1 depicts the total fungal loads (log CFU/g) on debris, kernel, soil, and tassel samples obtained from Kampong Dadong and Rhu Tapai grain corn farms in Terengganu following dilution plating on DRBC and incubation at 30 °C for 7 d. No significant difference (p = 0.997) was observed between the total fungal loads from both farms. However, there was a significant difference between the total fungal loads of each sample analyzed. For both farms, the tassel samples significantly yielded the highest total fungal loads (log 6.71 ± 0.01 CFU/g for Kampong Dadong and log 6.72 ± 0.01 CFU/g for Rhu Tapai), followed by the debris samples and kernel and soil samples.
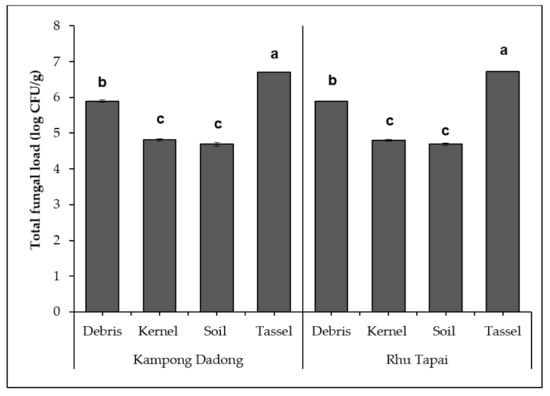
Figure 1.
Total fungal loads (colony-forming unit; log CFU/g) of debris, kernel, soil, and tassel samples obtained from Kampong Dadong and Rhu Tapai grain corn farms in Terengganu following dilution plating on dichloran rose bengal chloramphenicol agar (DRBC) and incubation at 30 °C for seven days. Data are means of triplicate (n = 3) with bars indicating a standard error (±SE). Different letters indicate a significant difference (p < 0.05) by Tukey’s honest significant difference (Tukey’s HSD). a,b,c = Different letters indicate a significant difference.
A total of 131 fungal isolates were recovered from all samples of both farms. Based on sequence identification, approximately 13 fungal genera (e.g., Aspergillus, Bjerkandera, Curvularia, Fusarium, Lasiodiplodia, Neosartorya, Penicillium, Phomopsis, Pyrrhoderma, Schizophyllum, Talaromyces, Trametes, Trichoderma) and 30 fungal species were identified (Table 2). Table 3 summarizes the qualitative distribution of fungal isolates on debris, kernel, soil, and tassel samples obtained from Kampong Dadong and Rhu Tapai grain corn farms. Ascomycota was the dominant phylum found on both farms, followed by Basidiomycota. Generally, the fungal species isolated from Kampong Dadong grain corn farm were slightly richer (i.e., 22 species) as compared to those isolated from Rhu Tapai grain corn farm (i.e., 20 species). The predominant species found from Kampong Dadong samples were F. verticillioides (n = 10/12 of samples; 83%) followed by the pathogenic fungi L. theobromae (n = 9/12 of samples; 75%), and A. niger (n = 6/12 of samples; 50%). Similarly, F. verticillioides (n = 10/12 of samples; 83%) was the predominant species found from Rhu Tapai samples, followed by L. theobromae (n = 9/12 of samples; 75%), and A. flavus (n = 6/12 of samples; 50%). Regarding mycotoxigenic fungi, five species were recovered from Kampong Dadong samples with their occurrence in the following order: F. verticillioides > A. niger > A. flavus > F. incarnatum > F. proliferatum, while only four mycotoxigenic species were recovered from Rhu Tapai samples in the following order: F. verticillioides > A. flavus > A. niger > F. incarnatum.

Table 2.
Fungal species, sequence similarity (%), GenBank accession number, and isolate code of fungal isolates obtained from Kampong Dadong and Rhu Tapai grain corn farms in Terengganu.

Table 3.
Qualitative distribution of fungal isolates on debris (n = 3), kernel (n = 3), soil (n = 3), and tassel (n = 3) samples obtained from Kampong Dadong and Rhu Tapai grain corn farms in Terengganu.
The isolation frequency (%) of fungal isolates on kernel and tassel samples obtained from Kampong Dadong and Rhu Tapai grain corn farms following direct plating on DG-18 and PDA and incubation at 30 °C for 7 d are depicted in Figure S1 Originally, DG-18 (0.95 aw) was normally employed to isolate moderately xerophilic fungi, particularly some fungi from the genera Aspergillus and Penicillium [34], while PDA was employed as the general medium for fungal isolation (as it supports luxuriant fungal growth). The present work, however, employed both growth media and found that most species were able to grow on them, which is possibly due to the ability of most fungi obtained in the present work to grow in a wide range of water activity [34]. Therefore, the % isolation frequency of fungal species was mainly reported on PDA. However, for fungal species that did not grow on PDA, their % isolation frequency was reported on DG-18 instead. For moderate xerophiles, their % isolation frequency was also reported on DG-18 as they thrived on that medium. Altogether, A. flavus, A. niger, F. verticillioides, and L. theobromae were the fungal isolates frequently isolated from all kernel and tassel samples. F. verticillioides was isolated more frequently as compared to the other species in kernel and tassel samples of Kampong Dadong and in-kernel samples of Rhu Tapai. In contrast, in tassel samples of Rhu Tapai, L. theobromae was the most frequent species isolated.
3.3. Mycotoxigenic Potentials of Mycotoxigenic Species Isolated from Kampong Dadong and Rhu Tapai Grain Corn Farms
The mycotoxigenic fungal isolates, which comprised of seven A. flavus isolates, five A. niger isolates, three F. incarnatum isolates, and one F. proliferatum isolate recovered on all samples obtained from both grain corn farms, were tested for their mycotoxigenic potentials in vitro. For F. verticillioides, 14 of 24 isolates were randomly selected from all samples from both farms for mycotoxins analysis. Mycotoxigenic potentials of mycotoxigenic species are tabulated in Table 4 (Aspergillus spp.) and Table 5 (Fusarium spp.). For aflatoxins, three of seven A. flavus isolates produced AFB1 ranging from 0.08 to 0.24 µg/g, of which the only one isolate also produced AFB2, with the production pattern of AFB1 > AFB2. No AFG1/AFG2 was detected. For OTA, all five A. niger isolates tested produced OTA ranging from 4.08 to 32.60 µg/g. For Fusarium mycotoxins, all three F. incarnatum isolates produced ZEN (29,717.31–68,550.30 µg/g), with one isolate produced HT-2 and T-2 toxins. For fumonisins, 13 of 14 F. verticillioides isolates produced both FB1 and FB2 (1118.18–10,087.77 µg/g and 298.91–2657.21 µg/g, respectively). One F. proliferatum isolate also produced FB1 and FB2. The fumonisin production pattern was FB1 > FB2. In addition, three of 15 F. verticillioides isolates also produced ZEN (21.31–2601.86 µg/g). None of the Fusarium spp. isolates were able to produce DON.

Table 4.
Aflatoxins and ochratoxin A (µg/g) production by Aspergillus flavus and A. niger isolate, respectively, recovered from Kampong Dadong and Rhu Tapai grain corn farms in Terengganu.

Table 5.
Fumonisins, trichothecenes A, and zearalenone (µg/g) production by Fusarium spp. isolates recovered from Kampong Dadong and Rhu Tapai grain corn farms in Terengganu.
4. Discussion
4.1. Diversity of Mycobiota and the Occurrence of Economically Important Mycotoxigenic Species, Aspergillus flavus and Fusarium verticillioides, in Two Pioneer Grain Corn Farms of Terengganu, Malaysia
The present work has successfully established the fungal community structure, including mycotoxigenic species of two pioneer grain corn farms in Terengganu, Malaysia. In general, two economically important mycotoxigenic fungi in grain corn in tropical countries, which are F. verticillioides and A. flavus, were isolated in high frequency. However, F. verticillioides was more predominant on both grain corn farms. Being a pathogen, F. verticillioides may cause Fusarium ear rot in corns, while A. flavus is an opportunistic pathogen that may cause Aspergillus ear rot in corns [35]. The occurrence of other mycotoxigenic species (i.e., A. niger, F. proliferatum, F. incarnatum) was also observed. Besides mycotoxigenic species, a high occurrence of the pathogenic L. theobromae was also observed on both farms. This could be due to the dual nature of this species, which may exist as both endophyte and pathogen. This species is ubiquitous in tropical and subtropical regions and prevalent in a wide range of plant hosts [36]. In corns, L. theobromae can be the cause of ear rot disease [37]. Among the many secondary metabolites produced by L. theobromae, a phytotoxin, known chemically as (3S,4R)-3-carboxy-2-methylene-heptan-4-olide, is associated with the black spot disease in tropical fruits [38]. However, its toxicity towards animals and humans is not yet known.
Besides pathogens, fungal species that have potential as biocontrol agents, especially the mycoparasitic Trichoderma spp., were also isolated from both grain corn farms and may be used in the development of an effective control measure to combat fungal infestation indigenously and biologically in the grain corn farms without the use of chemically synthesized fungicides. In our previous review article [39], we have discussed the potentials of Trichoderma spp. to biologically control the fungal diseases on corn, oil palm, soybean, mango, and rice paddy within the Malaysian agro-ecosystems. However, no work has been done thus far to control the proliferation of mycotoxigenic fungi in Malaysia. This thus opens a future research opportunity as an effort to sustainably manage and control mycotoxin contamination of economically relevant Malaysian agricultural commodities.
The high occurrence of F. verticillioides in both grain corn farms demonstrates that this species, indeed, is endemic in corn [40]. A similar high occurrence had also been reported in many countries, including that in tropical regions [5,41,42,43]. This result agrees with previous findings [10,11], which reported that F. verticillioides was among the Fusarium spp. frequently found in Malaysian corn agroecosystems. Nevertheless, in contrast to the present findings, Nur Ain Izzati et al. [10] reported that F. proliferatum was predominant in almost all corn farms investigated. Since both species have a similar life cycle, thus requiring similar ecophysiological conditions to survive [44], it is not surprising that both species could actively compete within the corn agroecosystems. The slight discrepancy between the findings of both studies may be attributed to the shift in weather conditions (i.e., climate change) over the ten years period, which is becoming more favorable for F. verticillioides colonization. According to Marín et al. [45], F. verticillioides may be better adapted to higher temperature and water stress than F. proliferatum. Although this was not ascertained in the present work, it is apparent that the recent higher daily temperatures [46] may have affected the water activity of the microenvironments (e.g., corn kernels, soil) in which those fungi inhabited, thus resulting in higher F. verticillioides occurrence in all samples from both grain corn farms. Regardless, both species are significant fumonisin producers, thus indicating a definite risk of fumonisin contamination in the corn kernels, and by extension, in the animal feeds.
Apart from its occurrence in the corn kernel samples, F. verticillioides, being an endophyte and saprophyte [47], was also present in the debris, kernel, and tassel samples, thus contributing to the high occurrence in both grain corn farms (Table 4). The high occurrence of F. verticillioides in the debris samples may serve as a source of inoculum for its vertical contamination in corn via systemic infection [48]. Further, Cotton and Munkvold [49] had described the long-term survival of this species (up to 630 days) in farm residues, either with a monocropping or rotation system, which may intensify the risk of F. verticillioides infection in corn farm should there be no proper crop residue management implemented.
As compared to F. verticillioides, A. flavus was found relatively lower in the present work. This was possibly due to the ability of the former species to dominate corn niches in a favorable growth environment. In fact, F. verticillioides may invariably outcompete many species co-existing within the corn niches, provided that they co-exist in a favorable environment for the growth of F. verticillioides, for example, under high humidity conditions. This has been documented by studies in vitro [50] and in planta [51]. In the case of F. verticillioides versus A. flavus, the former may effectively and rapidly utilize the carbon sources once it germinates and colonizes the corn ecosystem, thus inducing the plant host resistance, such as the production of phytoalexins, as a defense mechanism against A. flavus [50]. This process thereby inhibits further A. flavus colonization in the agroecosystem. A. flavus could only become predominant in drier environments, as reported in northern Italy during the 2003–2004 drought episode [52]. In the present work, high monthly rainfalls during the 2017 cropping season may have resulted in high soil and grain moisture, which was favorable for the proliferation of F. verticillioides. However, the Rhu Tapai grain corn farm, which recorded hotter and dryer climatic conditions (Table 2) throughout the sampling period in 2017, also recorded higher A. flavus occurrence (Figure S1) as compared to Kampong Dadong grain corn farm.
Prior to grain corn cultivation, Malaysian corn production was dominated by sweet corn. Therefore, previous studies on the occurrence of fungal species and mycotoxigenic fungi preharvest were focused mainly on this type of corn. Among the earliest documented data, all had particularly emphasized the proliferation of Fusarium spp. [10,11]. Two similar studies that were conducted in seven and 12 states of Malaysia, respectively, found various Fusarium spp. contaminating corn ears, bracts, and kernels [10,11]. The species found were F. equiseti, F. longipes, F. nygamai F. oxysporum, F. proliferatum, F. pseudograminearum, F. semitectum, F. solani, F. subglutinans, and F. verticillioides. In both studies, F. proliferatum was predominant in almost all regions. Interestingly, also in both studies, F. pseudograminearum, the closely related species to F. graminearum [53], was only isolated in the colder region of Malaysia (Cameron Highland, the average temperature of 18 °C–23 °C). Only recently, a study was conducted to determine the aflatoxigenic and non-aflatoxigenic A. flavus from sweet corn preharvest in Cameron Highland. Among 40 A. flavus isolates, 45% (n = 18/40) were aflatoxigenic, and the remaining 55% (n = 22/40) were non-aflatoxigenic [54]. Nevertheless, since all previous studies focused on one fungal genus contaminating corn, the present work, therefore, provides valuable information on the occurrence and structure of various fungal species occupy the Malaysian grain corn agroecosystems.
Although grain corn and sweet corn may become infected by the same fungal pathogens (e.g., F. verticillioides), the severity of infection may vary according to their type. This variation is attributed to their unique characteristics. As compared to grain corn, sweet corn contains higher reducing sugar and sucrose, a low amount of the complex carbohydrate amylopectin, and higher kernel water content [55]. Consequently, sweet corn is more susceptible and will be more severely affected by fungal infection than grain corn. However, depending on the corn breed, some sweet corn may also be resistant to the infection and the subsequent mycotoxin accumulation [56].
Regarding the fungal abundance in the analyzed samples, tassels from both grain corn farms recorded the highest total fungal loads, which reflects the abundance of airborne spores during the cropping season. The spores are released from their primary inocula (i.e., corn plant or debris) [57] by several mechanisms, including rain and wind [58]. Crandall and Gilbert [59] opined that high relative humidity and warm air temperature, which are the dominant weather conditions in Malaysia, may result in a high abundance of airborne spores. Furthermore, airborne spores are usually derived from plant pathogens and mycotoxigenic fungi [60]; hence their abundance in the atmosphere at the grain corn farms may heighten the risk of diseases and contamination in the corn plants. In fact, many cultivable fungal species recovered from the tassel samples of both grain corn farms analyzed in the present work were either pathogenic (i.e., L. theobromae) or mycotoxigenic (i.e., A. flavus, A. niger, F. incarnatum, F. verticillioides). Additionally, during corn flowering season, corn ears or kernels may be susceptible to fungal infection via pollens from tassels that carry fungal spores through the silk channel [35], thus predisposing the corns to fungal diseases and mycotoxin accumulation during favorable weather conditions.
4.2. Mycotoxigenic Potential of Mycotoxigenic Isolates from Two Pioneer Grain Corn Farms in Terengganu, Malaysia
Regarding fumonisin production, almost all (93%) F. verticillioides isolates analyzed in the present work, including one F. proliferatum, produced FB1 and FB2, with FB1 being the major analog (75% of the total fumonisins) [61]. These isolates are considered as high FB1 producers (>500 µg/g) [62], and this corroborates the findings of Darnetty and Salleh [8], who investigated the mycotoxigenic potentials of Fusarium spp. isolates from Indonesia and Malaysia. Nevertheless, this contradicts Ismail et al. [63], who reported the prevalence of low fumonisin-producing (<50 µg/g) F. verticillioides isolates recovered from corn kernels in Sabah and Sarawak, Malaysia. This discrepancy may be attributed to the differences in fumonisin production profiles by different fungal strains. Similar observation on the variability in fumonisin production profiles has been recorded in fumonisin-producing species from China [64], Italy [65], and Iran [43]. Interestingly, those studies reported high variability in the yields of fumonisins from the isolates obtained between and within provinces or fields. These emphasize that strain specificity, rather than geographical origins, is more relevant to distinguish their fumonisin production profiles. Different strains may be genetically varied, thus possessing distinct capability in terms of fumonisin production.
Among all aflatoxin producers in the Aspergillus section Flavi, only A. flavus was recovered in the present work. This may be due to the ubiquitous nature of this fungus in hot and dry environmental conditions. Previous studies on corn [66,67], peanut [66], and cotton [68] farms distinctly reported high A. flavus occurrence. In fact, those studies had indicated a very low to negative occurrence of A. parasiticus, the other prominent aflatoxin producer from the Aspergillus section Flavi. Among the A. flavus isolates recovered, 57% (n = 4/7) were non-toxigenic, while the remaining were toxigenic. These non-toxigenic isolates may be utilized as potential biocontrol agents against their toxigenic counterparts. Nevertheless, this “non-toxigenic” status could very well be a “false-negative”, and that these isolates were, in fact, very low aflatoxin producers, below the detection limits of the chromatography system employed in the present work. Therefore, a validation study is highly warranted to verify the atoxigenic property of these isolates by determining the absence/presence of genes responsible for aflatoxin biosynthesis [69].
Among A. flavus toxigenic isolates, a low amount of aflatoxins (<0.5 µg/g) was detected. This may be attributed to the complex interaction between the pathogens and climatic conditions, agricultural practices, as well as the plant host that further drives the natural selection of a certain group of strains (i.e., vegetative compatibility group, VCG) over the other. Since the A. flavus population is dynamic within the stated interaction, and no VCG may be dominant across years [70], it could be suggested that a temporal study spanning different years of sampling period should be conducted in the Malaysian grain corn agroecosystems to accurately assess the risk of aflatoxin contamination. If indeed Malaysian grain corn agroecosystems are infested with low-aflatoxin producers instead of the higher ones, suitable prevention and mitigation strategies could, therefore, be suggested, particularly using the indigenous atoxigenic strains. It is worth noting that among the primary challenges in selecting excellent atoxigenic candidates against the toxigenic ones is the ability to effectively inhibit aflatoxin production, especially among the high-producing isolates. The selection would, therefore, be slightly easier if the toxigenic strains are, in fact, the low-producing ones as recovered in the present work. No G-series aflatoxins were detected from Aspergillus spp. recovered from both grain corn farms assessed in the present work. This corresponds well to the absence of A. parasiticus from the recovered mycobiota.
The co-occurrence and interaction between A. flavus and F. verticillioides in grain corn may trigger stress conditions (i.e., due to niche competition), which consequently influences their mycotoxin production. This interaction, which is affected by climatic conditions, may stimulate/inhibit mycotoxin production of both species [51,71]. For example, in planta study on the co-occurrence of F. verticillioides and A. flavus revealed enhanced AFB1 production in corn co-inoculated with both species during the hotter and rainier growing season of 2016 as compared to the one inoculated with A. flavus alone. In contrast, the co-inoculated species F. verticillioides produced low fumonisins during the growing season of the same year. In the subsequent year of the slightly drier and cooler season, AFB1 production from the co-inoculation was significantly low, particularly near- and during the harvesting period. Simultaneously, fumonisin production from the co-inoculation was slightly enhanced at the harvesting period [51]. This shows that the outcome of the interaction between both species in corn may vary and is highly dependent on climatic conditions. That being said, Malaysia’s tropical climate that seems favorable for F. verticillioides proliferation and fumonisin formation in grain corn, as reported in the present work, could also easily induce greater aflatoxin production by A. flavus in grain corn, thus contributing to higher aflatoxin levels in corn and corn products. However, more research needs to be done to confirm this hypothesis.
Other mycotoxigenic species have also shown potential as producers of mycotoxin in the grain corn agroecosystem of Malaysia. The occurrence of ochratoxigenic A. niger in corns had been previously reported [72]. F. incarnatum (synonyms: F. pallidoroseum, F. semitectum) had also been reported to produce trichothecenes A and ZEN in corns [73]. These species were both recovered in the present work. The other common Fusarium pathogen in corn is F. graminearum, which was not recovered in the present work. This also explains the absence of DON from the analyzed samples. This could be due to F. graminearum being more prevalent in colder geographical regions as compared to the tropical regions [74,75]. Although various Fusarium spp. have been reported to produce ZEN, its production by F. verticillioides is very rare [76]. In the present work, one F. verticillioides was found to produce ZEN (Table 5). The occurrence of multi-mycotoxigenic isolates in samples obtained from both grain corn farms signifies the potential risk of the resulting animal feed being contaminated by multi-mycotoxins.
In Malaysia, aflatoxins, fumonisins, and their principal fungal producers constitute a major proportion of fungal- and mycotoxin contaminants in corns and corn-based products. Other mycotoxins and their fungal producers were reported to a lesser extent. During 2009–2010, Reddy and Salleh [77] investigated 80 grain corn samples collected from ten states of Malaysia and found a high proportion of samples contaminated with fumonisins (100%, n = 80/80) and AFB1 (81.2%, n = 65/80). Moreover, approximately 87% of the samples were contaminated by A. flavus and 47% by F. verticillioides. Later, two separate studies that were conducted for mycotoxin contamination in cornmeal had described the incidence of aflatoxins in most samples and found (in decreasing order) fumonisins (i.e., FB1 and FB2), OTA, DON, HT-2, T-2, and ZEN [78,79]. Further, another study on cornmeal [80] found that all F. verticillioides isolates investigated (n = 16/16) were able to produce FB1, and a high number of A. flavus isolates were able to produce AFB1 (n = 29/40). Moreover, they also reported that F. proliferatum, F. semitectum, and F. verticillioides were able to produce moniliformin (MON, n = 3/3, n = 6/6, and n = 6/7, respectively) and beauvericin (BEA, n = 3/3, n = 4/6, and n = 5/7, respectively) in the samples.
Similar trends of mycotoxin contamination in corn were observed in other South-east Asian countries with tropical climates. In Indonesia, a study conducted during 1985–1986 found approximately 96% of 52 corn samples and 95% of 290 poultry feed samples from feed mills contained aflatoxins. Moreover, ZEN and OTA were also found in 22% and 2% of the corn samples, respectively [81]. A subsequent study by the same author on the same corn samples found cyclopiazonic acid (CPA) in 81% (n = 21/26) of the samples [82]. Later in 1995, Ali et al. [83] found 100% (n = 16/16) aflatoxins, 69% (n = 11/16) fumonisins, and 12% (n = 2/16) each of DON, nivalenol (NIV), and ZEN from corn samples of central Java. All aflatoxin-contaminated samples were also co-contaminated with fumonisins. In addition, 75% (n = 12/16) and 56% (n = 9/16) of the samples were contaminated with A. flavus and fumonisin-producing species (i.e., F. moniliforme, F. proliferatum, and F. nygamai), respectively. In Thailand, high fumonisins (FB1:89% and FB2:67%) and aflatoxins (AFB1:83%, AFB2:55%) were found in 18 post-harvest corn samples intended for animal and human consumption. In most samples, fumonisin levels were higher than aflatoxins, and 72.2% of samples were contaminated with both mycotoxins [84]. Later, Tansakul et al. [85] detected fumonisins and BEA in 100% (n = 58/58) samples of dried distiller’s grains with solubles (DDGS) used for animal feed, while approximately 83% (n = 48/58) and 48% (n = 28/58) samples were also contaminated with ZEN and DON, respectively. In Southern Vietnam, a study conducted in the year 2005 by Thieu and Pettersson [86] found 92% of AFB1 and 33% of ZEN from 12 samples of corn for feed. Later in 2009, a study conducted on dried corn kernel from wholesale, retail, and households of Northern Vietnam found A. flavus, A. parasiticus and F. verticillioides in 29% (n = 30/102), 4% (n = 4/102), and 26% (n = 27/102) of the samples. Moreover, they also found aflatoxins (26%, n = 27/102) and fumonisins (23%, n = 24/102) in the samples, respectively [87]. Recently, a long and extensive three-year worldwide survey of mycotoxins in feedstuff and feed had verified a high occurrence of fumonisins (83%) and aflatoxins (71%), followed by DON (45%), ZEN (20%), and OTA (12%) in corn samples of South-east Asia (i.e., Malaysia, Indonesia, Philippines, Thailand, and Vietnam) [88].
Based on the available data, it is inferred that mycotoxigenic fungi and mycotoxin contamination, particularly aflatoxins and fumonisins and their fungal producers, are widespread and consistent across years and in various corn samples and corn-based products in Malaysia and other countries sharing similar climate. It is important to note that the occurrence of the fungal producer during preharvest may serve as the primary inoculum for the subsequent mycotoxigenic fungi and mycotoxin contamination in agricultural commodities post-harvest. Although the occurrence of mycotoxigenic species in the field may not always indicate the presence of their toxigenic metabolites in corn and corn products post-harvest, there is still a risk that could occur under certain environmental conditions. Therefore, prevention strategy is important to manage the fungal proliferation and subsequent mycotoxin accumulation earlier in the field [89].
One of the prevention strategies applied in the agricultural field, including in corn farms, is the integrated pest management strategies (IPM), which encompasses various physical, chemical and biological approaches. These approaches may include planting strategies (e.g., adherence to planting dates, early harvest, and crop rotation), crop residue management (e.g., physical removal of residues and the application of indigenous microorganisms for decomposition), soil tillage, soil fertilization to reduce nitrogen that predisposed corn to mycotoxigenic fungal infection, and the application of chemical pesticides or biocontrol agents, whenever the latter alternative is possible [89,90].
5. Conclusions
The present work provides primary information on the fungal community structure of two pioneer grain corn farms in Terengganu, Malaysia. The toxigenic potentials of the mycotoxigenic fungal isolates from both grain corn farms have also been elucidated. Overall, F. verticillioides was predominant in both grain corn farms. The fungal pathogen of corn, which is L. theobromae, was also isolated in high-frequency. Moreover, other mycotoxigenic species that may produce important mycotoxin in corn, namely A. flavus, A. niger, F. incarnatum and F. proliferatum, also occurred on both farms. Among the mycotoxigenic isolates tested for mycotoxin production, 13 of 14 F. verticillioides isolates, and one F. proliferatum isolate produced fumonisins, three of seven A. flavus isolates produced aflatoxins, all A. niger and F. incarnatum isolates produced OTA and ZEN, respectively, one of three F. incarnatum isolates produced HT-2 and T-2, and one isolate of F. verticillioides produced ZEN.
The data presented herein can be used to improve and support the potential use of modeling tools and big data analysis for the management of mycotoxin in grain corns under tropical climate. In a nutshell, aflatoxins and fumonisins are a challenge to the newly embarked mass-cultivation, and commercialization of grain corns for animal feeds in Malaysia. However, due to small sampling areas and a low number of samples, the presented results may not thoroughly capture the overall scenario of diversity and toxigenicity of the mycobiota in the grain corn farms. Therefore, a wider spatial and temporal sampling, which covers diverse agro-ecological zones and year-to-year climatic variations, could also be undertaken to fully comprehend what we are up against in terms of phytopathogenic and mycotoxigenic fungi. Good agronomic practice can be the first-line defense against fungal diseases and the subsequent production of mycotoxins preharvest. Moreover, biological control measures utilizing the indigenous atoxigenic and mycoparasitic strains could also be an immediate avenue for further research in the effort to replace the controversial and environment-deteriorating chemical control measures. A more thorough molecular differentiation method, such as multi-locus analysis (i.e., in addition to ITS analysis), can be employed in future works to better identify genetically close species, such as those within the Fusarium genus. Moreover, future work can also be done to analyze the secondary metabolites of some other fungal species, such as L. theobromae, that were found in the present work.
Supplementary Materials
The following is available online at https://www.mdpi.com/2077-0472/11/3/237/s1, Table S1 Linearity, limit of detection (LOD), and limit of quantification (LOQ) for the analyzed mycotoxins. Figure S1: Isolation Frequency (%) of fungal isolates on kernel (a) and tassel (b) samples obtained from Kampong Dadong grain corn farm, and on kernel (c) and tassel (d) samples obtained from Rhu Tapai grain corn farm following direct plating on dichloran glycerol 18% agar (DG-18) and potato dextrose agar (PDA), and incubation at 30 °C for seven days.
Author Contributions
Data curation, S.N.E.Y. and W.J.N.; formal analysis, S.N.E.Y. and W.J.N.; funding acquisition, J.S., S.I.I. and N.I.P.S.; investigation, S.N.E.Y. and W.J.N.; methodology, S.N.E.Y. and W.J.N.; project administration, J.S., S.I.I. and N.I.P.S.; resources, J.S., S.I.I. and N.I.P.S.; supervision, J.S., S.I.I. and N.I.P.S.; validation, S.N.E.Y. and W.J.N.; visualization, S.N.E.Y. and W.J.N.; writing—original draft, S.N.E.Y. and W.J.N.; writing—review and editing, J.S., S.I.I. and N.I.P.S. All authors have read and agreed to the published version of the manuscript.
Funding
The present work was financially supported by the Malaysian Ministry of Higher Education under the High Impact Centre of Excellence (HICoE) grant scheme (HICoE/ITAFoS/2017/FS9).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in the present work are available upon reasonable request to the corresponding author.
Acknowledgments
The authors would like to acknowledge University Putra Malaysia for the research facilities. The first author also thanks the School of Graduate Studies, Universiti Putra Malaysia, for her PhD studentship under the Graduate Research Fund (GRF) scheme 2017–2021.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zahari, M.W.; Wong, H.K. Research and development on animal feed in Malaysia. Wartazoa 2009, 19, 172–179. [Google Scholar] [CrossRef]
- Mohd Supaat, M.Z. Developmental Plan for Grain Corn Industry. In Proceedings of the National Seminar on Grain Corn Industrial Development, Kemaman, Terengganu, Malaysia, 5–7 July 2017. [Google Scholar]
- Bank Negara Malaysia. The 2018 Budget Speech. Available online: https://www.bnm.gov.my/files/2017/Budget2018.pdf (accessed on 15 January 2020).
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003, 16, 497–516. [Google Scholar] [CrossRef] [PubMed]
- García-Díaz, M.; Gil-Serna, J.; Vázquez, C.; Botia, M.N.; Patiño, B. A comprehensive study on the occurrence of mycotoxins and their producing fungi during the maize production cycle in Spain. Microorganisms 2020, 8, 141. [Google Scholar] [CrossRef] [PubMed]
- Munkvold, G.P.; Arias, S.; Taschl, I.; Gruber-Dorninger, C. Mycotoxins in corn: Occurrence, impacts, and management. In Corn: Chemistry and Technology, 3rd ed.; Serna-Saldivar, S.O., Ed.; AACC International Press: Washington, DC, USA, 2019; pp. 235–287. ISBN 9780128119716. [Google Scholar]
- Zain, M.E. Impact of mycotoxins on humans and animals. J. Saudi Chem. Soc. 2011, 15, 129–144. [Google Scholar] [CrossRef]
- Darnetty, T.; Salleh, B. Toxigenicity of Fusarium species in Gibberella fujikuroi species complex (GFSC) associated with stalk and ear rot disease of corn. Int. J. Phytopathol. 2013, 2, 147–154. [Google Scholar] [CrossRef]
- Hsuan, H.M.; Salleh, B.; Zakaria, L. Molecular identification of Fusarium species in Gibberella fujikuroi species complex from rice, sugarcane and maize from Peninsular Malaysia. Int. J. Mol. Sci. 2011, 12, 6722–6732. [Google Scholar] [CrossRef] [PubMed]
- Nur Ain Izzati, M.Z.; Azmi, A.R.; Siti Nordahliawate, M.S.; Norazlina, J. Contribution to the knowledge of diversity of Fusarium associated with maize in Malaysia. Plant Prot. Sc. 2011, 47, 20–24. [Google Scholar] [CrossRef]
- Zainudin, N.A.I.M.; Sidique, S.N.M.; Johari, N.A.; Darnetty; Razak, A.A.; Salleh, B. Isolation and identification of Fusarium species associated with Fusarium ear rot disease of corn. Pertanika J. Trop. Agric. Sci. 2011, 34, 325–330. [Google Scholar]
- Cotty, P.J.; Jaime-Garcia, R. Influences of climate on aflatoxin producing fungi and aflatoxin contamination. Int. J. Food Microbiol. 2007, 119, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Nesci, A.; Barros, G.; Castillo, C.; Etcheverry, M. Soil fungal population in preharvest maize ecosystem in different tillage practices in Argentina. Soil Tillage Res. 2006, 91, 143–149. [Google Scholar] [CrossRef]
- Warris, M.N.; Azami, A.; Abdul Wahab, N.M.A.; Abd Rahman, N.; Abdul Wahab, N.H.; Mohd Zin, M.F. Production Economy and Management Model of Grain Corn. Presented at the National Seminar on Grain Corn Industrial Development, Kemaman, Terengganu, Malaysia, 5–7 July 2017. [Google Scholar]
- Carranza, C.S.; Barberis, C.L.; Chiacchiera, S.M.; Dalcero, A.M.; Magnoli, C.E. Isolation of culturable mycobiota from agricultural soils and determination of tolerance to glyphosate of non-toxigenic Aspergillus section Flavi strains. J. Environ. Sci. Health B 2016, 51, 35–43. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Microbiological Specifications for Foods. Microbiology of the Food Chain—Preparation of Test Samples, Initial Suspension and Decimal Dilutions for Microbiological Examination—Part 1: General Rules for the Preparation of the Initial Suspension and Decimal Dilutions (ISO 6887—1:2017). Available online: https://www.iso.org/standard/63335.html (accessed on 1 December 2020).
- International Commission on Microbiological Specifications for Foods. Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Yeasts and Moulds—Part 1: Colony Count Technique in Products with Water Activity Greater than 0.95 (ISO 21527—1:2008). Available online: https://www.iso.org/standard/38275.html (accessed on 1 December 2020).
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage, 3rd ed.; Springer: Heidelberg, Germany, 2009; ISBN 9780387922065. [Google Scholar]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi; CBS-KNAW Fungal Biodiversity Centre: Utrecht, The Netherlands, 2010; ISBN 9789070351823. [Google Scholar]
- Visagie, C.M.; Houbraken, J.; Frisvad, J.C.; Hong, S.B.; Klaassen, C.H.W.; Perrone, G.; Seifert, K.A.; Varga, J.; Yaguchi, T.; Samson, R.A. Identification and nomenclature of the genus Penicillium. Stud. Mycol. 2014, 78, 343–371. [Google Scholar] [CrossRef] [PubMed]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2008; ISBN 9780470276464. [Google Scholar]
- Watanabe, T. Pictorial Atlas of Soil and Seed Fungi: Morphologies of Cultured Fungi and Key to Species; CRC Press: Boca Raton, FL, USA, 2010; ISBN 9781439804209. [Google Scholar]
- Yazid, S.N.E.; Thanggavelu, H.; Mahror, N.; Selamat, J.; Samsudin, N.I.P. Formulation of maize-and peanut-based semi-synthetic growth media for the ecophysiological studies of aflatoxigenic Aspergillus flavus in maize and peanut agro-ecosystems. Int. J. Food Microbiol. 2018, 282, 57–65. [Google Scholar] [CrossRef]
- Bernáldez, V.; Córdoba, J.J.; Magan, N.; Peromingo, B.; Rodríguez, A. The influence of ecophysiological factors on growth, aflR gene expression and aflatoxin B1 production by a type strain of Aspergillus flavus. LWT 2017, 83, 283–291. [Google Scholar] [CrossRef]
- Bragulat, M.R.; Abarca, M.L.; Cabañes, F.J. An easy screening method for fungi producing ochratoxin A in pure culture. Int. J. Food Microbiol. 2001, 71, 139–144. [Google Scholar] [CrossRef]
- Afsah-Hejri, L.; Jinap, S.; Arzandeh, S.; Mirhosseini, H. Optimization of HPLC conditions for quantitative analysis of aflatoxins in contaminated peanut. Food Control 2011, 22, 381–388. [Google Scholar] [CrossRef]
- Mohale, S.; Medina, A.; Rodríguez, A.; Sulyok, M.; Magan, N. Mycotoxigenic fungi and mycotoxins associated with stored maize from different regions of Lesotho. Mycotoxin Res. 2013, 29, 209–219. [Google Scholar] [CrossRef]
- Visconti, A.; Solfrizzo, M.; Girolamo, A.D. Determination of fumonisins B1 and B2 in corn and corn flakes by liquid chromatography with immunoaffinity column cleanup: Collaborative study. J. AOAC Int. 2001, 84, 1828–1837. [Google Scholar] [CrossRef]
- Moazami, E.F.; Jinap, S. Natural occurrence of deoxynivalenol (DON) in wheat based noodles consumed in Malaysia. Microchem. J. 2009, 93, 25–28. [Google Scholar] [CrossRef]
- Medina, A.; Magan, N. Temperature and water activity effects on production of T-2 and HT-2 by Fusarium langsethiae strains from north European countries. Food Microbiol. 2011, 28, 392–398. [Google Scholar] [CrossRef]
- Iqbal, S.Z.; Asi, M.R.; Jinap, S.; Rashid, U. Detection of aflatoxins and zearalenone contamination in wheat derived products. Food Control 2014, 35, 223–226. [Google Scholar] [CrossRef]
- Shrivastava, A.; Gupta, V.B. Methods for the determination of limit of detection and limit of quantitation of the analytical methods. Chron. Young Sci. 2011, 2, 21–25. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Beuchat, L.R. Media for detecting and enumerating yeasts and moulds. Int. J. Food Microbiol. 1992, 17, 145–158. [Google Scholar] [CrossRef]
- Thompson, M.E.; Raizada, M.N. Fungal pathogens of maize gaining free passage along the silk road. Pathogens 2018, 7, 81. [Google Scholar] [CrossRef]
- Alves, A.; Crous, P.W.; Correia, A.; Phillips, A.J.L. Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Divers. 2008, 28, 1–13. [Google Scholar]
- Ma, H.X.; Zhang, H.J.; Shi, J.; Dang, J.J.; Chang, J.Y.; Chen, D.; Hu, Q.Y.; Guo, N.; Han, H.L. First report of Lasiodiplodia theobromae causing maize ear rot in Hainan Province in Southern China. Plant Dis. 2016, 100, 2160–2161. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Alves, A.; Andolfi, A. Secondary metabolites of Lasiodiplodia theobromae: Distribution, chemical diversity, bioactivity, and implications of their occurrence. Toxins 2020, 12, 457. [Google Scholar] [CrossRef]
- Yazid, S.N.E.; Selamat, J.; Ismail, S.I.; Magan, N.; Samsudin, N.I.P. Phytopathogenic organisms and mycotoxigenic fungi: Why do we control one and neglect the other? A biological control perspective in Malaysia. Compr. Rev. Food Sci. Food Saf. 2020, 19, 643–669. [Google Scholar] [CrossRef] [PubMed]
- Munkvold, G.P.; Desjardins, A.E. Fumonisins in maize: Can we reduce their occurrence? Plant Dis. 1997, 81, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Degraeve, S.; Madege, R.R.; Audenaert, K.; Kamala, A.; Ortiz, J.; Kimanya, M.; Tiisekwa, B.; Meulenaer, B.D.; Haesaert, G. Impact of local pre-harvest management practices in maize on the occurrence of Fusarium species and associated mycotoxins in two agro-ecosystems in Tanzania. Food Control 2016, 59, 225–233. [Google Scholar] [CrossRef]
- Covarelli, L.; Beccari, G.; Salvi, S. Infection by mycotoxigenic fungal species and mycotoxin contamination of maize grain in Umbria, central Italy. Food Chem. Toxicol. 2011, 49, 2365–2369. [Google Scholar] [CrossRef] [PubMed]
- Ghiasian, S.A.; Rezayat, S.M.; Kord-Bacheh, P.; Maghsood, A.H.; Yazdanpanah, H.; Shephard, G.S.; Westhuizen, L.V.D.; Vismer, H.F.; Marasas, W.F. Fumonisin production by Fusarium species isolated from freshly harvested corn in Iran. Mycopathologia 2005, 159, 31–40. [Google Scholar] [CrossRef]
- Reyes Gaige, A.; Todd, T.; Stack, J.P. Interspecific competition for colonization of maize plants between Fusarium proliferatum and Fusarium verticillioides. Plant Dis. 2020, 104, 2102–2110. [Google Scholar] [CrossRef] [PubMed]
- Marín, P.; Magan, N.; Vázquez, C.; González-Jaén, M.T. Differential effect of environmental conditions on the growth and regulation of the fumonisin biosynthetic gene FUM1 in the maize pathogens and fumonisin producers Fusarium verticillioides and Fusarium proliferatum. FEMS Microbiol. Ecol. 2010, 73, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.H.D. Climate change in Malaysia: Trends, contributors, impacts, mitigation and adaptations. Sci. Total Environ. 2019, 650, 1858–1871. [Google Scholar] [CrossRef] [PubMed]
- Bacon, C.W.; Glenn, A.E.; Yates, I.E. Fusarium verticillioides: Managing the endophytic association with maize for reduced fumonisins accumulation. Toxin Rev. 2008, 27, 411–446. [Google Scholar] [CrossRef]
- Blacutt, A.A.; Gold, S.E.; Voss, K.A.; Gao, M.; Glenn, A.E. Fusarium verticillioides: Advancements in understanding the toxicity, virulence, and niche adaptations of a model mycotoxigenic pathogen of maize. Phytopathology 2018, 108, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Cotton, T.K.; Munkvold, G.P. Survival of Fusarium moniliforme, F. proliferatum, and F. subglutinans in maize stalk residue. Phytopathology 1998, 88, 550–555. [Google Scholar] [CrossRef]
- Marín, S.; Sanchis, V.; Arnau, F.; Ramos, A.J.; Magan, N. Colonisation and competitiveness of Aspergillus and Penicillium species on maize grain in the presence of Fusarium moniliforme and Fusarium proliferatum. Int. J. Food Microbiol. 1998, 45, 107–117. [Google Scholar] [CrossRef]
- Giorni, P.; Bertuzzi, T.; Battilani, P. Impact of fungi co-occurrence on mycotoxin contamination in maize during the growing season. Front. Microbiol. 2019, 10, 1265–1274. [Google Scholar] [CrossRef]
- Giorni, P.; Magan, N.; Pietri, A.; Bertuzzi, T.; Battilani, P. Studies on Aspergillus section Flavi isolated from maize in northern Italy. Int. J. Food Microbiol. 2007, 113, 330–338. [Google Scholar] [CrossRef]
- Clear, R.M.; Patrick, S.K.; Gaba, D.; Roscoe, M.; Demeke, T.; Pouleur, S.; Couture, L.; Ward, T.J.; O’Donnell, K.; Turkington, T.K. Trichothecene and zearalenone production, in culture, by isolates of Fusarium pseudograminearum from western Canada. Can. J. Plant Pathol. 2006, 28, 131–136. [Google Scholar] [CrossRef]
- Khan, R.; Mohamad Ghazali, F.; Mahyudin, N.A.; Samsudin, N.I.P. Morphological characterization and determination of aflatoxigenic and non-aflatoxigenic Aspergillus flavus isolated from sweet corn kernels and soil in Malaysia. Agriculture 2020, 10, 450. [Google Scholar] [CrossRef]
- James, M.G.; Robertson, D.S.; Myers, A.M. Characterization of the maize gene sugary1, a determinant of starch composition in kernels. Plant Cell 1995, 7, 417–429. [Google Scholar] [CrossRef] [PubMed]
- Santiago, R.; Cao, A.; Malvar, R.A.; Reid, L.M.; Butrón, A. Assessment of corn resistance to fumonisin accumulation in a broad collection of inbred lines. Field Crops Res. 2013, 149, 193–202. [Google Scholar] [CrossRef]
- Burge, H.A. An update on pollen and fungal spore aerobiology. J. Allergy Clin. Immunol. 2002, 110, 544–552. [Google Scholar] [CrossRef]
- McCartney, H.A. Dispersal of spores and pollen from crops. Grana 1994, 33, 76–80. [Google Scholar] [CrossRef]
- Crandall, S.G.; Gilbert, G.S. Meteorological factors associated with abundance of airborne fungal spores over natural vegetation. Atmos. Environ. 2017, 162, 87–99. [Google Scholar] [CrossRef]
- Bock, C.H.; Mackey, B.; Cotty, P.J. Population dynamics of Aspergillus flavus in the air of an intensively cultivated region of south-west Arizona. Plant Pathol. 2004, 53, 422–433. [Google Scholar] [CrossRef]
- Proctor, R.H.; Plattner, R.D.; Desjardins, A.E.; Busman, M.; Butchko, R.A. Fumonisin production in the maize pathogen Fusarium verticillioides: Genetic basis of naturally occurring chemical variation. J. Agric. Food Chem. 2006, 54, 2424–2430. [Google Scholar] [CrossRef]
- Nelson, P.E.; Plattner, R.D.; Shackelford, D.D.; Desjardins, A.E. Production of fumonisins by Fusarium moniliforme strains from various substrates and geographic areas. Appl. Environ. Microbiol. 1991, 57, 2410–2412. [Google Scholar] [CrossRef]
- Ismail, N.A.; Mohd, M.H.; Nor, N.M.I.M.; Zakaria, L. Fumonisin B1-producing Fusarium species from agricultural crops in Malaysia. Crop Prot. 2017, 98, 70–75. [Google Scholar] [CrossRef]
- Duan, C.; Qin, Z.; Yang, Z.; Li, W.; Sun, S.; Zhu, Z.; Wang, X. Identification of pathogenic Fusarium spp. causing maize ear rot and potential mycotoxin production in China. Toxins 2016, 8, 186. [Google Scholar] [CrossRef]
- Covarelli, L.; Stifano, S.; Beccari, G.; Raggi, L.; Lattanzio, V.M.T.; Albertini, E. Characterization of Fusarium verticillioides strains isolated from maize in Italy: Fumonisin production, pathogenicity and genetic variability. Food Microbiol. 2012, 31, 17–24. [Google Scholar] [CrossRef]
- Agbetiameh, D.; Ortega-Beltran, A.; Awuah, R.T.; Atehnkeng, J.; Cotty, P.J.; Bandyopadhyay, R. Prevalence of aflatoxin contamination in maize and groundnut in Ghana: Population structure, distribution, and toxigenicity of the causal agents. Plant Dis. 2018, 102, 764–772. [Google Scholar] [CrossRef] [PubMed]
- Razzaghi-Abyaneh, M.; Shams-Ghahfarokhi, M.; Allameh, A.; Kazeroon-Shiri, A.; Ranjbar-Bahadori, S.; Mirzahoseini, H.; Rezaee, M.B. A survey on distribution of Aspergillus section Flavi in corn field soils in Iran: Population patterns based on aflatoxins, cyclopiazonic acid and sclerotia production. Mycopathologia 2006, 161, 183–192. [Google Scholar] [CrossRef]
- Cotty, P.J. Aflatoxin-producing potential of communities of Aspergillus section Flavi from cotton producing areas in the United States. Mycol. Res. 1997, 101, 698–704. [Google Scholar] [CrossRef]
- Gallo, A.; Stea, G.; Battilani, P.; Logrieco, A.F.; Perrone, G. Molecular characterisation of an Aspergillus flavus population isolated from maize during the first outbreak of aflatoxin contamination in Italy. Phytopathol. Mediterr. 2012, 51, 198–206. [Google Scholar] [CrossRef]
- Ortega-Beltran, A.; Cotty, P.J. Frequent shifts in Aspergillus flavus populations associated with maize production in Sonora, Mexico. Phytopathology 2018, 108, 412–420. [Google Scholar] [CrossRef]
- Camardo Leggieri, M.; Giorni, P.; Pietri, A.; Battilani, P. Aspergillus flavus and Fusarium verticillioides interaction: Modelling the impact on mycotoxin production. Front. Microbiol. 2019, 10, 2653. [Google Scholar] [CrossRef]
- Palencia, E.R.; Hinton, D.M.; Bacon, C.W. The black Aspergillus species of maize and peanuts and their potential for mycotoxin production. Toxins 2010, 2, 399–416. [Google Scholar] [CrossRef]
- Munkvold, G.P. Fusarium species and their associated mycotoxins. In Mycotoxigenic Fungi: Methods and Protocols; Moretti, A., Susca, A., Eds.; Humana Press: Totowa, NJ, USA, 2017; pp. 51–106. ISBN 9781493967056. [Google Scholar]
- Broders, K.D.; Lipps, P.E.; Paul, P.A.; Dorrance, A.E. Evaluation of Fusarium graminearum associated with corn and soybean seed and seedling disease in Ohio. Plant Dis. 2007, 91, 1155–1160. [Google Scholar] [CrossRef] [PubMed]
- Pfordt, A.; Romero, L.R.; Schiwek, S.; Karlovsky, P.; von Tiedemann, A. Impact of environmental conditions and agronomic practices on the prevalence of Fusarium species associated with ear- and stalk rot in maize. Pathogens 2020, 9, 236. [Google Scholar] [CrossRef]
- Gupta, R.C.; Mostrom, M.S.; Evans, T.J. Zearalenone. In Veterinary Toxicology: Basic and Clinical Principles, 3rd ed.; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1055–1063. ISBN 9780128114117. [Google Scholar]
- Reddy, K.R.N.; Salleh, B. Co-occurrence of moulds and mycotoxins in corn grains used for animal feeds in Malaysia. J. Anim. Vet. Adv. 2011, 10, 668–673. [Google Scholar] [CrossRef]
- Soleimany, F.; Jinap, S.; Abas, F. Determination of mycotoxins in cereals by liquid chromatography tandem mass spectrometry. Food Chem. 2012, 130, 1055–1060. [Google Scholar] [CrossRef]
- Rahmani, A.; Jinap, S.; Soleimany, F. Validation of the procedure for the simultaneous determination of aflatoxins, ochratoxin A and zearalenone in cereals using HPLC-FLD. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2010, 27, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Zainudin, N.A.I.M.; Perumal, N. Mycotoxins production by Fusarium and Aspergillus species isolated from cornmeal. Int. J. Agric. Biol. 2015, 17, 440–448. [Google Scholar] [CrossRef]
- Widiastuti, R.; Maryam, R.; Blaney, B.J.; Stoltz, D.R. Corn as a source of mycotoxins in Indonesian poultry feeds and the effectiveness of visual examination methods for detecting contamination. Mycopathologia 1998, 102, 45–49. [Google Scholar] [CrossRef]
- Widiastuti, R.; Maryam, R.; Blaney, B.J.; Stoltz, D.R. Cyclopiazonic acid in combination with aflatoxins, zearalenone and ochratoxin A in Indonesian corn. Mycopathologia 1988, 104, 153–156. [Google Scholar] [CrossRef]
- Ali, N.; Sardjono; Yamashita, A.; Yoshizawa, T. Natural co-occurrence of aflatoxins and Fusarium mycotoxins (fumonisins, deoxynivalenol, nivalenol and zearalenone) in corn from Indonesia. Food Addit. Contam. 1998, 15, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, T.; Yamashita, A.; Chokethaworn, N. Occurrence of fumonisins and aflatoxins in corn from Thailand. Food Addit. Contam. 1996, 13, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Tansakul, N.; Jala, P.; Laopiem, S.; Tangmunkhong, P.; Limsuwan, S. Co-occurrence of five Fusarium toxins in corn-dried distiller’s grains with solubles in Thailand and comparison of ELISA and LC-MS/MS for fumonisin analysis. Mycotoxin Res. 2013, 29, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Thieu, N.Q.; Ogle, B.; Pettersson, H. Screening of aflatoxins and zearalenone in feedstuffs and complete feeds for pigs in Southern Vietnam. Trop. Anim. Health Prod. 2008, 40, 77–83. [Google Scholar] [CrossRef]
- Huong, B.T.M.; Do, T.T.; Madsen, H.; Brimer, L.; Dalsgaard, A. Aflatoxins and fumonisins in rice and maize staple cereals in Northern Vietnam and dietary exposure in different ethnic groups. Food Control 2016, 70, 191–200. [Google Scholar] [CrossRef]
- Rodrigues, I.; Naehrer, K. A three-year survey on the worldwide occurrence of mycotoxins in feedstuffs and feed. Toxins 2012, 4, 663–675. [Google Scholar] [CrossRef]
- Conte, G.; Fontanelli, M.; Galli, F.; Cotrozzi, L.; Pagni, L.; Pellegrini, E. Mycotoxins in feed and food and the role of ozone in their detoxification and degradation: An update. Toxins 2020, 12, 486. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, B.J. Good agricultural and harvest practices to reduce mycotoxin contamination in wheat in temperate countries. In Mycotoxin Reduction in Grain Chains; Leslie, J.F., Logrieco, A.F., Eds.; Wiley Blackwell: New Delhi, India, 2014; pp. 209–219. ISBN 9781118832790. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).