4.1. Andean Lupin Agronomic Performance in Two Greek Locations
Andean lupin accessions performance, using transplantation because of limited seed, was evaluated for the first time in two Greek locations, showing that they are promising as plant material for genetic improvement. Most of the traits studied presented great variation between the accessions tested. Plant architecture (PH, MainPH, N1stBr e.t.c.) and yield (PPMain, NPP1st, NPP2nd, SPMain, SP1st, SP2nd e.t.c) traits of
L. mutabilis has been reported to be highly dependent upon the environmental conditions [
4,
29,
33], as also exhibited in the present study. All the agronomic traits studied in the over location analysis differed between locations except from plant height and above ground biomass.
Plant height is one of the most important traits for breeders as it is correlated with early maturity [
1], and it has been reported to vary within
L. mutabilis genetic material from 23 to 225 cm [
29]. Lower plants are generally observed in south Europe than in north Europe [
21,
22,
38], especially when plants are sown late [
20] such as in the present study. Terminal drought due to low precipitation and high temperature is also highlighted by Palta et al. [
68]. Regarding the ANOVA in each location, specific accessions such as ‘Mutitalia’, LIB219 and LIB222 (
Table 2) presented higher plants by ca. 10 cm in Athens than in Kalamata. The soil in the Athens field contained more potassium, and lower plants of the specific accessions in Kalamata can be attributed to higher needs of these accessions to potassium as also has been observed in other lupin species [
69,
70]. Among species
L. angustifolius ‘Polo’
, presented lower plant height than
L. mutabilis accessions in both locations, probably as a result of the sensitivity of the former to the high soil CaCO
3 observed in both locations [
71,
72,
73,
74,
75].
Days to first flowering (DFF) is one of the most important traits for which lupins are bred, aiming to their adaptation to the European environments [
4,
29,
31]. The accessions ’Multitalia’ and LIB223 as well as the population LIB214 were the latest flowering accessions. Hammermeister et al. [
76] reported that white lupin results in high yields with a high crude protein content in the seeds, however it is late maturing. The until now evaluated genetic material of
L. mutabilis, has presented variable days from sowing to flowering ranging from 42 to 140 [
29,
38,
77,
78]. Hardy et al. [
29] mentioned that time to flowering is a trait that is significantly affected by environmental factors. Under favorable conditions in a greenhouse in Finland,
L. mutabilis Bolivian and Peruvian accessions flowered at 44 to 64 days [
35]. On the other hand, under Mediterranean climate in a field experiment in Portugal,
L. mutabilis plants flowered at 80.8 to 103.4 days from sowing date [
38], values that are smaller compared to the ones measured in the present study where transplantation took place.
Reports in literature regarding the effect of photoperiod on
L. mutabilis growth and development are contradictory [
1,
31,
34]. Adhikari et al. [
79] supported that longer photoperiods combined with higher temperatures, reduced time interval from sowing to flowering, and vernalization affects only late-maturing
L. mutabilis genotypes. Flowering time along with plant height is also related to the number of mainstem leaves, soil pH and high calcium carbonate which can inhibit the growth of mainstem leaves through Fe deficiency [
29,
71,
72,
73,
74,
80]. Hence, increased number of DFF as observed also in Lazaridi et al [
20], compared to that mentioned in literature, could be attributed to the lower number of leaves in the main stem and the soil properties. Also, lower precipitation and higher temperatures in Kalamata location, forced some of the accessions (LIB209, LIB212 and LIB214) to flower earlier as it has already been exhibited in other legumes [
29].
The majority of the pods and seeds harvested from
L. mutabilis accessions was observed in the main and first order of inflorescence as it has been reported in other studies in Southern Europe [
4,
20,
38,
81]. In addition, more pods and seeds were measured in several
L. mutabilis accessions compared to the white lupin cultivar in Kalamata location. On the contrary in Athens more pods and seeds were observed in ‘Multitalia’. Yield partitioning among the order of branches varies among years as observed by Guilengue et al. [
38], indicating that the environmental effect plays an important role in the partitioning of yield, as it is also highlighted in the over-location analysis of the present study (
Table 7).
Lower number of seeds per plant can be attributed to the coleoptera
Oxythyrea sp. and
Tropinota sp. (Coleoptera: Scarabaeidae, Cetoniinae), observed in the fields of the present study and mostly in Athens location. These coleoptera feed on stamens and pistils of the
Lupinus spp. flowers among other plant species, while they have a preference on blue color [
82]. In this context these Coleoptera presented a preference for
L. mutabilis’ blue colored flowers, thus reduced the number of flowers that can be pollinated. Adomas et al. [
33] and Porter et al. [
83] mentioned that
L. mutabilis plants are tender in losing the top flowers in their inflorescences with flower abscission ranging from 44.5 to 74.9%. Furthermore, another factor reducing the number of pods per plant especially in first and second order of inflorescence [
27] was the warm weather in spring above 27 °C that resulted in flower abortion [
6,
35]. However, competition between flowers and branches for nutrients is also accountable for flower loss [
4] up to 50%, as Williams, [
36] observed. Between locations, plants of
L. mutabilis accessions in Athens presented more pods in all order of branches than in Kalamata location, probably attributed to the higher temperatures and lower precipitation in Kalamata during and after the flowering stage, as it is also reported by others [
6,
20,
35].
The study of the lodging trait is very important given the intensive way of cultivating the land in modern agriculture and the use of combine harvesters for harvesting lupin. The accessions, from the most sensitive to the less sensitive to lodging, were LIB221, LIB214, LIB219, LIB222, LIB220, LIB212, LIB223, LIB220, ‘Polo’, ’Multitalia’. While Mikić et al. [
84] supports that Andean lupin has a good standing ability, Caligari et al. [
14] mention that a
L. mutabilis mutant (KW-1) with a determinate growth habit was found prompt to lodging.
One of the most important factors for which
L. mutabilis is not yet cropped commercially in Europe is the low and unstable yields in comparison to the other European originated
Lupinus spp. such as
L. albus or
L. angustifolius [
26,
27,
38,
85]. Yields of
L. mutabilis in literature range from 165 to 6000 kg ha
−1 in southern Europe and 400 to 6000 kg ha
−1 in central-northern Europe [
4,
14,
22,
27,
29,
33,
85,
86,
87] at various sowing densities (20, 25, 35, 50, 55, 60, 90, 120 plants per m
2). Seed yield of
L. mutabilis has been reported to increase proportionally with sowing density [
86]. Under this perspective the yield of
L. mutabilis obtained in both Athens and Kalamata ranged in similar levels or in some cases above the yields which Lopez-Bellido and Fuentes [
27] had reported applying a similar sowing density. Sensitivity of
L. angustifolius (‘Polo’) in high soil CaCO
3 and pH is higher, compared to
L. albus (‘Multitalia’) and
L. mutabilis that present the same sensitivity [
6]. In this context the very low yield of ‘Polo’ can be attributed to high soil calcium carbonate (15.47 and 17.47%) and alkaline pH values (7.62 and 7.87) [
72] of the soil in Athens and Kalamata experimental locations (
Table 1). Even though white lupin is tolerant to high soil calcium carbonate [
88] compared to other lupin species [
6], it produced an extremely low yield in Kalamata location respectively to those mentioned in literature [
88,
89,
90]. Seed yield in Kalamata location was correlated to both seed and pod number per first and second order of inflorescence, while in Athens seed yield was correlated only to pod and seed number of first order of inflorescence. The low yield of ‘Multitalia’ in Kalamata was related to the lower number of pods in first and second order inflorescences in comparison to
L. mutabilis accessions, maybe attributed to higher temperature and lower precipitation levels in spring.
Lupinus mutabilis’ seed crude protein is one of the main reasons that European Union is interested in its cultivation [
6]. The crude protein content in Andean lupin seeds exceeds this of the native in the Mediterranean region
L. albus and
L. angustifolius accessions while it ranges from similar however higher levels than
L. luteus [
31,
91]. In an experiment in Poland
L. mutabilis’ seed crude protein content was found to range from 35 g 100 g
−1 seed in the determinate form to 38 g 100 g
−1 seed in the indeterminate form [
86]. This is explained by Hardy et al. [
25] who mentions that indeterminate growth type plants present a preference of assimilates partitioning to vegetative growth at favorable conditions. Caligari et al. [
14] found that
L. mutabilis’ seed crude protein content was higher under European conditions in relation to Andean region, and Neves-Martins [
4] mentioned that seed crude protein of
L. mutabilis is lower in southern than in central-northern Europe. The
L. mutabilis’s seed crude protein in the present study was within literature levels [
4,
14,
33,
91]. The seed crude protein of the
L. albus cv ‘Multitalia
’ was more than 10% lower than in literature [
89,
90], however in the literature studies, the soil of the experimental field contained only traces of CaCO
3 in contrast to the soil of both experimental locations of the present study which was characterized by high levels of CaCO
3 (
Table 1). Lower levels of white lupin’ seed crude protein are also mentioned in literature in other locations [
92].
Lupinus angustifolius cultivar ‘Polo’ presented higher crude protein content than other narrow-leafed cultivars in literature [
89]. Huyghe, [
93] mentions that white lupin crude protein varies among locations, the same was observed in the present study where the over-location analysis presented a statistically significant interaction of accession and location, and a significant effect of both accession and location separately (
Table 7). In an experiment in pots, there was no effect of water stress in seed crude protein content of neither
L. mutabilis nor
L. albus’ seeds [
94,
95]. However, under water stress such as in Athens and Kalamata due to low precipitation (
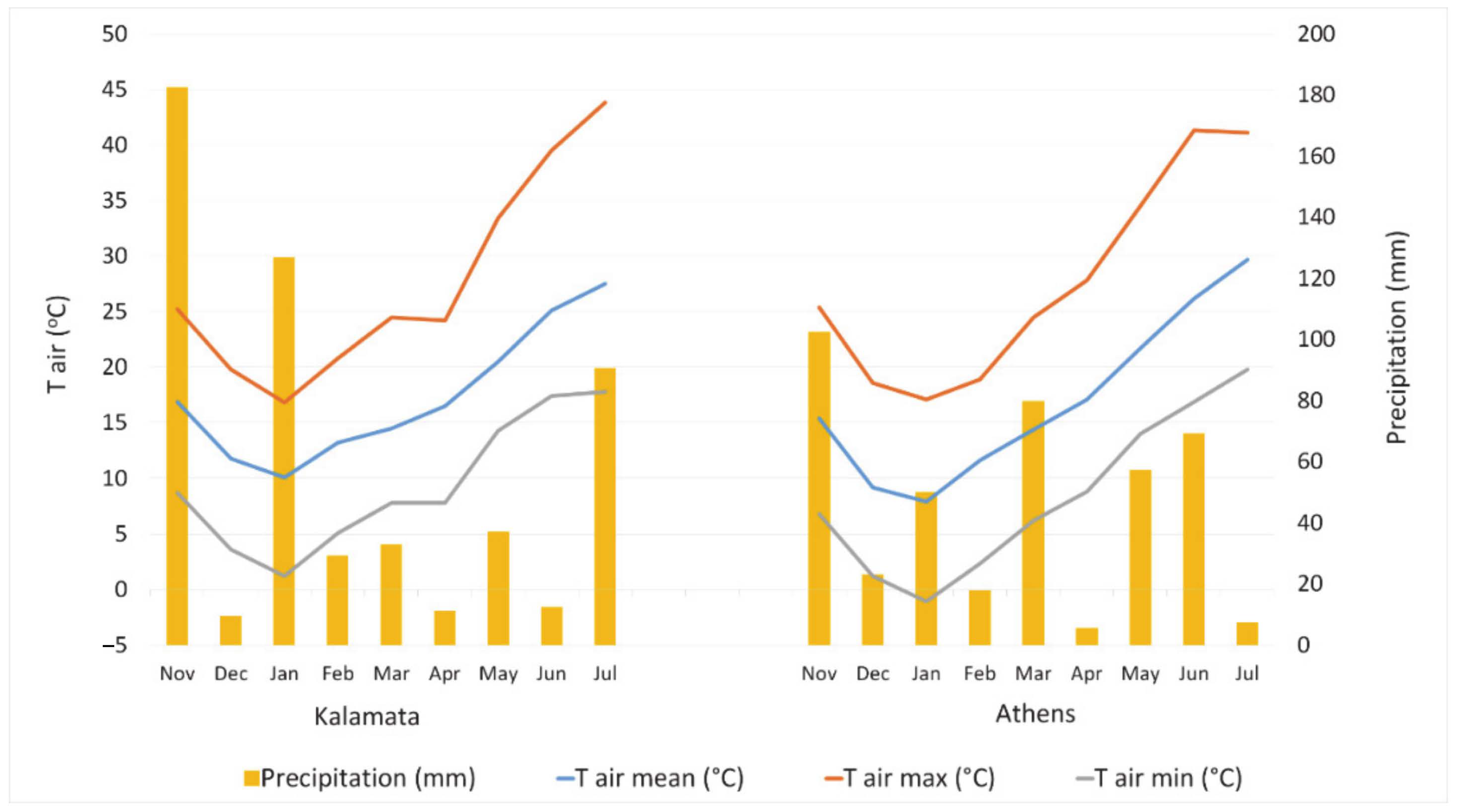
Figure 2) at flowering stage and high soil porosity in loamy and sandy clay loamy soils [
96] growth of lupins was stopped, reducing in this way the time available for the translocation of assimilates from stems and pods to the seeds [
68,
94,
97]. Conclusively, several factors such as high temperatures, low precipitation, high soil pH, pollen eating coleoptera have caused yield loss in the
L. mutabilis accessions tested.
4.2. Andean Lupin Morphological Plasticity and Performance
Plant morphological descriptors have been reported to vary between different environments in
L. mutabilis accessions and even among other species cultivars [
38,
98]. Hence study of their stability is important for breeders who could use them in plant selection for better and more stable performance. In this context the over location analysis revealed a genetic stability for morphological quantitative traits such as stem thickness, petiole length and length of flower indicating that they are not affected by the environmental conditions between the two locations studied. Stipule length was observed to be stable among accessions and locations however the accession LIB214 presented different pattern among locations but still within literature levels [
99]. Most morphological qualitative traits presented stable phenotypes between locations, while variability among accessions was recorded. For example, flower color differed among the
L. mutabilis accessions but not between the two locations (
Tables S2 and S9). Kurlovich [
52] mentions that the white seeded
L. mutabilis forms produce light-green plantlets, however this was not observed in the present study as LIB223 that presented white seeds produced medium-green plantlets. To our knowledge stem thickness has not been studied before in
L. mutabilis, however it has been reported to be negatively correlated to plant density [
33] and proportionally correlated to plant height in lupins [
77] (as it is also seen in the present study) and heat stress in other legumes [
100,
101]. Εven though several accessions presented thinner stems in Kalamata compared to Athens, the over-location analysis did not present any significant effect of location. However, these differences between Kalamata and Athens locations can partially explain the statistically significant interaction of accession and location regarding this trait. Leaf diameter has not been reported in other studies in literature to our knowledge, however petiole length was within literature levels as reported by Eastwood and Hughes [
99]. The height of the lowest primary branch (HlPB) was more stable in Athens than in Kalamata location among the Andean lupin accessions. A within variability of the HlPB has also been reported between years in
L. albus landraces [
102]. The main inflorescence length (IL) of
L. mutabilis in the present study was within those observed in literature [
30,
33,
38,
103]. IL varied among the accessions tested and was positively correlated to pod length as it is also reported in Lazaridi et al [
20]. Stem and stipule color differed among the
L. mutabilis accessions tested. A reddish color at the vegetative organs was observed in LIB209, LIB212, LIB222 accessions, that also present either black color in their seeds or pink-purple flowers. Τhis observation has been previously related to a dominant allele of the
Pur gene in
L. angustifolius [
52]. Seed length and width values of the various accessions of
L. mutabilis tested in the present study were similar to those reported in literature [
38,
99]. Small phenotypic variation as it is also reported in Guilengue et al [
38] was observed in the present study. Moreover, in a collection of
L. mutabilis accessions seed shape presented high total diversity while the diversity of primary seed color was low [
28].
4.4. Insect Pollinators Preferences on Lupinus spp.
From an agricultural perspective, pollinators play a key role in ecosystem sustainability and resilience as well as in productivity enhancement that is in line with the New European Commission’s Green Deal Strategy [
6,
47,
48,
51,
104]. Andean lupin is an entomogamous and partially allogamous crop that through its floral resources can serve the ecosystem by providing forage to the declining population of bees and in parallel increase its seed yield through pollination [
6,
105,
106]. The ways that this synergy is supported are evaluated and highlighted in the present study.
According to Kozin (cited by Williams [
54]), bees substantially increase the number and length of the pods as well as the number and weight of the seeds of the majority of the lupin cultivars. As lupin pollen is not transported by wind, bees should be widely used to increase crop yields of lupin seed [
107]. In this respect, the lupin flowering period should be synchronized with the activity of the potential lupin pollinators. Even though literature mentions
A. mellifera and
Bombus sp. as important visitors of lupin flowers, other wild bee pollinators such as
Andrena spp.,
Anthophora ursina,
Eucera sp.,
Lasioglossum spp.,
Xylocopa virginica have also been recorded to visit
Lupinus spp. flowers [
6,
54].
In the two cultivation areas of lupin accessions examined in Greece, the pollinating insect species varied mainly with the lupin species, a fact which could probably be associated among others with two phenotypic traits of their flower, the size and the color. However, lupin flower has several functions depending on its flower parts. Standard functions for visual discovery and attraction of pollinators, the wings for the landing of the pollinator and keel handling, and finally the keel enables the efficient transfer of pollen [
6,
108].
Flowers of
L. mutabilis accessions are larger than the corresponding
L. albus and
L. angustifolius accessions flowers, also highlighted in Cowling et al. [
77]. Large flowers such as those of
L. mutabilis accessions have the potential to accommodate large sized pollinators [
109] such as
Bombus sp.,
Xylocopa spp., and
Anthophora sp. The smaller flowers of
L. albus accessions were visited by smaller sized pollinators such as
A. mellifera and the
Megachile sp. [
56]. Moreover, attraction of more bee genera by
L. mutabilis flowers compared to
L. albus and
L. angustifolius may be associated to the variety of colors (yellow, purple, white, blue, pink) while flowers of the other two varieties are white. The null visits of pollinating bees of
L. angustifolius accessions could partly be attributed to its early flowering at the mid of March and could be also associated with its smaller (length size) main inflorescence (about 4.3 cm compared to about 15.0 cm for
L. albus and a range from 6.0–18.5 cm for
L. mutabilis) and its lower number of flowers in first order inflorescence (about 2 flowers compared with 7–13 flowers for
L. albus and 5–13 flowers for
L. mutabilis).
Apis mellifera was not observed to pollinate
L. mutabilis flowers. This may be the result of both the insect and the flower anatomy [
109]. Several species flowers need high mechanical strength to be opened, in many cases honeybees do not possess that strength [
110]. This observation is consistent with Coleman’s observation (1921), cited by [
111]) who stated that lupins are visited by large-scale pollinators (e.g., bumble bees) because of their large flowers but only when lupin flowers are small sized, they can be pollinated by honeybees. More specifically, except from flower and pollinator size, the angle formed between the standard and the wings is also important for pollination efficiency as it is related to the strength needed for the effective transfer of pollen to the pollinator [
110]. The correlation of flower color of the lupin varieties tested to the number of pods in main inflorescence observed in Athens locations highlights the importance of floral traits to yield through pollination [
54]. In the same line, the advantage of the multicolored (flower)
L. mutabilis in the crude protein value of the legumes and the attraction of pollinating insect species over the white (flower) varieties support the need for further investigation on the floral traits of
L. mutabilis, which would enable the selection and creation of varieties with attractive and functional flowers for insect-pollinators [
6].