Phthalanilic Acid with Biostimulatory Functions Affects Photosynthetic and Antioxidant Capacity and Improves Fruit Quality and Yield in Cowpea (Vigna unguiculata (L.) Walp.)
Abstract
:1. Introduction
2. Material and Methods
2.1. Plants and Chemicals
2.2. Experimental Design
2.3. Physiological and Biochemical Measurements
2.4. Assessment of Fruit Quality and Yield
2.4.1. Fruit Quality
2.4.2. Podding Rate and Yield
2.5. Statistical Analysis
3. Results
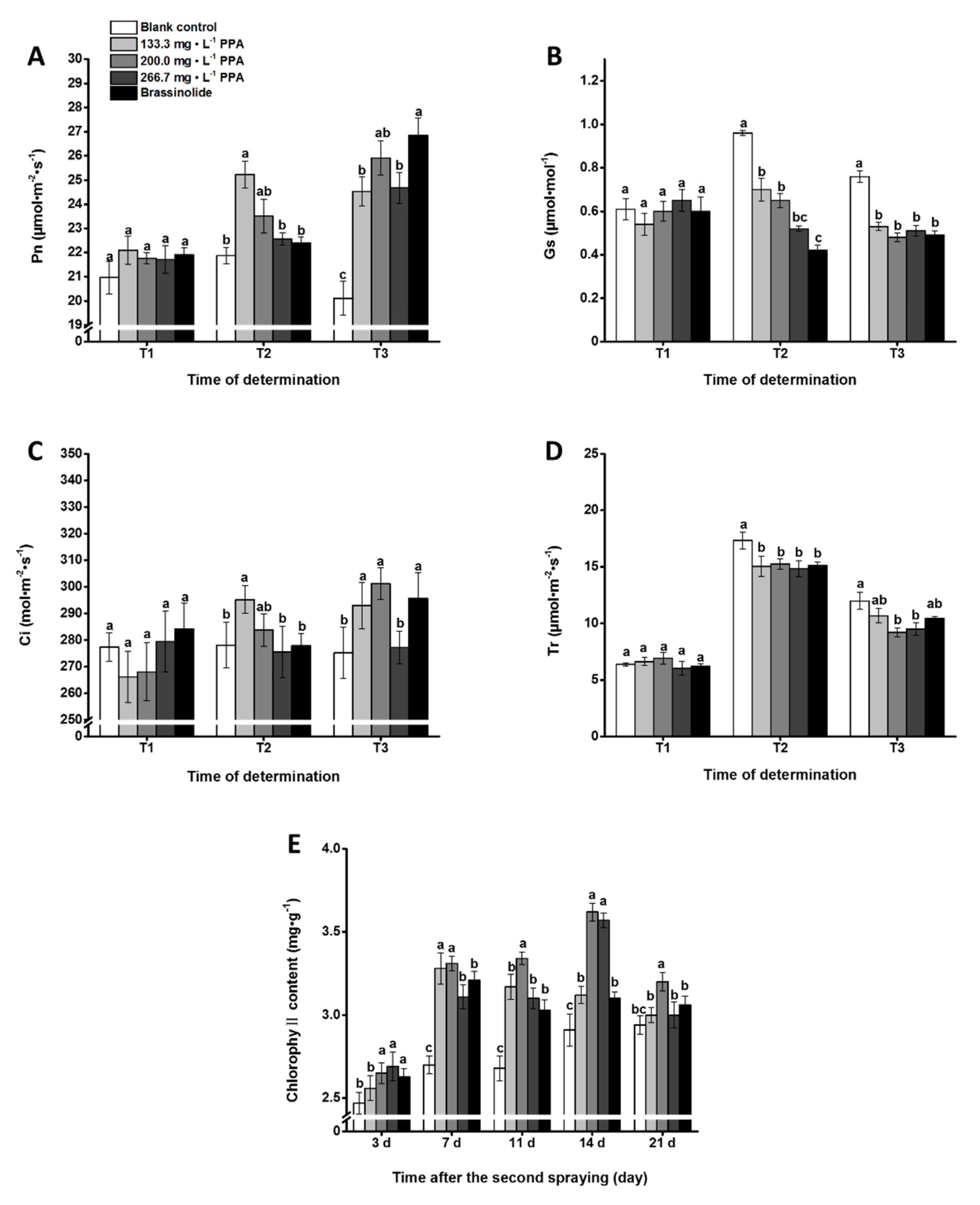
3.1. Effect of PPA on Photosynthetic Properties of Cowpea
3.2. Effect of PPA on the Lipid Peroxidation and Electrolyte Leakage of Cowpea
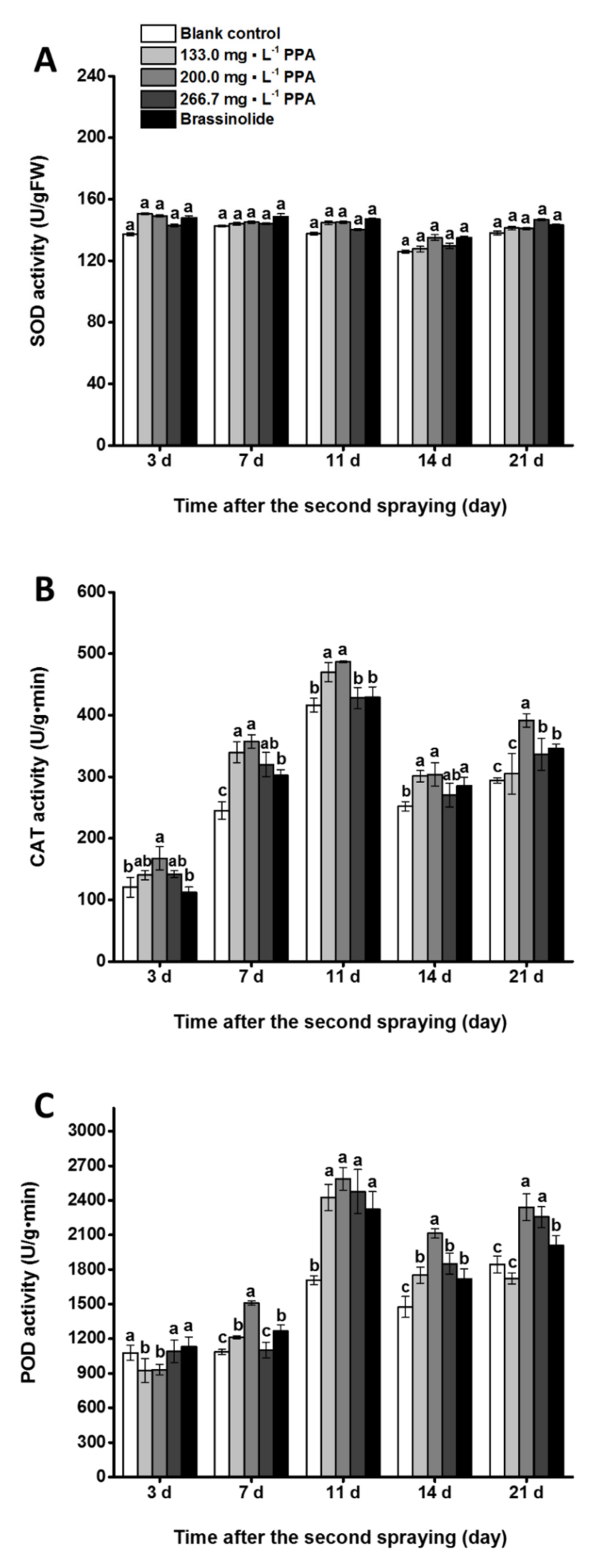
3.3. Effect of PPA on Antioxidant Enzyme Activities in Cowpea
3.4. Effect of PPA on the Free Proline Content in Cowpea
3.5. Effect of PPA on the Quality of Cowpea
3.6. Effect of PPA on the Podding Rate and Yield
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Campobenedetto, C.; Agliassa, C.; Mannino, G.; Vigliante, I.; Contartese, V.; Secchi, F.; Bertea, C.M. A Biostimulant Based on Seaweed (Ascophyllum nodosum and Laminaria digitata) and Yeast Extracts Mitigates Water Stress Effects on Tomato (Solanum lycopersicum L.). Agriculture 2021, 11, 557. [Google Scholar] [CrossRef]
- Cristofano, F.; El-Nakhel, C.; Rouphael, Y. Biostimulant Substances for Sustainable Agriculture: Origin, Operating Mechanisms and Effects on Cucurbits, Leafy Greens, and Nightshade Vegetables Species. Biomolecules 2021, 11, 1103. [Google Scholar] [CrossRef]
- Mrid, R.B.; Benmrid, B.; Hafsa, J.; Boukcim, H.; Yasri, A. Secondary metabolites as biostimulant and bioprotectant agents: A review. Sci. Total. Environ. 2021, 777, 146204. [Google Scholar] [CrossRef]
- Agliassa, C.; Mannino, G.; Molino, D.; Cavalletto, S.; Contartese, V.; Bertea, C.M.; Secchi, F. A new protein hydrolysate-based biostimulant applied by fertigation promotes relief from drought stress in Capsicum annuum L. Plant Physiol. Bioch. 2021, 166, 1076–1086. [Google Scholar] [CrossRef] [PubMed]
- Basile, B.; Rouphael, Y.; Colla, G.; Soppelsa, S.; Andreotti, C. Appraisal of emerging crop management opportunities in fruit trees, grapevines and berry crops facilitated by the application of biostimulants. Sci. Hortic. 2020, 267, 109330. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, X.; Zhang, J.; He, Y.; Zhu, X.; Zhou, X.; Gong, H.; Yin, J.; Liu, Y. Silicon confers cucumber resistance to salinity stress through regulation of proline and cytokinins. Plant Physiol. Biochem. 2020, 156, 209–220. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ashour, M.; Sakai, N.; Zhang, L.; Hassanien, H.A.; Gaber, A.; Ammarr, G.A.G. Impact of seaweed liquid extract biostimulant on growth, yield, and chemical composition of cucumber (Cucumis sativus). Agriculture 2021, 11, 320. [Google Scholar] [CrossRef]
- Shukla, P.S.; Mantin, E.G.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-Based Biostimulants: Sustainable Applications in Agriculture for the Stimulation of Plant Growth, Stress Tolerance, and Disease Management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef] [Green Version]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant Action of Protein Hydrolysates: Unraveling Their Effects on Plant Physiology and Microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Stasio, E.; Cirillo, V.; Raimondi, G.; Giordano, M.; Esposito, M.; Maggio, A. Osmo-Priming with Seaweed Extracts Enhances Yield of Salt-Stressed Tomato Plants. Agronomy 2020, 10, 1559. [Google Scholar] [CrossRef]
- Stirk, W.A.; van Staden, J. Potential of phytohormones as a strategy to improve microalgae productivity for biotechnological applications. Biotechnol. Adv. 2020, 44, 107612. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant properties of seaweed extracts in plants: Implications towards sustainable crop production. Plants 2021, 10, 531. [Google Scholar] [CrossRef] [PubMed]
- Shah, Z.H.; Rehman, H.M.; Akhtar, T.; Alsamadany, H.; Hamooh, B.T.; Mujtaba, T.; Daur, I.; Al Zahrani, Y.; Alzahrani, H.A.S.; Ali, S.; et al. Humic Substances: Determining Potential Molecular Regulatory Processes in Plants. Front. Plant Sci. 2018, 9, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, F.; Baloch, D.M.; Sadiq, S.A.; Ahmed, S.S.; Hanan, A.; Taran, S.A.; Ahmed, N.; Hassan, M.J. Plant growth regulators induced drought tolerance in sunflower (Helianthus annuus L.) hybrids. J. Anim. Plant. Sci. 2014, 24, 886–890. [Google Scholar]
- Fariduddin, Q.; Zaid, A.; Mohammad, F. Plant growth regulators and salt stress: Mechanism of tolerance trade-off. In Salt Stress, Microbes, and Plant Interactions: Causes and Solution; Akhtar, M., Ed.; Springer: Singapore, 2019. [Google Scholar]
- Ilias, I.F.; Maksimović, V.M.; Giannakoula, A.E.; Maksimović, J.J.D.; Živanović, B.D. The effects of plant growth regulators on growth, yield, and phenolic profile of lentil plants. Food Compos. Anal. 2012, 28, 46–53. [Google Scholar]
- Mao, L.; Zhang, L.; Zhao, X.; Liu, S.; Werf, W.V.D.; Zhang, S.; Li, Z. Crop growth, light utilization and yield of relay intercropped cotton as affected by plant density and a plant growth regulator. Field Crop. Res. 2014, 155, 67–76. [Google Scholar] [CrossRef]
- Zaid, A.; Mohammad, F.; Fariduddin, Q. Plant growth regulators improve growth, photosynthesis, mineral nutrient and antioxidant system under cadmium stress in menthol mint (Mentha arvensis L.). Physiol. Mol. Biol. Plants 2020, 26, 25–39. [Google Scholar] [CrossRef]
- Li, X.; Zhong, Q.; Li, Y.; Li, G.; Ding, Y.; Wang, S.; Chen, L. Triacontanol reduces transplanting shock in machine-transplanted rice by improving the growth and antioxidant systems. Front. Plant Sci. 2016, 7, 872. [Google Scholar] [CrossRef] [Green Version]
- Asadi Karam, E.; Keramat, B.; Asrar, Z.; Mozafari, H. Study of interaction effect between triacontanol and nitric oxide on alleviating of oxidative stress arsenic toxicity in coriander seedlings. J. Plant Interact. 2017, 12, 14–20. [Google Scholar] [CrossRef] [Green Version]
- Anjum, S.A.; Wang, L.C.; Farooq, M.; Hussain, M.; Zou, C.M. Brassinolide application improves the drought tolerance in maize through modulation of enzymatic antioxidants and leaf gas exchange. J. Agron. Crop Sci. 2011, 197, 177–185. [Google Scholar] [CrossRef]
- Chen, Z.F.; Wang, Z.; Yang, Y.G.; Li, M.; Xu, B.C. Abscisic acid and brassinolide combined application synergistically enhances drought tolerance and photosynthesis of tall fescue under water stress. Sci. Hortic-Amst. 2018, 228, 1–9. [Google Scholar] [CrossRef]
- Nawaz, K.; Ashraf, M. Exogenous application of glycinebetaine modulates activities of antioxidants in maize plants subjected to salt stress. J. Agron. Crop Sci. 2010, 196, 28–37. [Google Scholar] [CrossRef]
- Zhao, H.; Xu, J.; Dong, F.; Liu, X.; Wu, Y.; Zhang, J.; Zheng, Y. Determination of phthalanilic acid residue in bean, fruits and vegetables using a modified quechers method and ultra-performance liquid chromatography/tandem mass spectrometry. Anal. Methods 2014, 4596, 4336–4342. [Google Scholar] [CrossRef]
- Racskó, J.; Holb, I.; Szabo, Z.; Thurzo, S.; Dren, G.; Nyeki, J. Effect of auxin-synergistic preparation (Nevirol 60 WP) on flowering date and yield of sour-cherry and European plum fruits in Hungary. Acta Hortic. 2006, 727, 279–282. [Google Scholar] [CrossRef]
- Racskó, J.; Szabo, Z.; Nyeki, J. Direct and indirect effects of N-phenyl-phthalamic acid and fertilization on fruit setting and fruit quality parameters of apple (Malus domestica Borkh.). Acta Hortic. 2006, 3, 209–216. [Google Scholar] [CrossRef]
- Zhang, O.; Ma, Q.; Liu, N.; Ma, Z.Q.; Zhang, X. Effect of phthalanilic acid on stress resistance and yield of pepper. Chin. J. Pestic. Sci. 2017, 19, 449–456. [Google Scholar]
- Li, L.F.; Zhou, D.W.; Wang, Y.S. Study on the effect of phthalanilic acid on promoting the flower bud formation of Hongfushi apple. Northwest Horticult. 2019, 11, 54–55. [Google Scholar]
- Khadivikhub, A.; Nosrati, Z. Study of N-phenyl-phthalamic acid effects on fruit setting and fruit quality of sweet, sour and duke cherries. Acta Agr. Serb. 2013, 17, 3–9. [Google Scholar]
- Racskó, J.; Lakatos, L.; Kövics, G.J. Effect of N-phenilphtalanic acid (NEVIROL 60 WP) on quantitative and qualitative parameters of some horticultural plants. In Proceedings of the International Plant Protection Symposium at Debrecen University and Trans-Tisza Plant Protection Forum, Debrecen, Hungary, 15–16 October 2003; pp. 216–224. [Google Scholar]
- Albuquerque, J.D.A.A.D.; Oliva, L.S.D.C.; Alves, J.M.A.; Uchôa, S.C.P.; Melo, D.A.D. Cultivation of cassava and cowpea in intercropping systems held in Roraima’s savannah, Brazil. Rev. Cienc. Agron. 2015, 46, 93–99. [Google Scholar] [CrossRef]
- Karapanos, I.; Papandreou, A.; Skouloudi, M.; Makrogianni, D.; Fernández, J.A.; Rosa, E. Cowpea fresh pods: A new legume for the market assessment of their quality and dietary characteristics of 37 cowpea accessions grown in southern Europe. J. Sci. Food Agr. 2017, 97, 4343. [Google Scholar] [CrossRef]
- Bhattarai, G.; Shi, A.; Qin, J.; Weng, Y.; Morris, J.B.; Pinnow, D.L.; Dong, L. Association analysis of cowpea mosaic virus (CPMV) resistance in the USDA cowpea germplasm collection. Euphytica 2017, 213, 230. [Google Scholar] [CrossRef]
- Guzzetti, L.; Fiorini, A.; Panzeri, D.; Tommasi, N.; Grassi, F.; Taskin, E.; Misci, C.; Puglisi, E.; Tabaglio, V.; Galimberti, A.; et al. Sustainability perspectives of Vigna unguiculata L. Walp. cultivation under no tillage and water stress conditions. Plants 2020, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Elsaeid, H.M.; Abouhussein, S.D.; Eltohamy, W.A. Growth characters, yield and endogenous hormones of cowpea plants in response to IAA application. Res. J. Agr. Biol. Sci. 2010, 6, 27–31. [Google Scholar]
- Patel, H.D.; Patel, H.C.; Sitapara, H.H.; Nayee, D.D. Influence of plant growth regulators on growth and green pod yield of cowpea (Vigna unguiculata L.) cv. ANAND VEG. COWPEA-1. Asian J. Hortic. 2011, 6, 491–495. [Google Scholar]
- Yang, X.; Li, G.; Luo, W.; Chen, L.; Li, S.; Cao, M.; Zhang, X. Quantifying the relationship between leaf nitrogen content and growth dynamics and yield of muskmelon grown in plastic greenhouse. Hortscience 2015, 50, 1677–1687. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts polyphenoloxidase in beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Ekmekci, Y.; Terzioglu, S. Effects of oxidative stress induced by paraquat on wild and cultivated wheats. Pestic. Biochem. Phys. 2005, 83, 69–81. [Google Scholar] [CrossRef]
- Zhao, H.; Dai, T.; Jing, Q.; Jiang, D.; Cao, W. Leaf senescence and grain filling affected by post-anthesis high temperatures in two different wheat cultivars. Plant Growth Regul. 2007, 51, 149–158. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Xiong, X.; Yao, M.; Fu, L.; Ma, Z.Q.; Zhang, X. The botanical pesticide derived from sophora flavescens, for controlling insect pests can also improve growth and development of tomato plants. Ind. Crop. Prod. 2016, 92, 13–18. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Zhang, X.; Chen, C.J.; Zhou, M.G.; Wang, H.C. Effects of fungicides js399-19, azoxystrobin, tebuconazloe, and carbendazim on the physiological and biochemical indices and grain yield of winter wheat. Pestic. Biochem. Phys. 2010, 98, 151–157. [Google Scholar] [CrossRef]
- Masuthi, D.A.; Vyakaranahal, B.S.; Deshpande, V.K. Influence of pelleting with micronutrients and botanical on growth, seed yield and quality of vegetable cowpea. Karnataka J. Agr. Sci. 2010, 22, 35–38. [Google Scholar]
- Nakano, H.; Hirata, K.; Ohnishi, M. Effects of planting density on light interception and on the growth and yield of soybean (agronomy). Jpn. J. Crop Sci. 2004, 73, 175–180. [Google Scholar] [CrossRef]
- Fariduddin, Q.; Yusuf, M.; Ahmad, I.; Ahmad, A. Brassinosteroids and their role in response of plants to abiotic stresses. Biol. Plant. 2013, 58, 9–17. [Google Scholar] [CrossRef]
- Wani, W.; Masoodi, K.Z.; Zaid, A.; Wani, S.H.; Shah, F.; Meena, V.S.; Mosa, K.A. Engineering plants for heavy metal stress tolerance. Rend Lincei-Sci. Fis. 2018, 29, 709–723. [Google Scholar] [CrossRef]
- Wani, S.H.; Tripathi, P.; Zaid, A.; Challa, G.S.; Kumar, A.; Kumar, V.; Bhatt, M. Transcriptional regulation of osmotic stress tolerance in wheat (Triticum aestivum L.). Plant Mol. Biol. 2018, 97, 469–487. [Google Scholar] [CrossRef]
- Zaid, A.; Wani, S.H. Reactive oxygen species generation, scavenging and signaling in plant defense responses. In Bioactive Molecules in Plant Defense; Jogaiah, S., Abdelrahman, M., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Shah, K.; Nahakpam, S. Heat exposure alters the expression of SOD, POD, APX and CAT isozymes and mitigates low cadmium toxicity in seedlings of sensitive and tolerant rice cultivars. Plant Physiol. Bioch. 2012, 57, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Ghoulam, C.; Foursy, A.; Fares, K. Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ. Exp. Bot. 2002, 47, 39–50. [Google Scholar] [CrossRef]
- Anjum, F.; Rishi, V.; Ahmad, F. Compatibility of osmolytes with gibbs energy of stabilization of proteins. BBA Protein Struct. Mol. Enzymol. 2000, 1476, 75–84. [Google Scholar] [CrossRef]
- Hare, P.D.; Cress, W.A. Metabolic implications of stress-induced proline accumulation in plants. Plant Growth Regul. 1997, 21, 79–102. [Google Scholar] [CrossRef]
- Almeselmani, M.; Deshmukh, P.S.; Sairam, R.K.; Kushwaha, S.R.; Singh, T.P. Protective role of antioxidant enzymes under high temperature stress. Plant Sci. 2006, 171, 382–388. [Google Scholar] [CrossRef]
- Liu, D.; Pei, Z.F.; Naeem, M.S.; Ming, D.F.; Liu, H.B.; Khan, F. 5-aminolevulinic acid activates antioxidative defence system and seedling growth in Brassica napus L. under water-deficit stress. J. Agron. Crop Sci. 2011, 197, 284–295. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, R.; Li, Z.; Ma, Z.Q.; Zhang, X. Effect of phthalanilic acid on the endogenous hormone content and fruit quality and yield of peppers. Chin. J. Pestic. Sci. 2018, 20, 625–633. [Google Scholar]
- Schutzendubel, A.; Polle, A. Plant responses to abiotic stresses: Heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 2002, 53, 1351–1365. [Google Scholar] [CrossRef]
- Huang, S.; Li, X.H.; Tao, X.Y.; Lin, D.; Mao, L.C. Study on the effect of anti damage in strawberry with the anti-vibration package. Sci. Techol. Food Ind. 2015, 36, 272–275. [Google Scholar]
- Al-Yasi, H.; Attia, H.; Alamer, K.; Hassan, F.; Hessini, K. Impact of drought on growth, photosynthesis, osmotic adjustment, and cell wall elasticity in damask rose. Plant Physiol. Bioch. 2020, 150, 133–139. [Google Scholar] [CrossRef]
- Xu, F.; Liu, S.; Xiao, Z.; Fu, L. Effect of ultrasonic treatment combined with 1-methylcyclopropene (1-MCP) on storage quality and ethylene receptors gene expression in harvested apple fruit. J. Food Biochem. 2019, 43, e12967. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Lu, F.; Xiao, Z.; Li, Z. Influence of drop shock on physiological responses and genes expression of apple fruit. Food Chem. 2020, 303, 125424. [Google Scholar] [CrossRef]
- Lu, F.; Xu, F.; Li, Z.; Liu, Y.; Wang, J.; Zhang, L. Effect of vibration on storage quality and ethylene biosynthesis-related enzyme genes expression in harvested apple fruit. Sci. Hortic-Amst. 2019, 249, 1–6. [Google Scholar] [CrossRef]
- Xu, F.X.; Liu, S.Y. Control of postharvest quality in blueberry fruit by combined 1-methylcyclopropene (1-MCP) and UV-C irradiation. Food Bioproc. Tech. 2017, 10, 1695–1703. [Google Scholar] [CrossRef]
- Zhan, Y.F.; Dang, X.M.; Qi, Z.Q.; He, H.; Yang, Y. Main agronomic and quality characters of asparagus bean (Vigna unguiculata ssp. sesquipedalis) germplasms. J. South. Agr. 2015, 46, 2006–2010. [Google Scholar]
- Rao, L.B.; Chen, X.Z.; Chen, X.X.; Feng, G.; Zheng, J.H. Identification and comprehensive assessment on long cowpea germplasm resources. J. Zhejiang Agr. Sci. 2009, 1, 39–41. [Google Scholar]
- Zhou, X.M.; Mackenzie, A.F.; Madramootoo, C.A.; Smith, D.L. Effects of stem-injected plant growth regulators, with or without sucrose, on grain production, biomass and photosynthetic activity of field-grown corn plants. J. Agron. Crop Sci. 2010, 183, 103–110. [Google Scholar] [CrossRef]
- Barányiová, I.; Klem, K. Effect of application of growth regulators on the physiological and yield parameters of winter wheat under water deficit. Plant Soil Environ. 2016, 62, 114–120. [Google Scholar] [CrossRef] [Green Version]
- Fahad, S.; Hussain, S.; Saud, S.; Hassan, S.; Ihsan, Z.; Alghabari, F. Exogenously applied plant growth regulators enhance the morpho-physiological growth and yield of rice under high temperature. Front. Plant Sci. 2016, 7, 1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, D.; Orson, J. Plant growth regulators: Their effect on yield of winter wheat in a very dry spring and in the absence of lodging. Ann. Appl. Biol. 2012, 40, 91–94. [Google Scholar]


| Treatment | Concentration (mg·L−1) | Length (cm) | Width (cm) | Single Pod Weight (g) |
|---|---|---|---|---|
| Blank control | 0 | 50.62 ± 3.24 b | 0.60 ± 0.03 ab | 10.10 ± 0.67 b |
| Phthalanilic acid | 133.3 | 54.00 ± 1.91 a | 0.61 ± 0.05 a | 10.89 ± 1.72 ab |
| 200.0 | 55.01 ± 3.05 a | 0.61 ± 0.02 a | 11.71 ± 1.08 a | |
| 266.7 | 51.96 ± 1.83 ab | 0.57 ± 0.02 b | 10.30 ± 1.26 b | |
| Brassinolide | 0.075 | 50.95 ± 2.07 b | 0.59 ± 0.02 ab | 10.55 ± 0.46 ab |
| Treatment | Concentration (mg·L−1) | Vc Content (mg/100 g·FW) | Soluble Protein Content (mg/g·FW) | Soluble Sugar Content (mg/g·FW) |
|---|---|---|---|---|
| Blank control | 0 | 13.04 ± 0.50 b | 25.12 ± 0.74 c | 50.93 ± 1.11 b |
| Phthalanilic acid | 133.3 | 13.99 ± 0.93 b | 26.09 ± 0.88 c | 53.92 ± 2.23 a |
| 200.0 | 16.85 ± 0.55 a | 29.83 ± 0.68 b | 54.00 ± 1.43 a | |
| 266.7 | 14.31 ± 0.46 b | 26.06 ± 0.53 c | 51.87 ± 3.73 ab | |
| Brassinolide | 0.075 | 13.35 ± 0.95 b | 32.68 ± 1.19 a | 54.21 ± 2.30 a |
| Treatment | Concentration (mg·L−1) | Podding Rate( %) | Yield (kg/hm2) | Yield Growth Rate (%) |
|---|---|---|---|---|
| Blank control | 0 | 57.58 ± 2.92 b | 2.14 × 104 ± 1.24 × 103 b | ---- |
| Phthalanilic acid | 133.3 | 61.11 ± 3.93 b | 2.37 × 104 ± 1.60 × 103 ab | 10.75 |
| 200.0 | 68.89 ± 3.85 a | 2.48 × 104 ± 1.34 × 103 a | 15.89 | |
| 266.7 | 63.33 ± 4.77 ab | 2.29 × 104 ± 1.49 × 103 ab | 7.01 | |
| Brassinolides | 0.075 | 61.11 ± 5.09 b | 2.28 × 104 ± 1.03 × 103 ab | 6.54 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, T.; Wu, Q.; Liu, N.; Zhang, R.; Ma, Z. Phthalanilic Acid with Biostimulatory Functions Affects Photosynthetic and Antioxidant Capacity and Improves Fruit Quality and Yield in Cowpea (Vigna unguiculata (L.) Walp.). Agriculture 2021, 11, 1082. https://doi.org/10.3390/agriculture11111082
Ma T, Wu Q, Liu N, Zhang R, Ma Z. Phthalanilic Acid with Biostimulatory Functions Affects Photosynthetic and Antioxidant Capacity and Improves Fruit Quality and Yield in Cowpea (Vigna unguiculata (L.) Walp.). Agriculture. 2021; 11(11):1082. https://doi.org/10.3390/agriculture11111082
Chicago/Turabian StyleMa, Ting, Qiong Wu, Na Liu, Rong Zhang, and Zhiqing Ma. 2021. "Phthalanilic Acid with Biostimulatory Functions Affects Photosynthetic and Antioxidant Capacity and Improves Fruit Quality and Yield in Cowpea (Vigna unguiculata (L.) Walp.)" Agriculture 11, no. 11: 1082. https://doi.org/10.3390/agriculture11111082
APA StyleMa, T., Wu, Q., Liu, N., Zhang, R., & Ma, Z. (2021). Phthalanilic Acid with Biostimulatory Functions Affects Photosynthetic and Antioxidant Capacity and Improves Fruit Quality and Yield in Cowpea (Vigna unguiculata (L.) Walp.). Agriculture, 11(11), 1082. https://doi.org/10.3390/agriculture11111082





