Transcriptomic Analysis of Late-Ripening Sweet Orange Fruits (Citrus sinensis) after Foliar Application of Glomalin-Related Soil Proteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Application of EE-GRSP
2.2. Analysis of Fruit Quality
2.3. Construction of Fruit Transcriptomics
2.4. Reference Transcriptome Analysis
3. Results
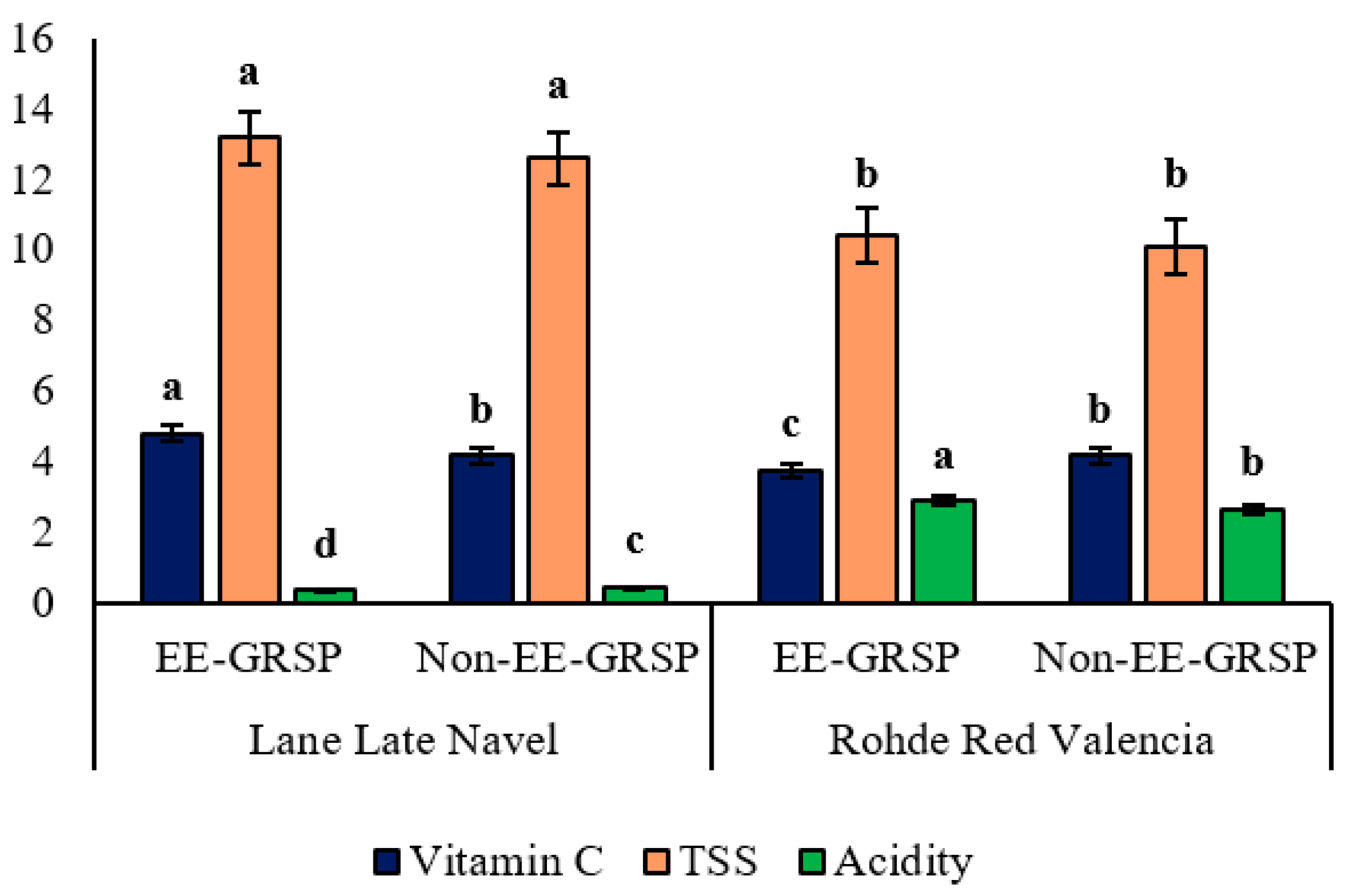
3.1. Improvement in Fruit Quality
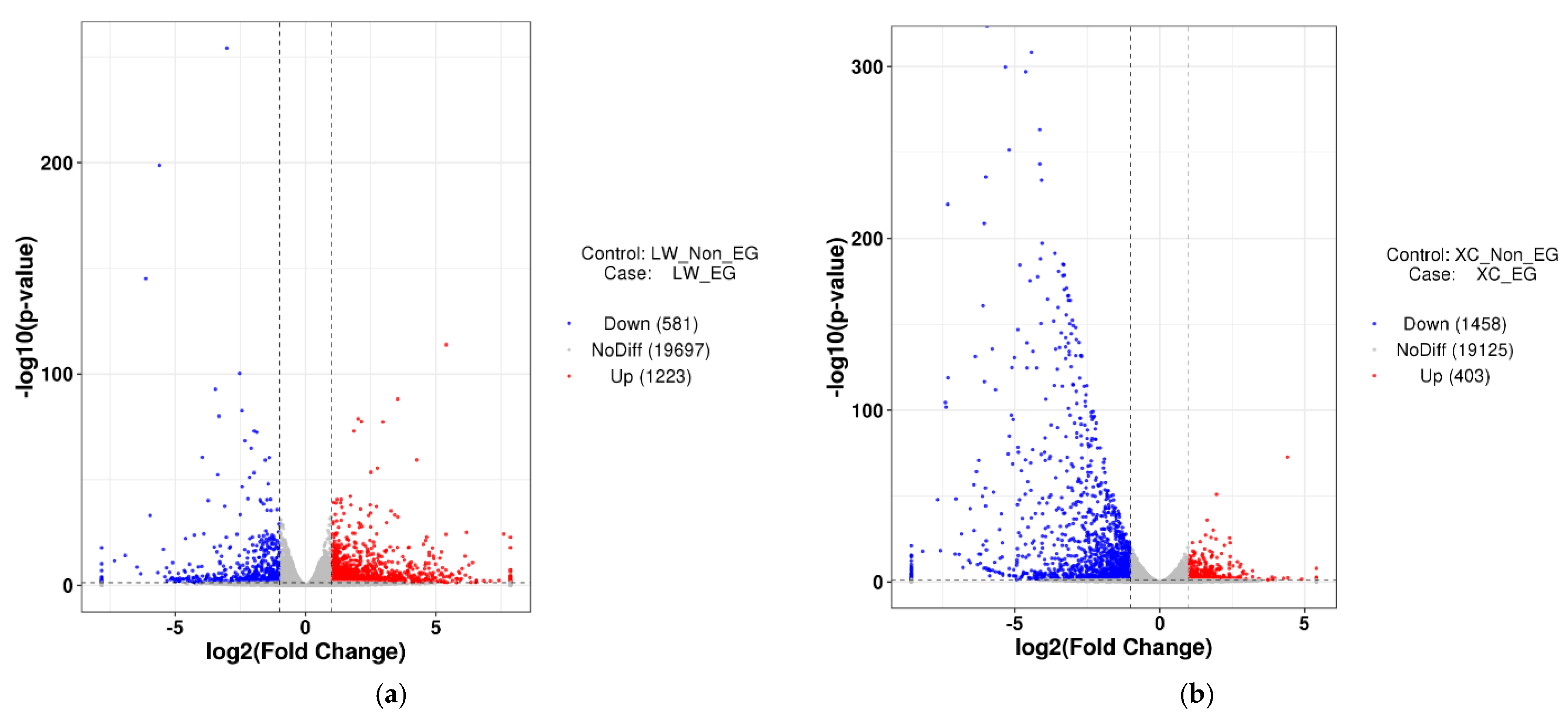
3.2. Data Filtering and Comparison Analysis
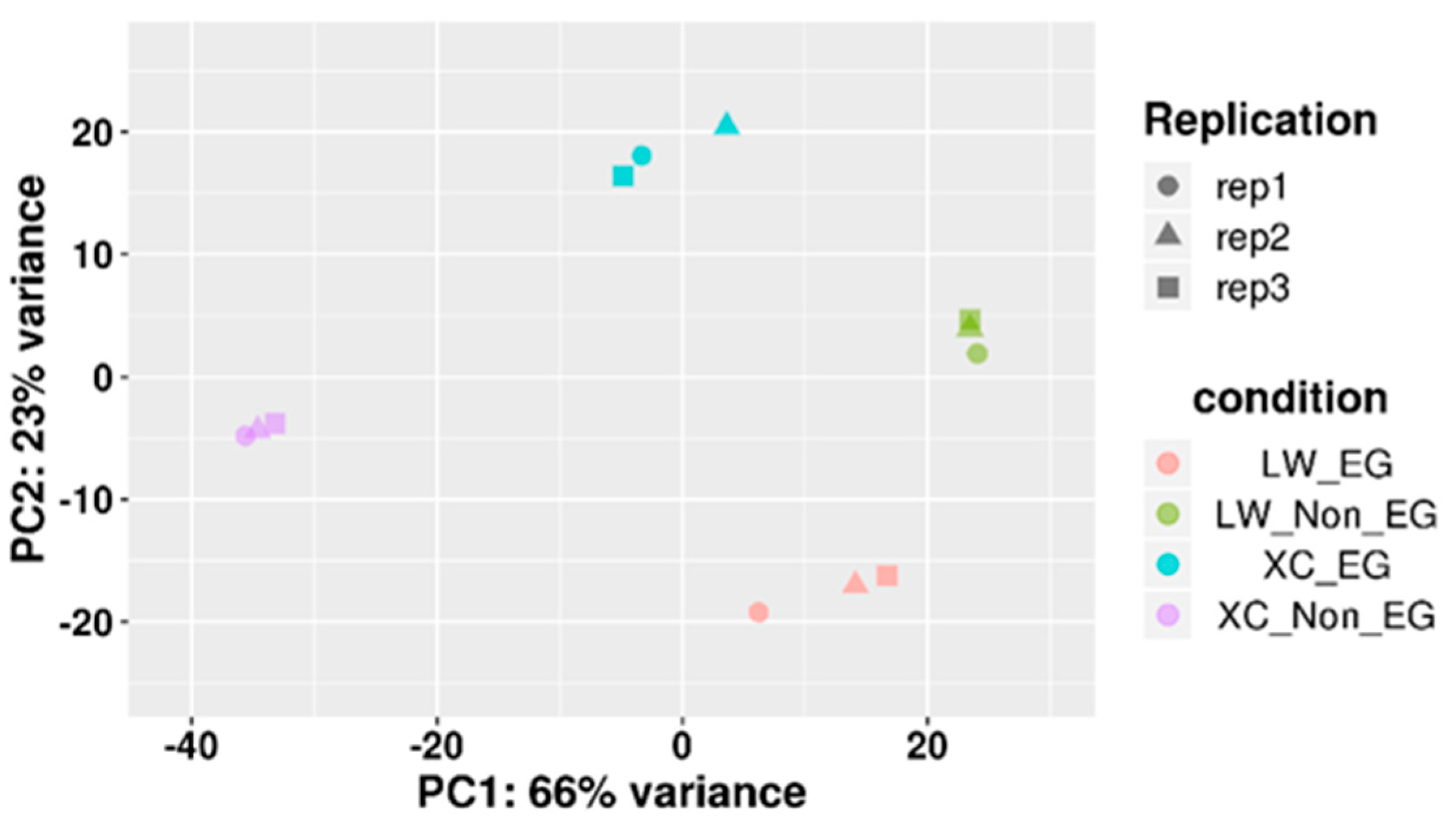
3.3. Principal Component Analysis
3.4. Analysis of Gene Expression
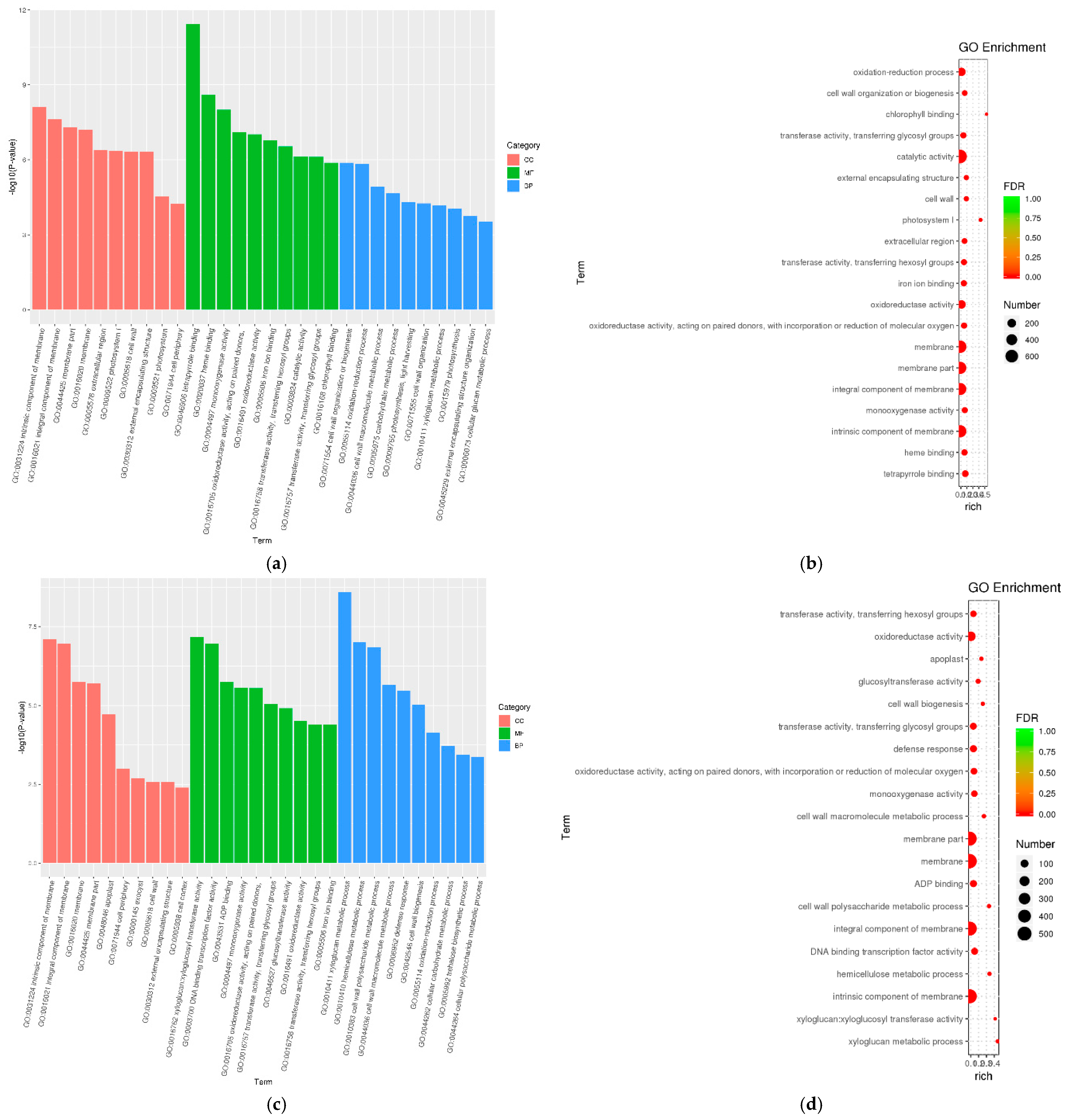
3.5. Gene Ontology (GO) Enrichment Analysis
3.6. Kyoto Encyclopedia of Genes and Genomes (KEGG) Pathway Enrichment
3.7. Starch and Sucrose Metabolism
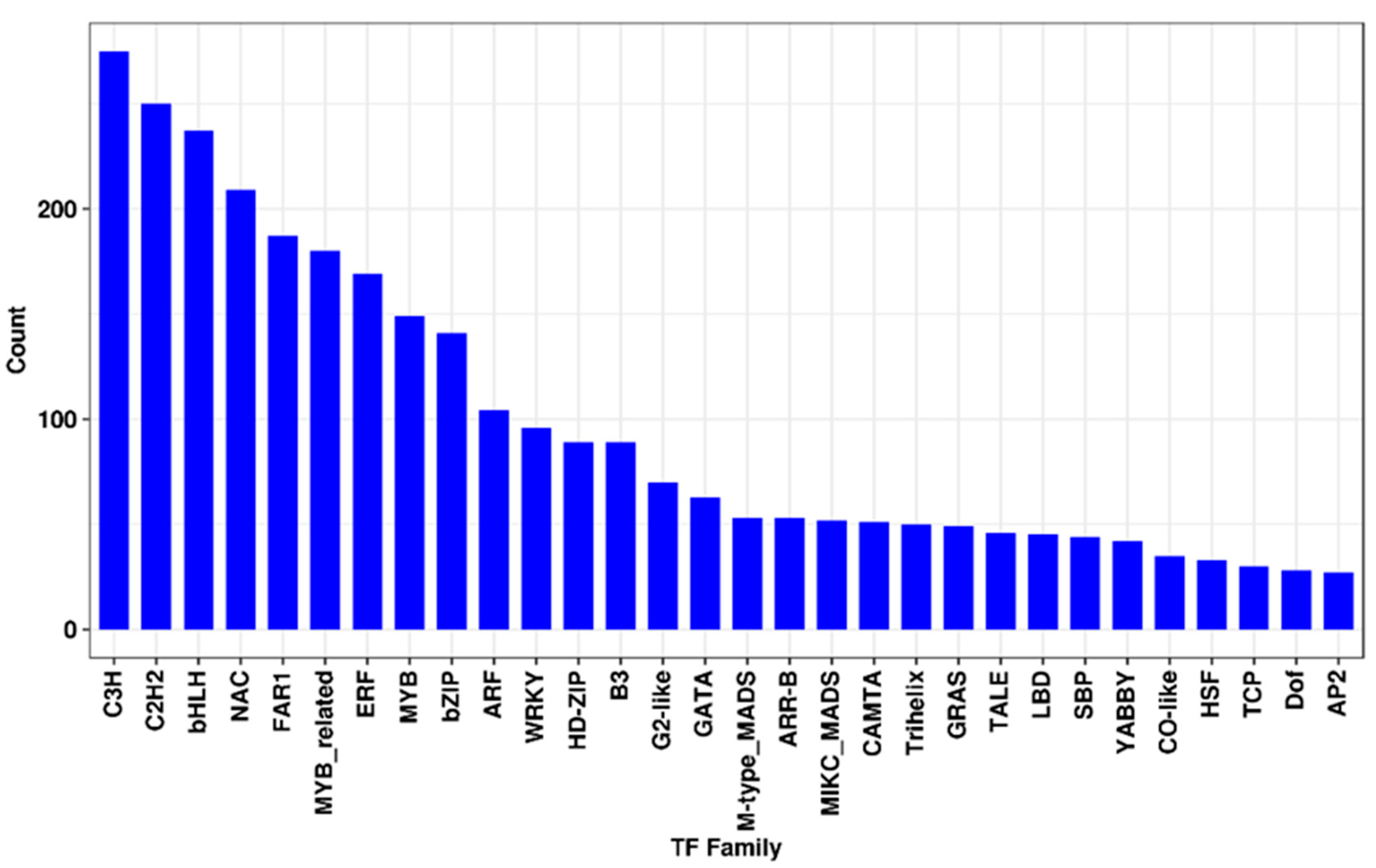
3.8. Transcription Factor Family Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Srivastava, A.K.; Singh, S.; Marathe, R.A. Organic citrus: Soil fertility and plant nutrition. J. Sustain. Agric. 2002, 19, 5–29. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Wu, Q.S.; Mousavi, S.M.; Hota, D. Integrated soil fertility management in fruit crops: An overview. Int. J. Fruit Sci. 2021, 21, 413–439. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Kohli, R.R. Agro-ecological approach for land use planning of citrus. Agric. Rev. 1999, 20, 41–47. [Google Scholar]
- Zhu, S.P.; Huang, T.J.; Yu, X.; Hong, Q.B.; Xiang, J.S.; Zeng, A.Z.; Gong, G.Z.; Zhao, X.C. The effects of rootstocks on performances of three late-ripening navel orange varieties. J. Integr. Agric. 2020, 19, 1802–1812. [Google Scholar] [CrossRef]
- Wu, L.M. Physiological and Molecular Mechanisms of Pre-Harvest Granulationi in Late-Ripening Navel Orange and Its Regulation Techniques. PhD’s Dissertation, Huazhong Agricultural University, Wuhan, China, 2020. [Google Scholar]
- Berger, F.; Gutjahr, C. Factors affecting plant responsiveness to arbuscular mycorrhiza. Curr. Opin. Plant Biol. 2021, 59, 101994. [Google Scholar] [CrossRef]
- Zou, Y.N.; Srivastava, A.K.; Wu, Q.S.; Huang, Y.M. Glomalin-related soil protein and water relations in mycorrhizal citrus (Citrus tangerina) during soil water deficit. Arch. Agron. Soil Sci. 2014, 60, 1103–1114. [Google Scholar] [CrossRef]
- Zhang, Y.C.; Zou, Y.N.; Liu, L.P.; Wu, Q.S. Common mycorrhizal networks activate salicylic acid defense responses of trifoliate orange (Poncirus trifoliata). J. Integr. Plant Biol. 2019, 61, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Bourazza, M.; Chetto, O.; Talha, A.; Farih, A.; Douira, A.; Benyahia, H. The influence of arbuscular mycorrhizal colonisation on key growth parameters of five citrus rootstock cultivars under salt stress. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 125–138. [Google Scholar]
- Wright, S.F.; Upadhyaya, A. A survey of soils for aggregate stability and glomalin, a glycoprotein produced by hyphae of arbuscular mycorrhizal fungi. Plant Soil 1998, 198, 97–107. [Google Scholar] [CrossRef]
- Wright, S.F. A fluorescent antibody assay for hyphae and glomalin from arbuscular mycorrhizal fungi. Plant Soil 2000, 226, 171–177. [Google Scholar] [CrossRef]
- Rosier, C.L.; Hoye, A.T.; Rillig, M.C. Glomalin-related soil protein: Assessment of current detection and quantification tools. Soil Biol. Biochem. 2006, 38, 2205–2211. [Google Scholar] [CrossRef]
- Treseder, K.K.; Turner, K.M. Glomalin in ecosystems. Soil Sci. Soc. Am. J. 2007, 71, 1257–1266. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.S.; Wang, S.; Srivastava, A.K. Mycorrhizal hyphal disruption induces changes in plant growth, glomalin-related soil protein and soil aggregation of trifoliate orange in a core system. Soil Till. Res. 2016, 160, 82–91. [Google Scholar] [CrossRef]
- He, J.D.; Chi, G.G.; Zou, Y.N.; Shu, B.; Wu, Q.S.; Srivastava, A.K.; Kuča, K. Contribution of glomalin-related soil proteins to soil organic carbon in trifoliate orange. Appl. Soil Ecol. 2020, 154, 103592. [Google Scholar] [CrossRef]
- Meng, L.L.; He, J.D.; Zou, Y.N.; Wu, Q.S.; Kuča, K. Mycorrhiza-released glomalin-related soil protein fractions contribute to soil total nitrogen in trifoliate orange. Plant Soil Environ. 2020, 66, 183–189. [Google Scholar] [CrossRef]
- Vlček, V.; Pohanka, M. Glomalin–an interesting protein part of the soil organic matter. Soil Water Res. 2020, 15, 67–74. [Google Scholar] [CrossRef]
- Hossain, M.B. Glomalin and contribution of glomalin to carbon sequestration in soil: A review. Turk. J. Agric.-Food Sci. Technol. 2021, 9, 191–196. [Google Scholar]
- Wu, Q.S.; Li, Y.; Zou, Y.N.; He, X.H. Arbuscular mycorrhiza mediates glomalin-related soil protein production and soil enzyme activities in the rhizosphere of trifoliate orange grown under different P levels. Mycorrhiza 2015, 25, 121–130. [Google Scholar] [CrossRef]
- Wang, S.; Wu, Q.S.; He, X.H. Exogenous easily extractable glomalin-related soil protein promotes soil aggregation, relevant soil enzyme activities and plant growth in trifoliate orange. Plant Soil Environ. 2015, 61, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.S.; Srivastava, A.K.; Wang, S.; Zeng, J.X. Exogenous application of EE-GRSP and changes in citrus rhizosphere properties. Ind. J. Agric. Sci. 2015, 85, 802–806. [Google Scholar]
- Chi, G.G.; Srivastava, A.K.; Wu, Q.S. Exogenous easily extractable glomalin-related soil protein improves drought tolerance of trifoliate orange. Arch. Agron. Soil Sci. 2018, 64, 1341–1350. [Google Scholar] [CrossRef]
- Meng, L.L.; Liang, S.M.; Srivastava, A.K.; Li, Y.; Liu, C.Y.; Zou, Y.N.; Kuča, K.; Hashem, A.; Abd-Allah, E.F.; Wu, Q.S. Easily extractable glomalin-related soil protein as foliar spray improves nutritional qualities of late ripening sweet oranges. Horticulturae 2021, 7, 228. [Google Scholar] [CrossRef]
- Liu, R.C.; Gao, W.Q.; Srivastava, A.K.; Zou, Y.N.; Kuča, K.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.S. Differential effects of exogenous glomalin-related soil proteins on plant growth of trifoliate orange through regulating auxin changes. Front. Plant Sci. 2021, 12, 745402. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of protein utilizing the principle of protein-dye binding. Analy Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Caemmerer, S.V.; Griffiths, H. Stomatal responses to CO2 during a diel Crassulacean acid metabolism cycle in Kalanchoe daigremontiana and Kalanchoe pinnata. Plant Cell Environ. 2009, 32, 567–576. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 16th ed.; Association of Official Analytical Chemists (AOAC): Washington, DC, USA, 1995. [Google Scholar]
- Wu, Q.S.; Lou, Y.G.; Li, Y. Plant growth and tissue sucrose metabolism in the system of trifoliate orange and arbuscular mycorrhizal fungi. Sci. Hortic. 2015, 181, 189–193. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, M.; Song, F. Transcriptome analysis of the symbiosis-related genes between Funneliformis mosseae and Amorpha fruticosa. J. For. Res. 2019, 30, 483–495. [Google Scholar] [CrossRef]
- Liu, C.Y.; Zhang, F.; Zhang, D.J.; Zou, Y.N.; Shu, B.; Wu, Q.S. Transcriptome analysis reveals improved root hair growth in trifoliate orange seedlings by arbuscular mycorrhizal fungi. Plant Growth Regul. 2020, 92, 195–203. [Google Scholar] [CrossRef]
- Schindler, F.V.; Mercer, E.J.; Rice, J.A. Chemical characteristics of glomalin-related soil protein (GRSP) extracted from soils of varying organic matter content. Soil Biol. Biochem. 2007, 39, 320–329. [Google Scholar] [CrossRef]
- Wei, Q.J.; Ma, Z.Z.; Li, S.; Lei, C.Y.; Ma, Q.L.; Gu, Q.Q. Identification and expression analysis of sucrose-phosphate synthase (SPS) genes in citrus. Acta Hortic. Sin. 2020, 47, 334–344. [Google Scholar]
- Liu, X.Y.; Li, J.; Huang, M.; Liang, C.H.; Chen, J.Z. Research on influences of rootstock on sugar accumulation in ‘Shatangju’ tangerine fruits. Sci. Agric. Sin. 2015, 48, 2217–2228. [Google Scholar]
- Arul, L.; Benita, G.; Sudhakar, D.; Thayumanavan, B.; Balasubramanian, P. β-glucuronidase of family-2 glycosyl hydrolase: A missing member in plants. Bioinformation 2008, 3, 194–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Samples | Clean Reads No. | Clean Data (Bp) | Clean Reads (%) | Total Mapped | Multiple Mapped | Uniquely Mapped |
|---|---|---|---|---|---|---|
| LW_Non_EG_1 | 42168768 | 6367483968 | 92.79 | 39396132(93.42%) | 1948127(4.94%) | 37448005(95.06%) |
| LW_Non_EG_2 | 35949522 | 5428377822 | 92.76 | 33544020(93.31%) | 1687584(5.03%) | 31856436(94.97%) |
| LW_Non_EG_3 | 38009648 | 5739456848 | 92.54 | 35475281(93.33%) | 1710341(4.82%) | 33764940(95.18%) |
| LW_EG_1 | 38173108 | 5764139308 | 92.84 | 35648187(93.39%) | 1647770(4.62%) | 34000417(95.38%) |
| LW_EG_2 | 39379550 | 5946312050 | 92.69 | 36801866(93.45%) | 1710902(4.62%) | 35090964(95.35%) |
| LW_EG_3 | 39201784 | 5919469384 | 92.75 | 36588621(93.33%) | 1682057(4.60%) | 34906564(95.40%) |
| XC_Non_EG_1 | 38468020 | 5808671020 | 92.95 | 35969372(93.50%) | 1691810(4.70%) | 34277562(95.30%) |
| XC_Non_EG_2 | 37943920 | 5729531920 | 92.85 | 35444261(93.41%) | 1740822(4.91%) | 33703439(95.09%) |
| XC_Non_EG_3 | 36631568 | 5531366768 | 92.80 | 34254699(93.51%) | 1694032(4.95%) | 32560667(95.05%) |
| XC_ EG_1 | 37449412 | 5654861212 | 92.96 | 35088625(93.70%) | 1707696(4.87%) | 33380929(95.13%) |
| XC_ EG_2 | 40065458 | 6049884158 | 92.82 | 37285676(93.06%) | 1977558(5.30%) | 35308118(94.70%) |
| XC_ EG_3 | 41695874 | 6296076974 | 92.54 | 38705480(92.83%) | 1862787(4.81%) | 36842693(95.19%) |
| Sweet Oranges | Up-Regulated Genes | Up-Regulated Gene Number | Down-Regulated Genes | Down-Regulated Gene Number |
|---|---|---|---|---|
| LW | Cs3g23560 (α-amylase), Cs1g18220 (β-fructofuranosidase), Cs8g13800 (glycoside hydrolase family 3), orange1.1t00611 (ADP-glucose pyrophosphorylase signature 2), Cs5g33760 (glycosyl hydrolase family 9), Cs9g18290 (trehalose 6-phosphate phosphatase), Cs3g26050 (hexokinase family signature), Cs5g01280 (granule-bound starch synthase 1), Cs2g12180 (glycosyl hydrolase family 9), and Cs4g11970 (glycosyl hydrolase family 1 signature) | 10 | Cs7g01380 (glycoside hydrolase family 1), Cs4g06900 (sucrose synthase), Cs5g19060 (sucrose-phosphate synthase 4), Cs2g07740 (glycosyl hydrolase family 1 signature), and Cs1g01330 (trehalose 6-phosphate phosphatase) | 5 |
| XC | Cs5g19060 and Cs7g08390 (α-trehalose-phosphate synthase [UDP-forming] 9) | 2 | Cs9g06360 (ADP-glucose pyrophosphorylase signature 1), Cs7g11210 (glycosyl hydrolase family 1), Cs9g14600 (trehalose-phosphate phosphatase A), Cs1g18220, orange1.1t03668 (sucrose-phosphate synthase 3), Cs4g02730 (α-trehalose-phosphate synthase [UDP-forming] 11), Cs5g09200 (trehalose-phosphate phosphatase F), Cs3g18450 (trehalose-phosphate phosphatase C), orange1.1t00483 (trehalose 6-phosphate phosphatase), Cs2g12180, and orange1.1t03470 (plant β-amylase signature) | 11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.-H.; Srivastava, A.K.; Li, Y.; Zou, Y.-N.; Hashem, A.; Abd_Allah, E.F.; Wu, Q.-S. Transcriptomic Analysis of Late-Ripening Sweet Orange Fruits (Citrus sinensis) after Foliar Application of Glomalin-Related Soil Proteins. Agriculture 2021, 11, 1171. https://doi.org/10.3390/agriculture11111171
Wu H-H, Srivastava AK, Li Y, Zou Y-N, Hashem A, Abd_Allah EF, Wu Q-S. Transcriptomic Analysis of Late-Ripening Sweet Orange Fruits (Citrus sinensis) after Foliar Application of Glomalin-Related Soil Proteins. Agriculture. 2021; 11(11):1171. https://doi.org/10.3390/agriculture11111171
Chicago/Turabian StyleWu, Hui-Hui, Anoop Kumar Srivastava, Yan Li, Ying-Ning Zou, Abeer Hashem, Elsayed Fathi Abd_Allah, and Qiang-Sheng Wu. 2021. "Transcriptomic Analysis of Late-Ripening Sweet Orange Fruits (Citrus sinensis) after Foliar Application of Glomalin-Related Soil Proteins" Agriculture 11, no. 11: 1171. https://doi.org/10.3390/agriculture11111171
APA StyleWu, H.-H., Srivastava, A. K., Li, Y., Zou, Y.-N., Hashem, A., Abd_Allah, E. F., & Wu, Q.-S. (2021). Transcriptomic Analysis of Late-Ripening Sweet Orange Fruits (Citrus sinensis) after Foliar Application of Glomalin-Related Soil Proteins. Agriculture, 11(11), 1171. https://doi.org/10.3390/agriculture11111171









