The Impact of Exotic Tamarix Species on Riparian Plant Biodiversity
Abstract
1. Introduction
2. Methods and Materials
2.1. Study Site
2.2. Data Collection
Experimental Design
2.3. Data Analysis
Determining Species Richness, Plant Diversity, and Evenness
2.4. Complementarity and Similarity
3. Results
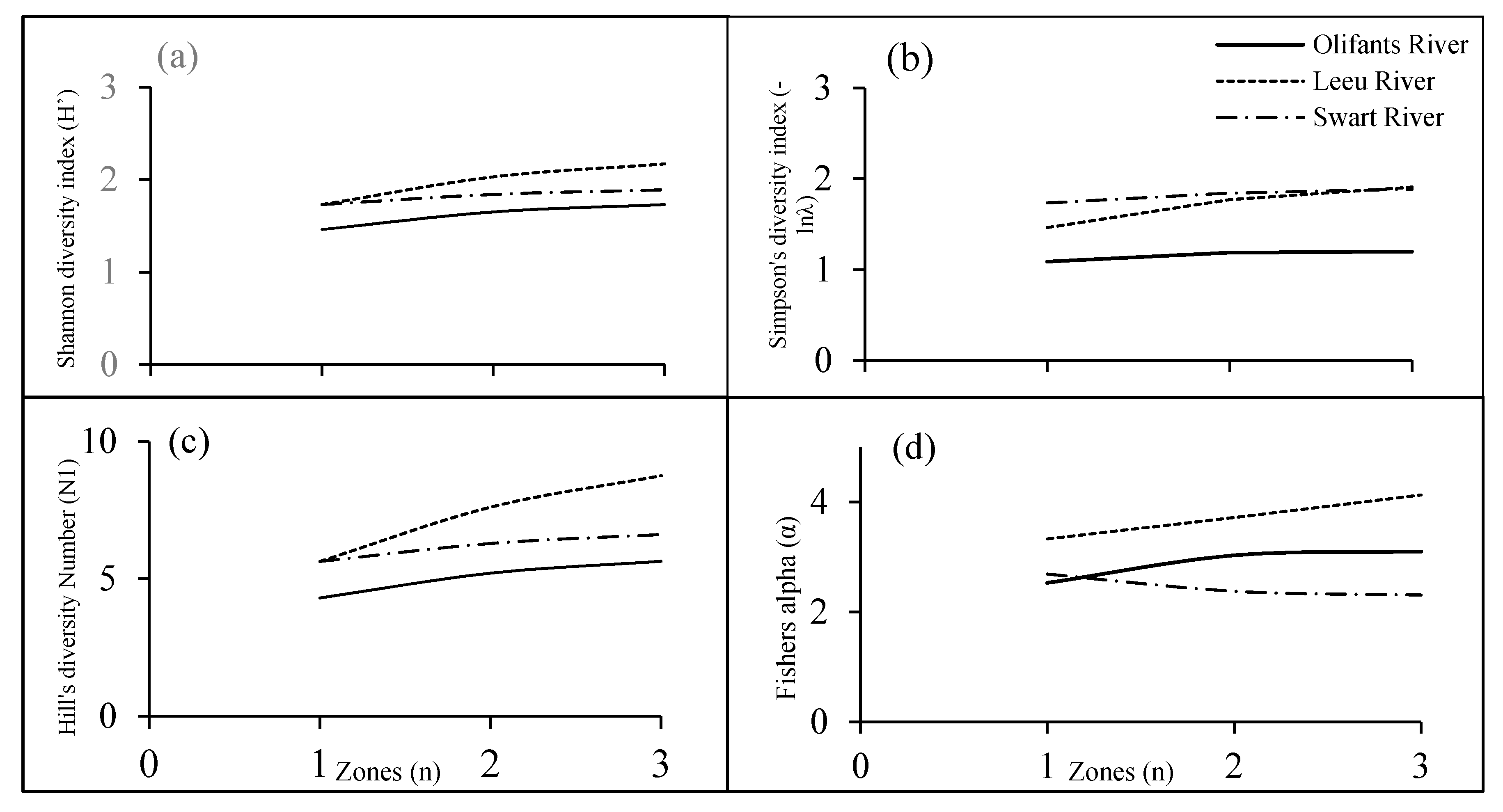
3.1. Species Richness, Diversity, and Evenness
Species Identification: Family Name, Binomial Nomenclature, Plant Type, Origin, and Status
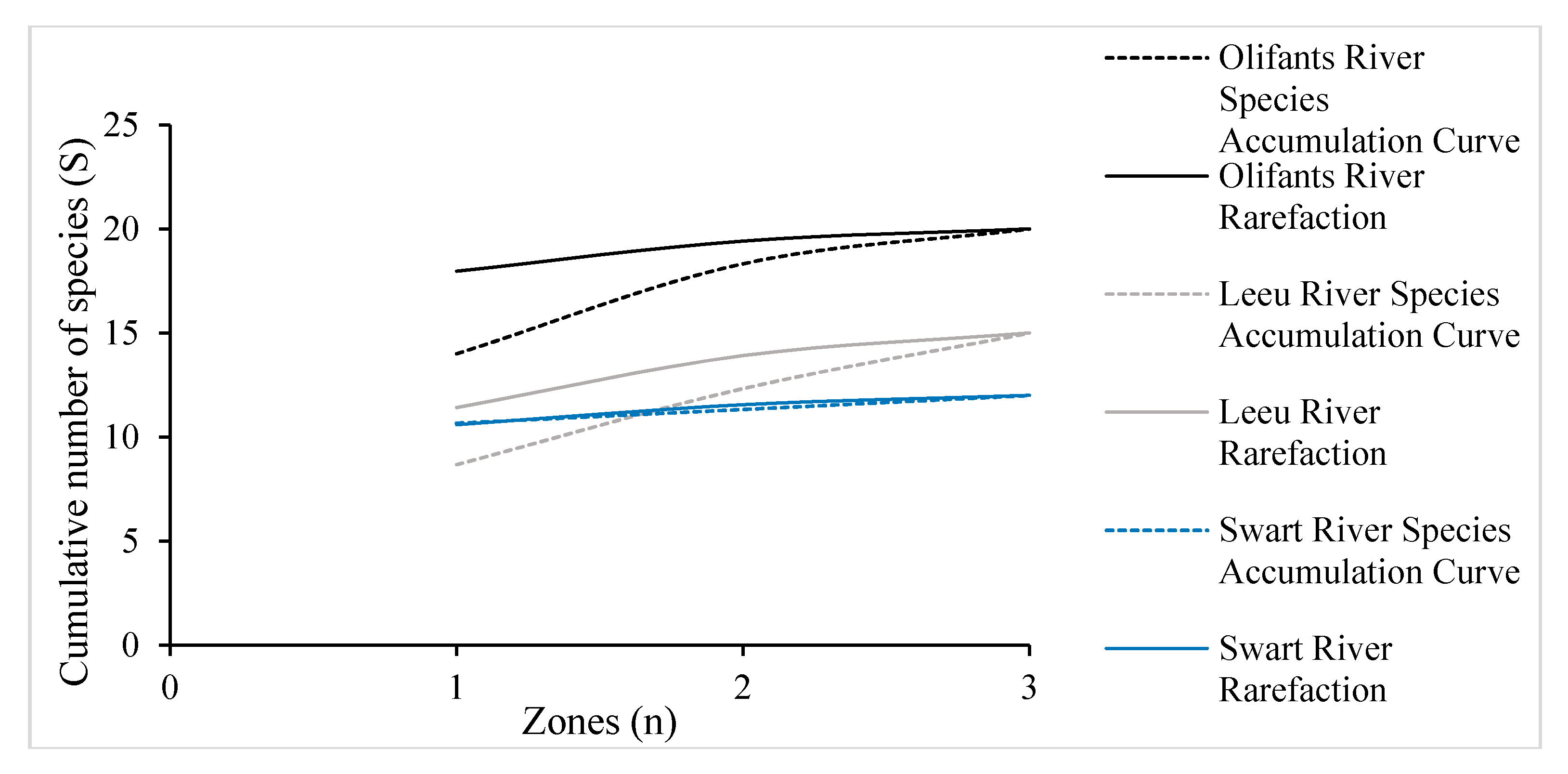
3.2. Species Accumulation and Rarefaction Curves for Woody Vegetation
3.3. Species Richness
3.4. Species Diversity
3.5. Species Evenness
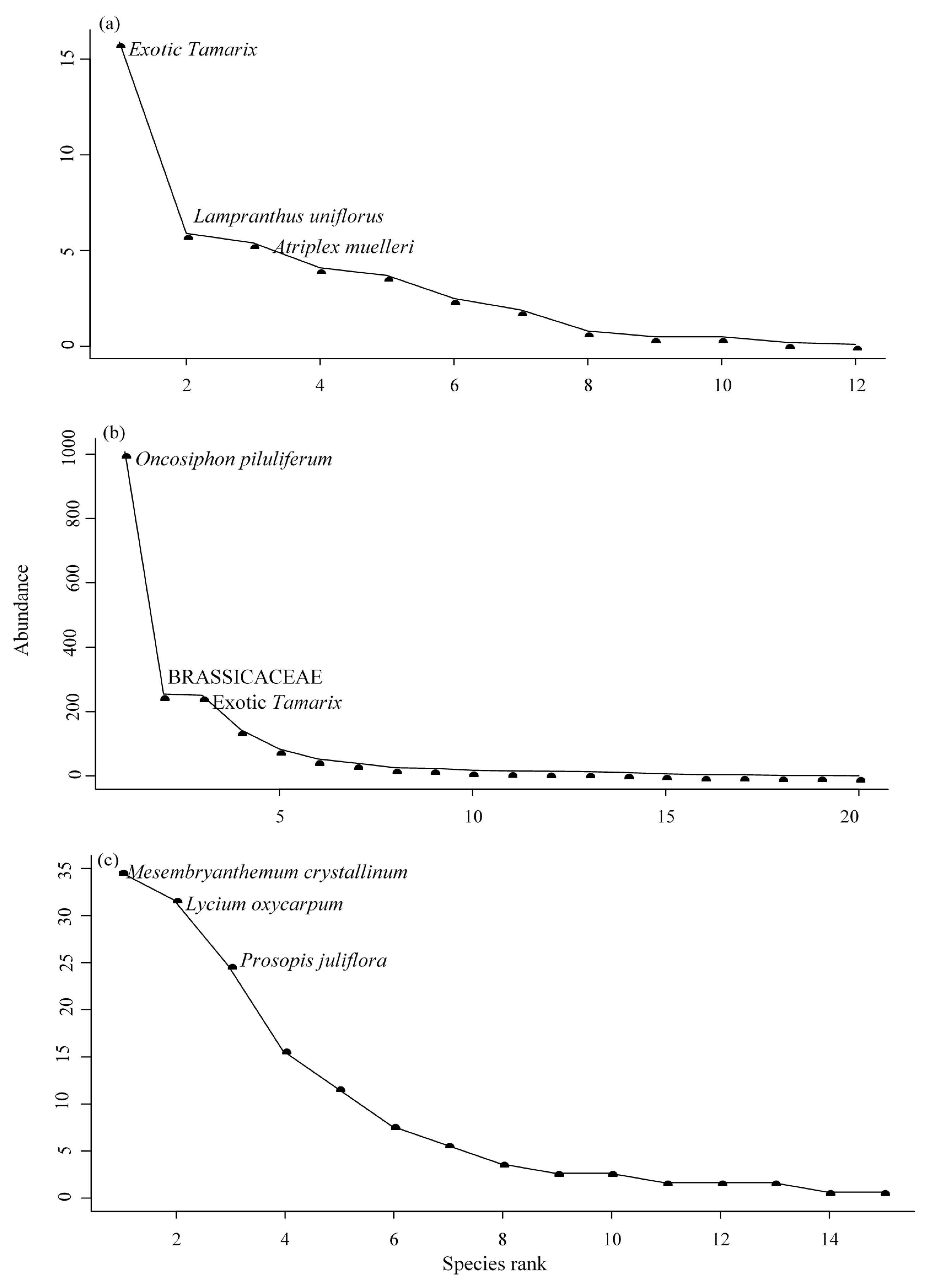
3.6. Rank Abundance Curves
3.7. Complementarity and Similarity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pamela, S.E. The Root Causes of Biodiversity Loss; Earthscan: London, UK, 2000. [Google Scholar]
- Torrance, A.W. Patent Law, HIPPO, and the Biodiversity Crisis. John Marshall Rev. Intell. Prop. Law 2010, 9, 1. [Google Scholar]
- Viciani, D.; Vidali, M.; Gigante, D.; Bolpagni, R.; Villani, M.; Acosta, A.T.R.; Adorni, M.; Aleffi, M.; Allegrezza, M.; Angiolini, C.; et al. A first checklist of the alien-dominated vegetation in Italy. Plant Sociol. 2020, 57, 29–54. [Google Scholar] [CrossRef]
- Wilson, E.O. The Future of Life; Vintage Books: New York, NY, USA, 2002. [Google Scholar]
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2014—Impacts, Adaptation and Vulnerability: Regional Aspects; Cambridge University Press: Cambridge, UK, 2014. [Google Scholar]
- Lohbeck, M.; Bongers, F.; Martínez-Ramos, M.; Poorter, L. The importance of biodiversity and dominance for multiple ecosystem functions in a human-modified tropical landscape. Ecology 2016, 97, 2772–2779. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.; Tilman, D.; Wardle, D.A.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 486, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Giam, X.; Bradshaw, C.J.; Tan, H.T.; Sodhi, N.S. Future habitat loss and the conservation of plant biodiversity. Biol. Conserv. 2010, 143, 1594–1602. [Google Scholar] [CrossRef]
- Hultine, K.R.; Bean, D.W.; Dudley, T.L.; Gehring, C.A. Species Introductions and Their Cascading Impacts on Biotic Interactions in desert riparian ecosystems. Integr. Comp. Biol. 2015, 55, 587–601. [Google Scholar] [CrossRef]
- Henderson, L. Invasive alien woody plants of the southern and southwestern Cape region, South Africa. Bothalia 1998, 28, 91–112. [Google Scholar] [CrossRef]
- Foxcroft, L.; Henderson, L.; Nichols, G.; Martin, B. A revised list of alien plants for the Kruger National Park. Koedoe 2003, 46, 21–44. [Google Scholar] [CrossRef]
- Holmes, P.M.; Richardson, D.M.; Esler, K.J.; Witkowski, E.T.F.; Fourie, S. A decision-making framework for restoring riparian zones degraded by invasive alien plants in South Africa. S. Afr. J. Sci. 2005, 101, 553–564. [Google Scholar]
- Newete, S.W.; Mayonde, S.; Byrne, M.J. Distribution and abundance of invasive Tamarix genotypes in South Africa. Weed Res. 2019, 59, 191–200. [Google Scholar] [CrossRef]
- Witkowski, E.T.F. Effects of Invasive Alien Acacias on Nutrient Cycling in the Coastal Lowlands of the Cape Fynbos. J. Appl. Ecol. 1991, 28, 1. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. Traits Associated with Invasiveness in Alien Plants: Where Do we Stand? Biol. Invasions 2008, 193, 97–125. [Google Scholar]
- Downey, P.O.; Richardson, D.M. Alien plant invasions and native plant extinctions: A six-threshold framework. AoB Plants 2016, 8. [Google Scholar] [CrossRef] [PubMed]
- Decamps, H.; Pinay, G.; Naiman, R.J.; Petts, G.E.; McClain, M.E.; Hillbricht-Ilkowska, A.; Hanley, T.A.; Holmes, R.M.; Quinn, J.; Gibert, J.; et al. Riparian zones: Where biogeochemistry meets biodiversity in management practice. Pol. J. Ecol. 2004, 52, 3–18. [Google Scholar]
- Van Wilgen, B.W.; Richardson, D.M.; Le Maitre, D.C.; Marais, C.; Magadlela, D. The economic consequences of alien plant invasions: Examples of impacts and approaches to sustainable management in South Africa. Environ. Dev. Sustain. 2001, 3, 145–168. [Google Scholar] [CrossRef]
- Marlin, D.; Newete, S.W.; Mayonde, S.; Smit, E.R.; Byrne, M.J. Invasive Tamarix (Tamaricaceae) in South Africa: Current research and the potential for biological control. Biol. Invasions 2017, 19, 2971–2992. [Google Scholar] [CrossRef]
- Newete, S.W.; Abd Elbasit, M.A.; Araya, T.W. Soil salinity and moisture content under non-native Tamarix species. Int. J. Phytoremediat. 2020, 22, 1–8. [Google Scholar] [CrossRef]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection From the Global Invasive Species Database, 12; Invasive Species Specialist Group: Auckland, New Zealand, 2000. [Google Scholar]
- Mayonde, S.; Cron, G.; Gaskin, J.; Byrne, M.J. Evidence of Tamarix hybrids in South Africa, as inferred by nuclear ITS and plastid trnS-trnG DNA sequences. S. Afr. J. Bot. 2015, 96, 122–131. [Google Scholar] [CrossRef]
- Crins, W.J. The Tamaricaceae in the southeastern United States. J. Arnold Arboretum. 1989, 70, 403–425. [Google Scholar] [CrossRef]
- Gaskin, J.F.; Schaal, B.A. Hybrid Tamarix widespread in U.S. invasion and undetected in native Asian range. Proc. Natl. Acad. Sci. USA 2002, 99, 11256–11259. [Google Scholar] [CrossRef]
- Mayonde, S.; Cron, G.V.; Gaskin, J.F.; Byrne, M.J. Tamarix (Tamaricaceae) hybrids: The dominant invasive genotype in southern Africa. Biol. Invasions 2016, 18, 3575–3594. [Google Scholar] [CrossRef]
- Di Tomaso, J.M. Impact, biology, and ecology of saltcedar (Tamarix spp.) in the southwestern United States. Weed Technol. 1998, 12, 326–336. [Google Scholar] [CrossRef]
- Roux, P.W.; Vorster, M.; Zeeman, P.J.L.; Wentzel, D. Stock production in the Karoo region. Proc. Annu. Congr. Grassl. Soc. S. Afr. 1981, 16, 29–35. [Google Scholar] [CrossRef]
- Gallo, J.A.; Pasquini, L.; Reyers, B.; Cowling, R.M. The role of private conservation areas in biodiversity representation and target achievement within the Little Karoo region, South Africa. Biol. Conserv. 2009, 142, 446–454. [Google Scholar] [CrossRef]
- Rutherford, M.C.; Powrie, L.W. Severely degraded rangeland: Implications for plant diversity from a case study in Succulent Karoo, South Africa. J. Arid. Environ. 2010, 74, 692–701. [Google Scholar] [CrossRef]
- Martindale, G.J. Influence of Livestock Grazing on Plant Diversity of Highland Sourveld Grassland in KwaZulu-Natal. Master’s Thesis, University of the Witwatersrand, Johannesburg, South Africa, 2007. [Google Scholar]
- Germishuizen, G.; Meyer, N.L. Plants of Southern Africa: An Annotated Checklist; Strelitzia 14; National Botanical Institute: Pretoria, South Africa, 2003. [Google Scholar]
- African Plant Database (version 3.4.0). Conservatoire et Jardin Botaniques (CJB) de la Ville de Genève and South African National Biodiversity Institute, Pretoria. Available online: https://www.ville-ge.ch/musinfo/bd/cjb/africa/recherche.php (accessed on 8 June 2019).
- Colwell, R.K. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. Version 6, User’s Guide and Application. Available online: http://viceroy.eeb.uconn.edu/estimates (accessed on 8 June 2019).
- Department of Agriculture, Forestry and Fisheries (DAFF). Conservation of Agricultural Resources Act; Act No. 43 of 1983 (CARA); Department of Agriculture, Forestry and Fisheries: Pretoria, South Africa, 1983.
- Department of Environmental Affairs (DEA). National Environmental Management: Biodiversity Act; Act No. 10 of 2004 (NEM: BA); Department of Environmental Affairs: Pretoria, South Africa, 2004.
- Newete, S.W.; Allem, S.M.; Venter, N.; Byrne, M.J. Tamarix efficiency in salt excretion and physiological tolerance to salt-induced stress in South Africa. Int. J. Phytoremediat. 2019, 22, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Zavaleta, E. Valuing ecosystem services lost to Tamarix invasion in the United States. In Invasive Species in a Changing World; Island Press: Washington, DC, USA, 2000; pp. 261–300. [Google Scholar]
- Steenkamp, H.; Chown, S. Influence of dense stands of an exotic tree, Prosopis glandulosa Benson, on a savanna dung beetle (Coleoptera: Scarabaeinae) assemblage in southern Africa. Biol. Conserv. 1996, 78, 305–311. [Google Scholar] [CrossRef]
- Van Klinken, R.D.; Graham, J.; Flack, L.K. Population Ecology of Hybrid Mesquite (Prosopis Species) in Western Australia: How Does it Differ from Native Range Invasions and What are the Implications for Impacts and Management? Biol. Invasions 2006, 8, 727–741. [Google Scholar] [CrossRef]
- Laldaparsad, S. A geo-referenced census frame of dwellings for the 2011 census of the Republic of South Africa. In Proceedings of the Innovative Methodologies for Censuses in the New Millennium, a Satellite Meeting of the 56 Session of the ISI, Lisbon, Portugal, 31 August–2 September 2007. [Google Scholar]
- Busch, D.; Smith, S. Effects of fire on water and salinity relations of riparian woody taxa. Oecologia 1993, 94, 186–194. [Google Scholar] [CrossRef]
- Milton, S.J.; Dean, W.R.J. Plant invasions in arid areas: Special problems and solutions: A South African perspective. Biol. Invasions 2010, 12, 3935–3948. [Google Scholar] [CrossRef]
- Erfanifard, Y.; Khosravi, E. Saltcedar (Tamarix mascatensis) inhibits growth and spatial distribution of eshnan (Seidlitzia rosmarinus) by enrichment of soil salinity in a semi-arid desert. Plant Soil 2019, 440, 219–231. [Google Scholar] [CrossRef]
- Clayton, W.D.; Vorontsova, M.S.; Harman, K.T.; Williamson, H. World Grass Species: Descriptions, Identification, and Information Retrieval. Available online: http://www.kew.org/data/grasses-db.html (accessed on 8 November 2019).
- Ripley, B.; Visser, V.; Christin, P.A.; Archibald, S.; Martin, T.; Osborne, C. Fire ecology of C3 and C4 grasses depends on evolutionary history and frequency of burning but not photosynthetic type. Ecology 2015, 96, 2679–2691. [Google Scholar] [CrossRef] [PubMed]
- Le Houérou, H.N.; Corra, M. Some Browse Plants of Ethiopia; Le Houerou, H.N., Ed.; Browse in Africa. ILCA: Addis Ababa, Ethiopia, 1980; pp. 109–114. [Google Scholar]
- Rubin, F.; Palmer, A.R. The physical environment and major plant communities of the Karoo National Park, South Africa. Koedoe 1996, 39, 25–52. [Google Scholar] [CrossRef][Green Version]
- Davison, A.W. The Ecology of Hordeum Murinum L.: III. Some Effects of Adverse Climate. J. Ecol. 1977, 65, 523. [Google Scholar] [CrossRef]
- Sharif, F.; Khan, A.U. Effect of salinity on tissue nutrient contents of the four dryland tree species of Indus flood plains. Arid. Land Res. Manag. 2016, 30, 65–78. [Google Scholar] [CrossRef]
- El-Keblawy, A.; Abdelfattah, M.A.; Khedr, A.-H. Relationships between landforms, soil characteristics and dominant xerophytes in the hyper-arid northern United Arab Emirates. J. Arid. Environ. 2015, 117, 28–36. [Google Scholar] [CrossRef]
- Mulroy, T.W.; Rundel, P.W. Annual Plants: Adaptations to Desert Environments. BioScience 1977, 27, 109–114. [Google Scholar] [CrossRef]
- Dudley, S. The response to differing delection on plant physiological traits: Evidence for local adaptation. Evolution 1996, 50, 103. [Google Scholar] [CrossRef]
- Muscolo, A.; Sidari, M.; Panuccio, M.R.; Santonoceto, C.; Orsini, F.; De Pascale, S. Plant responses in saline and arid environments: An overview. Eur. J. Plant Sci. Biotechnol. 2011, 5, 1–11. [Google Scholar]
- Lombard, A.; Hilton-Taylor, C.; Rebelo, A.; Pressey, R.L.; Cowling, R.M. Reserve selection in the Succulent Karoo, South Africa: Coping with high compositional turnover. Plant Ecol. 1999, 142, 35–55. [Google Scholar] [CrossRef]
- Vlok, J.; Vlok, A. Plants of the Klein Karoo; Umdaus Press: Hatfield, UK, 2015. [Google Scholar]
- Dziba, L.; Scogings, P.; Gordon, I.; Raats, J. Effects of season and breed on browse species intake rates and diet selection by goats in the False Thornveld of the Eastern Cape, South Africa. Small Rumin. Res. 2003, 47, 17–30. [Google Scholar] [CrossRef]
- Bidak, L.M.; Kamal, S.A.; Halmy, M.W.A.; Heneidy, S.Z. Goods and services provided by native plants in desert ecosystems: Examples from the northwestern coastal desert of Egypt. Glob. Ecol. Conserv. 2015, 3, 433–447. [Google Scholar] [CrossRef]
- Klein, A.; Vaissière, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B Biol. Sci. 2006, 274, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Kremen, C.; Williams, N.M.; Thorp, R.W. Crop pollination from native bees at risk from agricultural intensification. Proc. Natl. Acad. Sci. USA 2002, 99, 16812–16816. [Google Scholar] [CrossRef] [PubMed]
- Goel, V.L.; Behl, H.M. Screening of Prosopis germplasm for afforestation of degraded soil sites: Performance, leaf nutrient status and influence on soil properties. J. Sustain. For. 1998, 8, 1–13. [Google Scholar] [CrossRef]
- Yuan, G.-F.; Zhang, P.; Shao, M.-A.; Luo, Y.; Zhu, X. Energy and water exchanges over a riparian Tamarix spp. stand in the lower Tarim River basin under a hyper-arid climate. Agric. For. Meteorol. 2014, 194, 144–154. [Google Scholar] [CrossRef]
- Imada, S.; Matsuo, N.; Acharya, K.; Yamanaka, N. Effects of salinity on fine root distribution and whole plant biomass of Tamarix ramosissima cuttings. J. Arid. Environ. 2015, 114, 84–90. [Google Scholar] [CrossRef]


| Family | Species | Plant Type | Origin | Site Occurred | Status |
|---|---|---|---|---|---|
| Aizoaceae | Galenia africana L. | Shrub | South Africa | S, O, | |
| Aizoaceae | Galenia pubescens L. | Dwarf shrub | South Africa | O | |
| Aizoaceae | Lampranthus uniflorus L. Bolus | Succulent-Shrub | South Africa | S | |
| Aizoaceae | Mesembryanthemum crystallinum L. | Succulent | South Africa | S, O, L | |
| Aizoaceae | Prenia tetragona Thunb. | Succulent | South Africa | ||
| Aizoaceae | Psilocaulon coriarium Burch. ex N.E. Br. | Succulent-Shrub | South Africa | S | |
| Aizoaceae | Tetragonia tetragonioides Pall. | Shrub | Eastern Asia, Australia, and New Zealand | S, L | ** |
| Amaranthaceae | Atriplex muelleri L. | Dwarf shrub | Australia | S, O | * |
| Amaranthaceae | Atriplex semibaccata L. | Dwarf shrub | Australlia | S, O, L | ** |
| Amaranthaceae | Atriplex vestita L. | Shrub | South Africa | O, L | |
| Amaranthaceae | Bassia salsoloides Fenzl | Dwarf shrub | South Africa | O | |
| Amaranthaceae | Salsola barbata Aellen | Shrub | South Africa | S | |
| Anacardiaceae | Searsia pendulina Jacq. | Tree | South Africa | L | |
| Asteraceae | Chrysocoma ciliata L. | Shrub | O | ||
| Asteraceae | Oncosiphon piluliferum L.f. | Herb | South Africa | O | |
| Asteraceae | Senecio burchellii DC. | Shrub/Dwarf shrub | South Africa | O | |
| Brassicaceae | Sisymbrium sp. L. | Herb | Naturalised-introduced in South Africa | O | |
| Brassicaceae | Unidentified | Herb | O | ||
| Chenopodiaceae | Chenopodium murale L. | Herb | Europe and parts of Asia and northern Africa | L | * |
| Ebenaceae | Diospyros lycioides Desf. | Tree/Shrub | South Africa | L | |
| Fabaceae | Medicago sativa L. | Herb | Naturalised-introduced in South Africa | O | |
| Fabaceae | Prosopis hybrid L. | Tree/Shrub | Mexico, Central and northern South America | L | * |
| Fabaceae | Prosopis juliflora L. | Tree/Shrub | Mexico, Central and northern South America | L | ** |
| Fabaceae | Vachellia karroo Hayne | Tree/Shrub | South Africa | S, O, L | |
| Juncaceae | Juncus kraussii Hochst. | Herb | South Africa | O | |
| Papaveraceae | Argemone albiflora Hornem. | Forb/Herb | North America | O | * |
| Papaveraceae | Argemone mexicana L. | Forb/Herb | Mexico | O | ** |
| Poaceae | Cenchrussp. | Graminoid | South Africa | S | |
| Poaceae | Cenchrus ciliaris L. | Graminoid | South Africa | O, L | |
| Poaceae | Cynodon dactylon L. | Graminoid | South Africa | O, L | |
| Poaceae | Enneapogon desvauxii P.Beauv. | Graminoid | South Africa | O | |
| Poaceae | Hordeum murinum L. | Graminoid | South Africa | O | |
| Poaceae | Stipagrostis namaquensis Nees | Graminoid | South Africa | O, L | |
| Santalaceae | Viscum rotundifolium L.f. | Hemiparasite | South Africa | S | |
| Scrophulariaceae | Sutera sp.Roth | Shrub | South Africa | S | |
| Solanaceae | Lycium hirsutum Dunal | Shrub/Dwarf shrub | S, L | ||
| Solanaceae | Lycium oxycarpum Dunal | Shrub/Occasional tree | South Africa | O, L | |
| Solanaceae | Solanum tomentosum L. | Shrub/Dwarf shrub | South Africa | O | |
| Tamaricaceae | Exotic Tamarix (T. ramossissima L. and T. chinensis L.) | Tree/Shrub | Eurasia | S, O, L | ** |
| Tamaricaceae | Tamarix usneoides L. | Tree/Shrub | Eurasia and South Africa | L | |
| Zygophyllaceae | Zygophyllum retrofractum Thunb. | Succulent-Shrub | South Africa | S, L |
| Similarity and Complementarity Index | Leeu River | Swart River |
|---|---|---|
| Jaccard Index | ||
| Olifants River | 21 | 23 |
| Leeu River | 17 | |
| Sorenson’s index | ||
| Olifants River | 34 | 38 |
| Leeu River | 29 |
| Leeu River | Olifants River | Swart River | ||||
|---|---|---|---|---|---|---|
| Similarity and Complementarity Index | Zone 2 | Zone 3 | Zone 2 | Zone 3 | Zone 2 | Zone 3 |
| Jaccard Index | ||||||
| Zone 1 | 36 | 50 | 44 | 41 | 83 | 83 |
| Zone 2 | - | 36 | - | 70 | - | 100 |
| Sorenson’s index | ||||||
| Zone 1 | 53 | 67 | 62 | 52 | 91 | 91 |
| Zone 2 | - | 53 | - | 82 | - | 100 |
| Plant | Economic Resources | Ecological Resources |
|---|---|---|
| Atriplex semibaccata | Gr, Md, Ed | Sf, We |
| Chenopodium murale | Md, Ed, Ar | Sf |
| Cynodon dactylon | Gr, Md, Fu | Sf, Re, Sh |
| Hordeum murinum | Gr, Md | Sf |
| Juncus kraussii | Gr, Han, Ot | Sf, Re, St, Wb, Ot |
| Lycium hirsutum | Gr, Md, Ed, Fu, Fe, Ot | Sf, Re, Sh, Sr, Wb, Rs |
| Lycium oxycarpum | Gr, Md, Ed, Fu, Fe, Ot | Sf, Re, Sh, Sr, Wb, Rs |
| Medicago sativa | Gr | Sr |
| Salsola barbata | Gr, Md, Ed, Fu | Ot |
| Senecio burchellii | Gr, Md, Ed | Sf, We |
| Sisymbrium sp. | Gr | - |
| Solanum tomentosum | Md | Sf |
| Stipagrostis namaquensis | Gr, Md | Sf, Sr |
| Tamarix usneoides | Gr, Md, Fu, Or, Ot | Sf, Re, Sh, Wb, Rs |
| Zygophyllum retrofractum | Md, Ot | Sf, St, Wb, Rs |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Setshedi, K.T.A.; Newete, S.W. The Impact of Exotic Tamarix Species on Riparian Plant Biodiversity. Agriculture 2020, 10, 395. https://doi.org/10.3390/agriculture10090395
Setshedi KTA, Newete SW. The Impact of Exotic Tamarix Species on Riparian Plant Biodiversity. Agriculture. 2020; 10(9):395. https://doi.org/10.3390/agriculture10090395
Chicago/Turabian StyleSetshedi, Kgalalelo Tshimologo Annie, and Solomon Wakshom Newete. 2020. "The Impact of Exotic Tamarix Species on Riparian Plant Biodiversity" Agriculture 10, no. 9: 395. https://doi.org/10.3390/agriculture10090395
APA StyleSetshedi, K. T. A., & Newete, S. W. (2020). The Impact of Exotic Tamarix Species on Riparian Plant Biodiversity. Agriculture, 10(9), 395. https://doi.org/10.3390/agriculture10090395





