Abstract
Soil salinity has emerged as one of the most prominent threats to modern intensive farming systems, and it has necessitated the cultivation of halophytes to ensure food security and human nutrition. Peucedanum japonicum Thunb. is an edible wild plant with medicinal value that is widely distributed along the Pacific coast of western Japan. However, the adaptive mechanisms of this plant with respect to salt stress tolerance have not yet to be elucidated. The purpose of this study was to compare the physiological responses of P. japonicum to salt stress with those of barley (Hordeum vulgare), which is considered a salinity-tolerant plant. Seedlings of both species at the same height were exposed to different concentrations (0, 50, 75, and 150 mM) of NaCl for 16 days, after which the leaves were analyzed with respect to different physiological parameters. The results revealed a maintenance of leaf growth in P. japonicum compared with that in barley, the growth of which was severely impaired at low concentrations of NaCl (50 and 75 mM). In response to salt stress, a higher suppression of Na+ and Cl− assimilations was observed in P. japonicum than in barley under all NaCl treatments. Moreover, P. japonicum showed a greater ability to maintain leaf K+ and Ca2+ concentrations, whereas barley exhibited a significant reduction in the concentrations of these ions under saline conditions. Thus, the superior salinity tolerance of P. japonicum could be attributed to a more efficient maintenance of ionic balances. Taken together, our results indicate that P. japonicum may be classified as a halophyte, given its superior regulation of K+, Ca2+, SO4−, and sucrose concentrations and lower NO3− concentrations compared with those of barley.
1. Introduction
Soil salinity has serious detrimental effects on sustainable agricultural production systems worldwide. In most arable areas affected by high salinity, the saline conditions can primarily be attributed to either the effects of seawater or irrigation with water containing excessive amounts of sodium chloride (NaCl) [1]. Once plants take up large quantities of NaCl, the resultant high cytosolic Na+ and Cl− concentrations lead to ion (Na+ and Cl−) toxicity, which can in turn cause a reduction in cytoplasmic K+ concentrations and deficiencies in Ca2+, Mg2+, and NO3− concentrations, or those of other minerals, in plant tissues [2,3]. To limit the detrimental effects of cytoplasmic Na+ or Cl− toxicity, plants either compartmentalize Na+ and Cl− within vacuoles or inhibit the entry of Na+ and Cl− into cells [1]. However, such mechanisms have been found to interfere with the accumulation of K+, Ca2+, Mg2+, or NO3− in the tissues of barley (Hordeum vulgare L.) [4], maize (Zea mays L.) [5], and Eutrema parvulum [6]. Consequently, the inhibition of Na+ uptake and maintenance of cytoplasmic K+, Ca2+, and Mg2+ concentrations for diverse metabolic activities represent key factors in enhancing the salinity tolerance in plants [3,7], and in this regard, Na+ toxicity may be alleviated by regulating the uptake of K+, Ca2+, and Mg2+ into the xylem stream [2]. Notably, a high Na+ uptake is particularly disruptive to cytosolic Na+ and K+ homeostasis, which is on account of the similarity between the hydrated ionic radii of Na+ (1.65–2.05 Å) and K+ (2.35–2.66 Å), resulting in lower K+ concentrations in plant tissues. Therefore, the maintenance of stable K+ acquisition and subsequent distribution are essential for plant cells to balance the toxic effects of Na+ accumulation and to maintain cytosolic ion homeostasis under conditions of salt stress [1]. Similarly, in salt-stress plants, the concentrations of NO3− may be reduced by the presence of Cl− in the rhizosphere [8]. Therefore, stable NO3− acquisition and distribution are also essential for plant cells to balance the toxic effects of accumulated Cl−.
Moreover, soil salinity can promote immediate hyperosmotic stress, which disrupts vital physiological mechanisms in crop plants [2], and in turn detrimentally affects plant growth. To cope with the injurious effects of hyperosmotic stress, plants often accumulate organic osmolytes, such as glycine betaine, proline, and sugars, and inorganic osmolytes, such as K+, Ca2+, Mg2+, and NO3−, to maintain low intercellular osmotic potentials [1]. However, the accumulation of osmolytes is typically a species-specific trait in crop plants. In several species of Chenopodiaceae, Na+ and Cl− are the major osmolytes controlling the cellular osmotic potential under conditions of soil salinity [7], the compartmentalization of which within vacuoles maintains the concentrations of these ions in the cytoplasm within levels conducive to resistance [9]. Additionally, high levels of organic osmolytes, such as sucrose, sorbitol, and mannitol, can enhance the scavenging of reactive oxygen species to cope with oxidative stress [3].
Peucedanum japonicum Thunb. is a wild perennial plant that is widely distributed in coastal areas of Japan, the Philippines, Korea, and the south of China [10]. Of particular note is the ability of P. japonicum to grow vigorously, even in oligotrophic soils, such as those found along coral reef terraces where salt spray from the ocean is common (Figure 1). P. japonicum leaves are used as leafy vegetables, green juices, and garnishes, and as the main ingredient in the Chomeiso noodles of the Ryukyu Islands in Japan. Recently, P. japonicum has been attracting increasing attention with respect to its potential health benefits, notably its anti-obesity [11], antinociceptive [12], and anti-allergic (i.e., lung inflammation) properties [13]. However, at present, there is still relatively limited information available with regard to the responses of P. japonicum to abiotic stress, particularly salt stress. Barley is considered a salt-tolerant cereal [4], and its young leaves are extensively used as green juices, owing to their high contents of valuable nutritional compounds [14]. Despite the fact that barley growth is adversely affected to a certain extent by salinity, owing to Na+ interference in K+ and Ca2+ assimilation [4], H. vulgare is characterized by notable physiological traits that are useful for examining the responses of other species to salinity.

Figure 1.
Peucedanum japonicum growing on a coral reef terrace on Tokunoshima Island, Central Ryukyus, Southwestern Japan.
Thus, in light of our current limited knowledge regarding the response of P. japonicum to salt stress, we conducted a comparative study of P. japonicum and barley growth in response to different levels of salinity. In particular, we sought to elucidate the mechanisms whereby P. japonicum adapts to soil salinity by (1) comparing leaf growth and physiological parameters, such as relative water content, osmotic potential, photosynthetic pigments, the concentrations of Na+, Cl−, and other ions (K+, Ca2+, Mg2+, SO4−, NO3−), total N concentration, and sucrose concentration in the leaves of P. japonicum and barley exposed to different concentrations NaCl; (2) identifying the possible physiological mechanisms underlying the observed differential responses of P. japonicum and barley to salinity; and (3) determining the characteristic of P. japonicum that contribute to its high degree of salinity tolerance.
2. Materials and Methods
2.1. Plant Materials and Experimental Setup
Seeds of P. japonicum (collected from Tokunoshima Island, Central Ryukyus, Southwestern Japan) and barley (obtained from the Snow Brand Seed Corporation Ltd., Chiba, Japan) were sown in a seedbed. Twenty-nine-week-old P. japonicum seedlings of uniform size were transferred to 1.7-L pots filled with soil and perlite (1:1, v/v, 1 kg in total), with each pot containing two seedlings. The pots were installed in the greenhouse of the Faculty of Applied Biological Sciences of Hiroshima University, Japan. Conditions in the greenhouse were maintained at a relative humidity of 60%, day/night temperatures of 17–26 °C/12–19 °C, and illumination was provided by natural sunlight. At day 8 following germination, three barley seedlings were maintained in individual pots for employing treatments. All pots were irrigated daily with a nutrient solution containing 4.2 mM NO3–N, 0.3 mM NH4–N, 0.2 mM P2O5, 1.1 mM K2O, 0.4 mM MgO, 1.0 mM CaO, 5.3 μM MnO, 5.4 μM B2O3, 12.1 μM Fe, 0.1 μM Cu, 0.3 μM Zn, and 0.08 μM Mo [15]. Compared with barley, the rate of growth of P. japonicum is considerably slower; therefore, we decided to use the seedling stage at which the plants reached comparable heights to compare the salinity tolerance capacities of the two species. Accordingly, 30-day-old barley seedlings and 31-week-old P. japonicum seedlings were exposed to different concentrations (0, 50, 75, or 150 mM) of NaCl, which was added to the nutrient medium to mimic salinity stress. Seedlings supplied with the unsupplemented nutrient medium were designated the control seedlings (0 mM NaCl), whereas those grown with the same medium supplemented with the different concentrations of NaCl (50, 75, or 150 mM) were designated the treated seedlings. All pots containing control or treated seedlings were irrigated twice daily. After 16 days of treatment, leaves were harvested and then oven-dried at 70 for 3 days to determine leaf dry weight. The leaf relative dry growth was calculated using the following formula:
leaf relative dry growth = average dry weight of treated plants/average dry weight of control plants.
The young leaves were flash-frozen in liquid nitrogen and stored at −80 °C until used for further analysis.
2.2. Relative Water Content and Osmotic Potential
Relative water content (RWC) was measured using fresh leaves, as described by Turner [16]. The RWC was calculated using the equation RWC = 100 × (fresh weight − dry weight)/(turgor weight − dry weight). The osmotic potentials (Ψπ) of the cell sap extracted from the young leaves of both species were determined using a Wescor 5500 vapor pressure osmometer (Wescor Inc., Logan, UT, USA).
2.3. Sucrose Analysis
Sucrose was extracted from 20 mg of freeze-dried leaf samples by immersing in methanol, which was maintained at 4 °C for 24 h and thereafter separated using chloroform and water. The air-dried supernatant was extracted with 10 mL of acetonitrile:methanol (20:1, v/v) to remove proteins, and the sucrose concentration was determined by HPLC (Gulliver system, Jasco Co., Tokyo, Japan) using an Asahipak NH2P-50 4E column (Shodex, Tokyo, Japan).
2.4. Chlorophyll and Carotenoid Analysis
Chlorophyll (Chl) a, Chl b, and carotenoids were extracted from fresh leaves using 80% acetone. Briefly, 0.5 g of fresh leaf samples were cut into 1-cm2 sections, which were placed in glass vials containing 5 mL of 80% acetone. After being maintained in the dark at room temperature for 1 day, the extract was centrifuged at 10,000× g for 5 min, and the absorbance of the supernatant was measured at 645, 663, and 451 nm [17].
2.5. Mineral Analysis
For cation analyses, 50 mg of the freeze-dried leaf samples were digested with HNO3–H2O2 for estimations of Na+ and K+ concentrations using an ANA-135 flame photometer (Eiko Instruments Inc., Tokyo, Japan) and the concentrations of Ca2+ and Mg2+ using an AA-6200 atomic absorption flame emission spectrophotometer (Shimazu Corporation Industrial Company, Kyoto, Japan). For anion analyses, Cl−, NO3−, and SO42− were extracted from 20 mg of freeze-dried leaf samples in 2 mL of distilled water contained in microtubes with tight-fitting lids, which were subjected to heating at 100 °C for 1 h. After centrifugation, the anion concentrations in the extract were determined using an ICS-900 ion chromatograph (Nippon Dionex K.K., Osaka, Japan). For nitrogen analysis, freeze-dried leaf samples were digested with H2SO4–H2O2 using a Kjeldahl nitrogen digester, after which the total nitrogen concentration was determined using Kjeldatherm Type TT100 and Vapodset Type 20 distillators (Gerhardt, Germany).
2.6. Statistical Analysis
All data collected (mean of four replicates) from barley and P. japonicum were subjected to a one-way analysis of variance (ANOVA) using the IBM SPSS statistical package v. 25 (Chicago, IL, USA). Significance was determined using the Duncan multiple range test at p ≤ 0.05. The relationships between ions and the dry weight the barley and P. japonicum leaves were analyzed by linear association using Excel.
3. Results
3.1. Plant Growth
Compared with those of control plants, we detected reductions of 27%, 30%, and 31% in the dry weights of barley plants treated with 50, 75, and 150 mM NaCl, respectively (Figure 2A). For P. japonicum exposed to 50 and 75 mM NaCl, a maintenance of dry weight was observed compared with that of the control plants. Therefore, P. japonicum was capable of maintaining leaf biomass production in the presence of NaCl at concentrations up to 75 mM, whereas in the presence of 150 mM NaCl, P. japonicum leaf biomass production was found to be less than that of the control plants (Figure 2B).
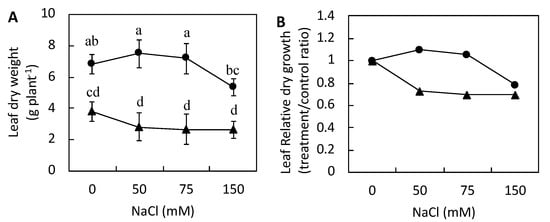
Figure 2.
The effects of increasing NaCl stress on the dry weights of barley (▲) and Peucedanum japonicum (●). (A) Dry leaf weight and (B) dry leaf relative growth at each NaCl concentration compared with that of the control (0 mM NaCl). The values represent the means (±SE) of four replicates. Different letters in the figures indicate significant differences between the treatments of both plant species at P ≤ 0.05, as determined using a one-way ANOVA with the Duncan test (5% significance level).
3.2. Leaf RWC and Ψπ
We observed reductions in RWC values in barley subjected to 50, 75, and 150 mM NaCl, and in P. japonicum subjected to 75 and 150 mM NaCl (Figure 3A), whereas Ψπ values decreased in both barley and P. japonicum exposed to 50, 75, and 150 mM NaCl (Figure 3B). Moreover, for each NaCl concentration, the RWC and Ψπ values obtained for barley were higher than those for P. japonicum.
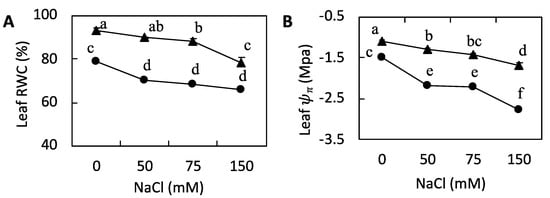
Figure 3.
The effect of increasing salinity on the water balance in barley (▲) and Peucedanum japonicum (●) leaves. (A) Relative water content (RWC) and (B) osmotic potential. The values represent the means (±SE) of four replicates. Different letters in the figures indicate significant differences between the treatments of both plant species at P ≤ 0.05, as determined using a one-way ANOVA with the Duncan test (5% significance level).
3.3. Leaf Chlorophyll and Carotenoid Concentrations
The concentrations of total carotenoids, Chl a, Chl b, and total Ch in barley and P. japonicum showed similar trends and did not vary in response to an increase in NaCl concentration (Table 1).

Table 1.
The effect of increasing salinity on carotenoid, chlorophyll a, and chlorophyll b concentrations (mg g−1 fresh weight) in barley and Peucedanum japonicum (PJT).
3.4. Cation Concentrations
We observed that the Na+ content in barley increased progressively in response to increasing NaCl concentration from 50 to 150 mM NaCl, with plants exposed to 150 mM showing the highest increase (Table 2). Although we detected similar increases in the Na+ content of P. japonicum in response to increasing NaCl concentration, the differences among treatment were not significant. The Na+ content in P. japonicum was found to be significantly lower than that in barley in response to the different treatments. The contents of other cations (K+, Ca2+, and Mg2+) in barley progressively decreased with increasing NaCl concentration, as did those in P. japonicum, although we detected no significant differences among the treatments. The K+ concentration in P. japonicum was higher than that in barley at NaCl concentrations from 50 to 150 mM. Furthermore, when exposed to 50, 75, and 150 mM NaCl, the K+/Na+ and Ca2+/Na+ ratios in both barley and P. japonicum were lower than those in the control plants, and at each NaCl concentration, the K+/Na+ and Ca2+/Na+ ratios in P. japonicum were higher than those in barley.

Table 2.
Cation concentrations and K+/Na+ and Ca2+/Na+ ratios in barley and Peucedanum japonicum (PJT) leaves at different NaCl concentrations.
3.5. Anion Concentrations
Cl− concentrations in barley increased steadily in response to increasing NaCl concentration (Table 3). Compared with the control plants, Cl− concentrations in P. japonicum were higher at 50, 75, and 150 mM NaCl, although were slightly lower than those in barley at 75 and 150 mM NaCl. NO3− concentrations in barley steadily decreased with an increase in NaCl concentration, and a similar pattern was observed in P. japonicum, although there were no significant differences among plants exposed to the different NaCl concentrations from 50 to 150 mM. SO42− concentrations in barley at 50, 75, and 150 mM NaCl showed no significant differences from those recorded in control plants, whereas in contrast, SO42− concentrations in P. japonicum were lower compared with those of the control plants. Furthermore, the NO3−/Cl− ratio in barley decreased progressively in response to an increase in NaCl concentration, whereas in P. japonicum, the NO3−/Cl− ratio was lower at 50, 75, and 150 mM NaCl compared with that in the control plants, and it showed no significant difference at NaCl concentrations from 50 to 150 mM.

Table 3.
Anion concentrations and NO3−/Cl− ratios in barley and Peucedanum japonicum (PJT) leaves at different NaCl concentrations.
3.6. Nitrogen and Sucrose Concentrations
We found that the N concentrations in barley treated with 50, 75, and 150 mM NaCl did not differ significantly from those in the control plants (Table 4). In contrast, compared with that of the control plants, we detected lower N concentrations in P. japonicum plants exposed to 50 and 150 mM NaCl. Although the sucrose concentration in barley did not vary between plants exposed to 50 and 75 mM NaCl, it was found to be slightly higher at 150 mM NaCl compared with that in the control plants. In P. japonicum, sucrose concentrations remained essentially unchanged among plants exposed to the different concentrations of NaCl.

Table 4.
Nitrogen (N) and sucrose concentrations in barley and Peucedanum japonicum (PJT) leaves at different NaCl concentrations.
3.7. Relationships between the Ion Concentrations and Dry Weights in Barley and P. Japonicum Leaves
Our analyses of the relationships between the ion contents and dry wrights of leaves revealed a negative linear relationship between the concentrations of both Na+ (R2 = 0.58) and Cl− (R2 = 0.58) and dry leaf weight in barley (Figure 4A,D). In contrast, we detected positive relationships between the concentrations of K+ and Ca2+ and dry leaf weight in barley (Figure 4B,C). In addition, positive relationships were detected between the ratios K+/Na+, Ca2+/Na+, and NO3−/Cl− and dry leaf weight in barley, with corresponding R2 values of 0.61, 0.63, and 0.53 (Figure 5A–C). In contrast, we detected no strong relationships between the Na+, K+, Ca2+, Cl−, or NO3− concentrations and dry leaf weight in P. japonicum, nor between the K+/Na+, Ca2+/Na+, or NO3−/Cl− ratios and dry leaf weight (Figure 4F–J and Figure 5D–F).
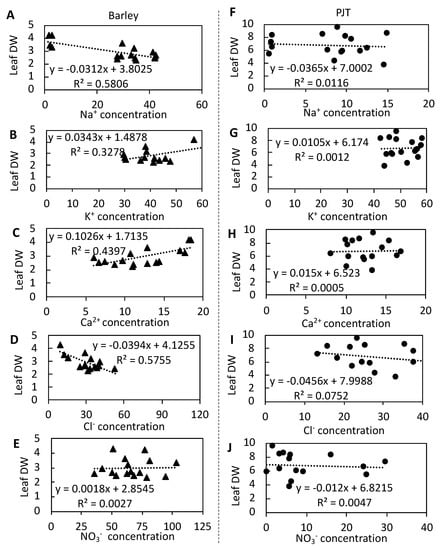
Figure 4.
Correlations between ion concentrations (Na+, K+, Ca2+, Cl−, and NO3−) in barley (▲) leaves (A–E) and dry leaf weights and in Peucedanum japonicum (●) leaves (F–J) and dry leaf weights at different NaCl concentrations.
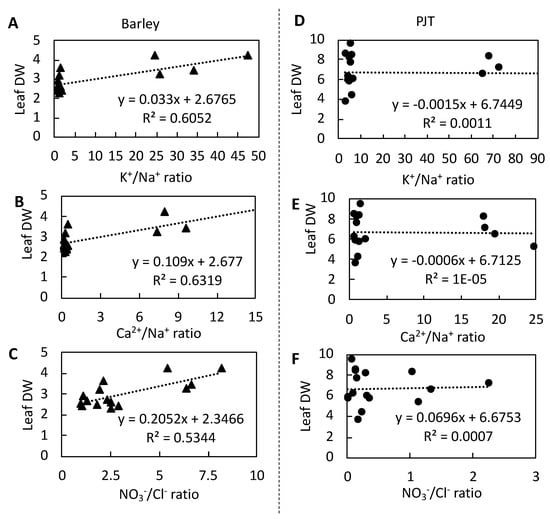
Figure 5.
Correlations between ionic balances (K+/Na+, Ca2+/Na+, and NO3−/Cl−) in barley leaves (▲) and dry leaf weights (A–C) and Peucedanum japonicum leaves (●; PJT) and dry leaf weights (D–F) at different NaCl concentrations.
4. Discussion
4.1. Leaf Growth and Leaf Water Uptake
Despite the fact that barley is considered a salt-tolerant cereal [4], its growth is typically affected by salinity, as a consequence of Na+ interference in K+ and Ca2+ assimilation [15]. P. japonicum is widely distributed in coastal areas characterized by high NaCl concentration [10]; thus, this plant is assumed to show a certain degree of tolerance to salinity stress, even at the young stage. In this study, we compared the physiological responses of barley and P. japonicum to salinity among plants at the same stage of growth, given the markedly slower growth rate of P. japonicum. The results revealed that barley and P. japonicum show varying extents of differential response to increasing salinity. We found that a progressive increase in soil salinity resulted in reduced barley growth, whereas that of P. japonicum was little affected at low soil salinity (Figure 2), and maximum dry leaf weights were obtained at 50 and 75 mM NaCl. Thus, the effects of increasing salinity appear to be more detrimental to the growth of barley than to that of P. japonicum, and the notable reduction in barley leaf growth can be taken as indicative of the greater sensitivity of barley to soil salinity compared with P. japonicum plants.
Under conditions of salt stress, increases in salt concentrations in the rhizosphere lead to reductions in soil water potential, which can interfere with the ability of plants to take up water from the soil, and this in turn reduces the water uptake ability of leaves [18]. In the present study, the observed reductions in leaf RWC and Ψπ values in both barley and P. japonicum reveal that both species are susceptible to aberrant water accumulation under conditions of salt stress (Figure 3). However, the leaf RWC and Ψπ values for P. japonicum were significantly lower than those for barley under different NaCl concentrations, whereas P. japonicum was able to maintain leaf growth at NaCl concentrations up to 75 mM NaCl. Therefore, P. japonicum may have developed a water supplementation mechanism for different molecular reactions, including the maintenance of sucrose production under conditions in which cell water status is low, which may protect plants from osmotic damage in response to water deficit.
4.2. P. Japonicum Maintains Lower Na+ and Cl− Concentrations in Leaves at High NaCl Concentrations
Normally, high Na+ concentrations are toxic or even lethal to plants. However, salt-tolerant plants have evolved mechanisms that enable them to tolerate elevated concentrations of Na+ based on inhibition of the uptake of this ion by plant organs [7]. In the present study, we found that in barley, a progressive increase in leaf concentrations Na+ with increasing salinity resulted in significantly reduced leaf growth (Table 2). Similarly, previous studies have reported that high Na+ concentrations in the root rhizosphere can reduce the growth of barley [4]. However, compared with barley, we found that P. japonicum had markedly lower leaf Na+ concentrations in plants exposed to different levels of salinity, thereby implying the presence of Na+ exclusion mechanisms. The negative linear correlation between leaf Na+ concentration and dry leaf weight (R2 = 0.64) in barley is also clearly indicative of the severe detrimental effect of high Na+ concentrations on dry matter production (Figure 4A). In contrast, the lack of any significant relationship between leaf Na+ concentrations and dry leaf weight in P. japonicum (R2 = 0.014) (Figure 4F) tends to indicate that low concentrations of Na+ do not pose a substantial threat with respect to the growth of P. japonicum. Indeed, a certain level of Na+ may be required for growth in P. japonicum, given that we observed a slight increase in the growth of plants treated with 50 mM NaCl (Figure 2A).
In plant tissues, high Cl− concentrations would prove damaging or even lethal in non-halophytes. In the present study, increased Cl− concentrations probably resulted in Cl− toxicity in barley (Table 3) and detrimentally affected dry leaf biomass production (Figure 2A) under conditions of salt stress. These observations are consistent with the findings of a previous study, which indicated the negative effects of high Cl− assimilation on the barley growth under high salinity [15]. The occurrence of Cl− toxicity in barley was strongly supported by the negative linear relationship observed between the Cl− concentration and dry leaf weight in barley (R2 = 0.59) (Figure 4D). However, compared with barley, we detected lower Cl− concentrations in the leaves P. japonicum at 75 and 150 mM NaCl (Table 3), thereby indicating that either more or stronger Cl− exclusion or inhibition mechanisms are present in P. japonicum under conditions of salt stress. P. japonicum may have a notable capacity to either exclude Cl− from its leaves or control Cl− transport into the leaves, as evidenced by the absence of a relationship between leaf Cl− concentrations and dry leaf weight (Figure 4I). Therefore, it is likely that Cl−, within a certain range at least, does not reduce leaf growth in P. japonicum.
4.3. P. Japonicum Maintains a Higher Ionic Balance in Leaves than Barley
Excessive amounts of Na+ and Cl− in the rhizosphere result in competition among other ions, such as K+, Ca2+, Mg2+, and NO3−, which can lead to ionic imbalances and nutrient deficiencies in plant tissues [4,6,19]. This was clearly apparent in the salt-stressed barley examined in the present study, wherein the leaf concentrations of K+, Ca2+, Mg2+, and NO3− underwent gradual reductions concomitant with increases in the concentrations of Na+ and Cl− in tissues exposed to NaCl. Similarly, a reduction in K+, Ca2+, and Mg2+ uptake attributable to high concentrations of Na+ has previously been observed in barley [4]. In contrast to the significant reductions in K+, Ca2+, and Mg2+ contents in barley leaves, the concentrations of these cations in P. japonicum leaves were found to be similar among all salinities to which the plants were exposed (Table 2). Notably, compared with barley, we detected markedly higher K+ concentrations in P. japonicum at all salinities examined, thereby implying the presence of superior K+ maintenance mechanisms in P. japonicum under conditions of salt stress. In this regard, the findings of a previous study have indicated that K+ retention in leaves is essential with respect to conferring salinity tolerance in plants [20]. Thus, the heightened salt tolerance of P. japonicum compared with barley may be associated with superior K+ maintenance mechanisms
In plants, the presence of Cl− in the rhizosphere is normally associated with a reduction in NO3− concentrations, thereby resulting in an anionic imbalance [8]. Consequently, the positive roles played by NO3− in maintaining the salinity tolerance of plants would be compromised [21]. In the present study, we observed reductions in leaf NO3− concentrations in plants exposed to NaCl concentration from 50 to 150 mM, which resulted in a reduction in the NO3−/Cl− ratio (Table 3) in barley. In contrast, we observed that in P. japonicum, there was no continual reduction in NO3− concentrations concomitant with a progressive increase in Cl− concentrations in plants exposed to salinities ranging from 50 to 150 mM NaCl. We accordingly assume that maintenance of the NO3− concentrations in plants exposed to 50 to 150 mM NaCl may have sustained the contribution of NO3− to osmotic regulation and thereby prevented Cl− toxicity in P. japonicum. Moreover, compared with barley, the significantly lower NO3− concentration in the leaves of P. japonicum could be considered indicative of the lower requirement of NO3− for the growth of P. japonicum plants. This low growth requirement for NO3− is conceivably one of the characteristics that enable P. japonicum to grow well in coastal areas.
In barley, the significant increase in Na+ and Cl− assimilation resulted in a reduction in K+, Ca2+, and NO3− assimilation, and it severely disrupted the ionic balance thereafter (Table 2 and Table 3). These observations are consistent with the findings of previous studies [3,8], which have indicated that elevated Na+ and Cl− concentrations significantly reduce the concentrations of K+, Ca2+, and NO3−. Consequently, in barley plants grown under saline conditions, a disruption of ionic balance promoted notable reductions in the Ca2+/Na+, K+/Na+, and NO3−/Cl− ratios of these plants (Table 2 and Table 3) compared with those of the control plants. However, previous studies have also reported that halophytes are able to reduce the concentrations of Na+ and Cl− in the xylem, thereby preventing the accumulation of Na+ and Cl− in leaves [3] and maintaining an ionic balance in metabolic tissues. In the present study, we found that the inhibition of Na+ assimilation and maintenance of the assimilations of K+ and Ca2+ in P. japonicum effectively prevented an ionic imbalance in leaves, and consequently, we observed higher K+/Na+ and Ca2+/Na+ ratios than in barley (Table 2). Thus, the significantly higher K+/Na+ and Ca2+/Na+ ratios (Table 2) in P. japonicum indicate a more efficient cation homeostasis in this plant compared with that in barley.
Moreover, reductions K+, Ca2+, and NO3− concentrations are of key importance with respect to salt sensitivity [3,8], which is manifested in reduced plant growth. In the present study, we observed positive linear relationships between the concentrations of K+ and Ca2+ (Figure 4B,C) and ratios of K+/Na+, Ca2+/Na+, and NO3−/Cl− (Figure 5A–C) and dry leaf weights in barley leaf tissues. These correlations clearly indicate the injurious effect of lower K+ and Ca2+ concentrations on leaf dry matter production in barley. In contrast, we found that the corresponding correlation coefficients for these relationships in P. japonicum were not significant (Figure 4G–J and Figure 5D–F). The lack of any prominent relationships between the concentration of Ca2+ or K+ and dry weight highlight the limited or negligible effects of these ions on leaf growth in P. japonicum. In this regard, the findings of a previous study have indicated that dicotyledonous halophytes grow optimally at NaCl concentrations in the range between 50 and 250 mM, whereas monocotyledonous halophytes generally show optimal growth at concentrations of less than 50 mM NaCl [9], thereby indicating that dicotyledonous halophytes tend to be more salinity tolerant than monocotyledonous halophytes. Consistently, in the present study, we observed that P. japonicum was able to maintain leaf dry weights when exposed to salinities of up to 75 mM NaCl, whereas in contrast, a significant reduction of leaf dry weight was observed in barley, even in plants treated with 50 mM NaCl, thereby indicating that P. japonicum is more salinity tolerant than barley and that this tolerance may be attributable to slight reductions in the concentrations of K+ and Ca2+ in the leaves of P. japonicum.
4.4. Sucrose and Total Nitrogen Concentrations in Leaves
Sucrose is a major translocatable product of photosynthesis, and it acts as an organic solute and non-enzymatic antioxidant that contributes to countering the detrimental effects of environmental oxidative stress [22,23]. Compared with barley, the higher sucrose concentrations detected in P. japonicum plants exposed all the experimental salinities can be taken as indicative of a higher photosynthetic production from the source tissues.
Nitrogen (N) is an essential element for plant growth and development, and in the present study, we observed reductions in the total N concentration in P. japonicum plants subjected to 50, 75, and 150 mM NaCl (Table 4), although there were increases in dry weight of leaves in plants exposed to 50 and 75 mM NaCl, compared with those in the control plants (Figure 2). Moreover, we found that the N contents of P. japonicum to be lower than those in barley. These observations accordingly tend to indicate that P. japonicum is a plant characterized by an effective N use efficiency for its growth, and that this could be a factor that enables P. japonicum to grow well under poor nutritional conditions, such as those typifying coastal areas. Moreover, high NO3− concentration in vegetables may cause methemoglobinemia, which is potentially harmful to human health [24]. Accordingly, the maintenance of low NO3− concentrations in the leaves of plants exposed to 50 to 150 mM NaCl could be one of the reasons why P. japonicum is considered a good source of plant nutrition for humans.
5. Conclusions
The results of this study provide clear evidence for the differential tolerance of P. japonicum and barley to salt stress, with P. japonicum being more tolerant to salinity than barley. This difference appears to be primarily attributable to differences in Na+ and Cl− assimilation in the leaves of these plants, with P. japonicum being characterized by significantly lower Na+ and Cl− concentrations compared with those in barley for the soil salinities evaluated. We suspect that these differences may contribute to a more efficient ionic balance in P. japonicum. Furthermore, compared with barley, we also observed higher K+ and Ca2+ concentrations and higher K+/Na+ and Ca2+/Na+ ratios in P. japonicum. Collectively, these differences can be assumed to counter the detrimental effect of increasing soil salinity on the growth of P. japonicum compared with that of barley, and accordingly, P. japonicum may be classified as a halophyte. Moreover, the high capacity to maintain nutrient (K+ and Ca2+) assimilation, by reducing Na+ and Cl− assimilation, may be one of the reasons why P. japonicum is considered a good source of plant nutrition for humans.
Author Contributions
Conceptualization and methodology, L.L., Y.N., H.S.; formal analysis and data curation, Y.N.; writing, L.L.; editing, L.L., H.S.; review, L.L., N.A.T., A.E.S., R.E.M., H.S.; project administration, H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroederet, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Munns, R.; Tester, M. Mechanisms of Salinity Tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [PubMed]
- Flowers, T.J.; Colmer, T.D. Plant salt tolerance: Adaptation in halophytes. Ann. Bot. 2015, 115, 327–331. [Google Scholar] [CrossRef] [PubMed]
- Tavakkoli, E.; Fatehi, F.; Coventry, S.; Rengasamy, P.; McDonald, G.K. Additive effects of Na+ and Cl− ions on barley growth under salinity stress. J. Exp. Bot. 2011, 62, 2189–2203. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Spivak, M.; Bernstein, N. Involvement of calcium-mediated effects on ROS metabolism in the regulation of growth improvement under salinity. Free Radical. Biol. Med. 2011, 51, 1221–1234. [Google Scholar] [CrossRef]
- Uzilday, B.; Ozgur, R.; Sekmen, A.H.; Yildiztugay, E.; Turkan, I. Changes in the alternative electron sinks and antioxidant defense in chloroplasts of the extreme halophyte Eutrema parvulum (Thellungiella parvula) under salinity. Ann. Bot. 2015, 115, 449–463. [Google Scholar] [CrossRef]
- Flowers, T.J.; Munns, R.; Colmer, T.D. Sodium chloride toxicity and the cellular basis of salt tolerance in halophytes. Ann. Bot. 2015, 115, 419–431. [Google Scholar] [CrossRef]
- Teakle, N.L.; Tyerman, S.D. Mechanisms of Cl− transport contributing to salt tolerance. Plant Cell Environ. 2010, 33, 566–589. [Google Scholar] [CrossRef]
- Flowers, T.J.; Colmer, T.D. Salinity tolerance in halophytes. New Phytol. 2008, 179, 945–963. [Google Scholar] [CrossRef]
- Chen, C.C.; Agrawal, D.C.; Lee, M.R.; Lee, R.J.; Kuo, C.L.; Wu, C.R.; Tsay, H.S.; Chang, H.C. Influence of LED light spectra on in vitro somatic embryogenesis and LC-MS analysis of chlorogenic acid and rutin in Peucedanum japonicum Thunb.: A medicinal herb. Bot. Stud. 2016, 57, 9. [Google Scholar] [CrossRef]
- Taira, N.; Nugara, R.N.; Inafuku, M.; Takara, K.; Ogi, T.; Ichiba, T.; Iwasaki, H.; Okabe, T.; Oku, H. In vivo and in vitro anti-obesity activities of dihydropyranocoumarins derivatives from Peucedanum japonicum Thunb. J. Funct. Foods 2017, 29, 19–28. [Google Scholar] [CrossRef]
- Kim, J.M.; Erkhembaatar, M.; Lee, G.S.; Lee, J.H.; Noh, E.M.; Lee, M.; Song, H.K.; Lee, C.H.; Kwon, K.B.; Kim, M.S. Peucedanum japonicum thunb. ethanol extract suppresses RANKL-mediated osteoclastogenesis. Exp. Ther. Med. 2017, 14, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.M.; Lee, A.R.; Kim, H.S.; Lee, A.Y.; Gu, G.J.; Moon, B.C.; Kwon, B.I. Peucedanum japonicum extract attenuates allergic airway inflammation by inhibiting Th2 cell activation and production of pro-inflammatory mediators. J. Ethnopharmacol. 2018, 211, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Blicharz-Kania, A.; Andrejko, D.; Kluza, F.; Rydzak, L.; Kobus, Z. Assessment of the potential use of young barley shoots and leaves for the production of green juices. Sustainability 2019, 11, 3960. [Google Scholar] [CrossRef]
- Liu, L.; Ueda, A.; Saneoka, H. Physiological responses of white Swiss chard (Beta vulgaris L. subsp. cicla) to saline and alkaline stresses. Aust. J. Crop Sci. 2013, 7, 1046–1052. [Google Scholar]
- Turner, N.C. Technique and experimental approaches for the measurement of plant water status. Plant Soil 1981, 58, 339–366. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef]
- Yamada, M.; Kuroda, C.; Fujiyama, H. Function of sodium and potassium in growth of sodium-loving Amaranthaceae species. Soil Sci. Plant Nutr. 2016, 62, 20–26. [Google Scholar] [CrossRef]
- Wu, H.; Shabala, L.; Barry, K.; Zhou, M.; Shabala, S. Ability of leaf mesophyll to retain potassium correlated with salinity tolerance in wheat and barley. Physiol. Plant. 2013, 149, 515–527. [Google Scholar] [CrossRef]
- Ding, X.; Tian, C.; Zhang, S.; Song, J.; Zhang, F.; Mi, G.; Feng, G. Effects of NO3−-N on the growth and salinity tolerance of Tamarix laxa Willd. Plant Soil 2010, 331, 57–67. [Google Scholar] [CrossRef]
- Ashraf, M. Biotechnological approach of improving plant salt tolerance using antioxidants as marker. Biotechnol. Adv. 2009, 27, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.S.; Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010, 48, 909–930. [Google Scholar] [CrossRef]
- Hmelak Gorenjak, A.; Cencič, A. Nitrate in vegetables and their impact on human health, a review. Acta Aliment. 2013, 42, 158–172. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).