Influence of Species Composition and Management on Biomass Production in Missouri
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Rainfall and Temperature
3.2. Biomass Yield and Weather
3.3. Mixture Composition and Yields
3.4. Cutting Height and Biomass Yield
3.5. Management Implications for Biomass Production
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- U.S. DOE (Department of Energy) 2009. Available online: http://www1.eere.energy.gov/biomass/biomass (accessed on 31 March 2017).
- Karp, A.; Shield, I. Bioenergy from plants and the sustainable yield challenge. New Phytol. 2008, 179, 15–32. [Google Scholar] [CrossRef]
- Zegada-Lizarazu, W.; Elbersen, H.W.; Cosentino, S.L.; Zatta, A.; Alexopoulou, E.; Monti, A. Agronomic aspects of future energy crops in Europe. Biofuels Bioprod. Bioref. 2010, 4, 674–691. [Google Scholar] [CrossRef]
- Soper, J.M.; Raynor, E.J.; Wienhold, C.; Schacht, W.H. Evaluating composition and conservation value of roadside plant communities in a grassland biome. Environ. Manag. 2019, 63, 789–803. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Hill, J.; Lehman, C. Carbon-negative biofuels from low-input high-diversity grassland biomass. Science 2006, 314, 1598–1600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilman, D.; Socolow, R.; Foley, J.A.; Hill, J.; Larson, E.; Lynd, L.; Pacala, S.; Reilly, J.; Searchinger, T.; Somerville, C.; et al. Beneficial biofuels–The food, energy, environment trilemma. Science 2009, 325, 270–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelfand, I.; Sahajpal, R.; Zhang, X.; Izaurralde, R.C.; Gross, K.L.; Robertson, G.P. Sustainable bioenergy production from marginal lands in the, US Midwest. Nature 2013, 493, 514–517. [Google Scholar] [CrossRef]
- Nielson, R.L. Historical Corn Grain Yields for Indianan and the US 2012. Available online: https://www.agry.purdue.edu/ext/corn/news/timeless/yieldtrends.html (accessed on 1 June 2017).
- NASS.USDA.GOV. Available online: usda.nass.usda.gov/Charts_and_Maps/graphics/ 2017 (accessed on 10 August 2017).
- Bothast, R.J.; Schlicher, M.A. Biotechnological processes for conversion of corn into ethanol. Appl. Microbiol. Biotechnol. 2005, 67, 19–25. [Google Scholar] [CrossRef]
- Heaton, E.A.; Dohleman, F.G.; Long, S.P. Meeting US biofuel goals with less land: The potential of Miscanthus. Glob. Chang. Biol. 2008, 14, 2000–2014. [Google Scholar] [CrossRef]
- Wright, L.; Turhollow, A. Switchgrass selection as a “model “bioenergy crop: A history of the process. Biomass Bioenergy 2010, 34, 851–868. [Google Scholar] [CrossRef]
- Adler, P.R.; Sanderson, M.A.; Boateng, A.A.; Weimer, P.J.; Jung, H.J.G. Biomass yield and biofuel quality of switchgrass harvested in fall or spring. Agron. J. 2006, 98, 1518–1525. [Google Scholar] [CrossRef] [Green Version]
- Gunderson, C.A.; Davis, E.B.; Jager, H.I.; West, T.O.; Perlack, R.D.; Brandt, C.C.; Wullschleger, S.D.; Baskaran, L.M.; Wilkerson, E.G.; Downing, M.E. Exploring Potential, US Switchgrass Production for Lignocellulosic Ethanol. 2008 Technical Manuscript ORNL/TM-2007/183. Available online: http://bioenergy.ornl.gov (accessed on 1 April 2016).
- Vogel, K.P.; Mitchell, R.B. Heterosis in switchgrass: Biomass yield in swards. Crop Sci. 2008, 48, 2159–2164. [Google Scholar] [CrossRef] [Green Version]
- Wullschleger, W.S.; Davis, E.B.; Borsuk, M.E.; Gunderson, C.A.; Lynd, L.R. Biomass production in switchgrass across the united states: Database description and determinants of yield. Agron. J. 2010, 102, 1158–1168. [Google Scholar] [CrossRef] [Green Version]
- Tilman, D.; Reich, P.B.; Knops, J.M.H. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 2006, 441, 629–632. [Google Scholar] [CrossRef] [PubMed]
- Jungers, J.M.; Clark, A.T.; Betts, K.; Mangan, M.E.; Sheaffer, C.C.; Wyse, D.L. Long-term biomass yield and species composition in native perennial bioenergy cropping systems. Agron. J. 2015, 107, 1627–1640. [Google Scholar] [CrossRef]
- Meehan, T.D.; Hurlbert, A.H.; Gratton, C. Bird communities in future bioenergy landscapes of the Upper Midwest. Proc. Natl. Acad. Sci. USA 2010, 107, 18533–18538. [Google Scholar] [CrossRef] [Green Version]
- Pierce, R.A., II; Reinbott, T.; Wright, R.; White, B.; Potter, L. Establishing and Managing Early Successional Habitats for Wildlife on Agricultural Lands; MU Extension Publication MP: Missouri, MO, USA, 2010; Volume 907, p. 20. [Google Scholar]
- McNaughton, S.J. Ecology of grazing ecosystem: The Serengeti. Ecol. Monogr. 1985, 55, 259–294. [Google Scholar] [CrossRef]
- Burkepile, D.E. Comparing aquatic and terrestrial grazing ecosystems: Is the grass really green? Oikos 2013, 122, 306–312. [Google Scholar] [CrossRef]
- Gardiner, M.A.; Tuell, J.K.; Isaacs, R.; Gibbs, J.; Ascher, J.S.; Landis, D.A. Implications of three biofuel crops for beneficial arthropods in agricultural landscapes. Bioenergy Res. 2010. [Google Scholar] [CrossRef] [Green Version]
- Haaland, C.; Naisbit, R.E.; Bersier, L.F. Sown wildflower strips for insect conservation: A review. Insect Conserv. Divers. 2011, 4, 60–80. [Google Scholar] [CrossRef]
- Packard, S.; Mutel, C.F. (Eds.) The Tallgrass Restoration Handbook: For Prairies, Savannas, and Woodlands; Society for Ecological Restoration; Island Press: Washington, DC, USA, 1997; p. 463. [Google Scholar]
- Dragoni, F.; Nasso, N.N.; O Di Tozzini, C.; Bonari, E.; Ragaglini, G. Aboveground yield and biomass quality of giant reed (arundo donax l.) as affected by harvest time and frequency. Bioenergy Res. 2015, 8, 1321–1331. [Google Scholar] [CrossRef] [Green Version]
- Hao, X.; Thelen, K.; Gao, J. Spatial variability in biomass yield of switchgrass, native prairie, and corn at field scale. Agron. J. 2016, 108, 548–558. [Google Scholar] [CrossRef]
- Schmer, M.R.; Mitchell, R.B.; Bogel, K.P.; Schacht, W.H.; Marx, D.B. Spatial and temporal effects on switchgrass stands and yield in the Great Plains. Bioenergy Res. 2010, 3, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Thelemann, R.; Johnson, G.; Sheaffer, C.; Banerjee, S.; Cai, H.; Wyse, D. The effect of landscape position on biomass crop yield. Agron. J. 2010, 102, 513–522. [Google Scholar] [CrossRef] [Green Version]
- Wilson, D.M.; Heaton, E.A.; Schulte, L.A.; Gunther, T.P.; Shea, M.E.; Hall, R.B.; Headlee, W.L.; Moore, W.L.; Boersma, N.N. Establishment and short-term productivity of annual and perennial bioenergy crops across a landscape gradient. Bioenergy Res. 2014, 7, 885–898. [Google Scholar] [CrossRef]
- Lewandowski, I.; Heinz, A. Delayed harvest of miscanthus—Influences on biomass quantity and quality and environmental impacts of energy production. Eur. J. Agron. 2003, 19, 45–63. [Google Scholar] [CrossRef]
- Tahir, M.H.N.; Casler, M.D.; Moore, K.J.; Brummer, E.C. Biomass yield and quality of reed canarygrass under five harvest management systems for bioenergy production. Bioenergy Res. 2011, 4, 111–119. [Google Scholar] [CrossRef]
- Kering, M.K.; Butler, T.J.; Biermacher, J.T.; Guretzky, J.A. Biomass yield and nutrient removal rates of perennial grasses under nitrogen fertilization. Bioenergy Res. 2012, 5, 61–70. [Google Scholar] [CrossRef]
- Vogel, K.P.; Brejda, J.J.; Walters, D.T.; Buxton, D.R. Switchgrass biomass production in the Midwest, USA: Harvest and nitrogen management. Agon. J. 2002, 94, 413–420. [Google Scholar] [CrossRef]
- Monti, A.; Zegada-Lizarazu1, W. Sixteen-year biomass yield and soil carbon storage of giant reed (Arundo donax L.) grown under variable nitrogen fertilization rates. Bioenergy Res. 2016, 9, 248–256. [Google Scholar] [CrossRef]
- Angelini, L.G.; Ceccarini, L.; Bonari, E. Biomass yield and energy balance of giant reed (Arundo donax L.) cropped in central Italy as related to different management practices. Eur. J. Agron. 2005, 22, 375–389. [Google Scholar] [CrossRef]
- Yang, Z.; Shen, Z.; Tetreault, H.; Johnson, L.; Friebe, B.; Frazier, T.; Huang, L.; Burklew, C.; Zhang, X.; Zhao, B. Production of autopolyploid lowland switchgrass lines through in vitro chromosome doubling. Bioenergy Res. 2014, 7, 232–242. [Google Scholar] [CrossRef] [Green Version]
- Johnson, L.C.; Olsen, J.T.; Tetreault, H.; DeLaCruz, A.; Bryant, J.; Morgan, T.J.; Knapp, M.; Bello, N.M.; Baer, S.G.; Maricle, B.R. Intraspecific variation of a dominant grass and local adaptation in reciprocal garden communities along a, US Great Plains’ precipitation gradient: Implications for grassland restoration with climate change. Evol. Appl. 2015, 8, 705–723. [Google Scholar] [CrossRef] [Green Version]
- Anderson, S.H. Claypan and its Environmental Effects. In Encyclopedia of Agrophysics; Encyclopedia of Earth Sciences Series; Gliński, J., Horabik, J., Lipiec, J., Eds.; Springer: Dordrecht, The Netherlands, 2011. [Google Scholar]
- USDA NRCS 2016. Available online: https://www.nrcs.usda.gov/wps/portal/nrcs/detail/mo/plantsanimals/?cid=stelprdb1083060 (accessed on 10 June 2018).
- Sudkamp, S.; Chapman, R.; Pierce, R.A., II. Quail Friendly Plants of the Midwest; University of Missouri Extension MP: Missouri, MO, USA, 2008; Volume 903, p. 121. [Google Scholar]
- SAS. SAS User’s Guide, Statistics; Version 5; SAS Institute: Cary, NC, USA, 2010. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach; Mcgraw Hill Series in Probability and Statistics; Mcgraw Hill: New York, NY, USA, 1997; p. 672. [Google Scholar]
- Heaton, E.; Voigt, T.; Long, S.P. A quantitative review of comparing the yields of two candidate C-4 perennial biomass crops in relation to nitrogen, temperature and water. Biomass Bioenergy 2004, 27, 21–30. [Google Scholar] [CrossRef]
- Kiniry, J.R.; Tischler, C.R.; van Esbroeck, G.A. Radiation use efficiency and leaf, CO2 exchange for diverse C4 grasses. Biomass Bioenergy 1999, 17, 95–112. [Google Scholar] [CrossRef]
- Muir, J.P.; Sanderson, M.A.; Ocumpaugh, W.R.; Jones, R.M.; Reed, R.L. Biomass production of Alamo switchgrass in response to nitrogen, phosphorus, and row spacing. Agron. J. 2001, 93, 896–901. [Google Scholar] [CrossRef]
- Lee, D.K.; Boe, A. Biomass production of switchgrass in central South Dakota. Crop Sci. 2005, 45, 2583–2590. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Reed, R.L.; Ocumpaugh, W.R.; Hussey, M.A.; Van Esbroeck, G.; Read, J.C.; Tischler, C.R.; Hons, F.M. Switchgrass cultivars and germplasm for biomass feedstock production in Texas. Bioresour. Technol. 1999, 67, 209–219. [Google Scholar] [CrossRef]
- Berdahl, J.D.; Frank, A.B.; Krupinsky, J.M.; Carr, P.M.; Hanson, J.D.; Johnson, H.A. Biomass yield, phenology and survival of diverse cultivars and experimental strains in western North Dakota. Agron. J. 2005, 97, 549–555. [Google Scholar] [CrossRef] [Green Version]
- Parrish, D.J.; Fike, J.H. The biology and agronomy of switchgrass for biofuels. Crit. Rev. Plant. Sci. 2005, 24, 423–459. [Google Scholar] [CrossRef]
- Monti, A.; Bezzi, G.; Pritoni, G.; Venturi, G. Long-term productivity of lowland and upland switchgrass cytotypes as affected by cutting frequency. Bioresour. Technol. 2008, 99, 7425–7432. [Google Scholar] [CrossRef]
- Nizami, A.S.; Korres, N.E.; Murphy, J.D. Review of the integrated process for the production of grass biomethane. Environ. Sci. Technol. 2009, 43, 8496–8508. [Google Scholar] [CrossRef] [PubMed]
- Prochnow, A.; Heiermann, M.; Plöchl, M.; Linke, B.; Idler, C.; Amon, T.; Hobbs, P.J. Bioenergy from permanent grassland—A review: 1. Biogas. Bioresour. Technol. 2009, 100, 4931–4944. [Google Scholar] [CrossRef] [PubMed]
- Prochnow, A.; Heiermann, M.; Plöchl, M.; Amon, T.; Hobbs, P.J. Bioenergy from permanent grassland—A review: 2. Combustion. Bioresour. Technol. 2009, 100, 4945–4954. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, P.G.; McCaughey, W.P. Switchgrass (Panicum virgatum L.) cultivar adaptation, biomass production, and cellulose concentration as affected by latitude of origin. ISRN Agron. 2012, 2012, 763046. [Google Scholar] [CrossRef] [Green Version]
- Khalsa, J.; Fricke, T.; Weisser, W.W.; Weigelt, A.; Wachendorf, M. Effects of functional groups and species richness on biomass constituents relevant for combustion: Results from a grassland diversity experiment. Grass Forage Sci. 2012, 67, 569–588. [Google Scholar] [CrossRef]
- Jungers, J.M.; Sheaffer, C.C.; Lamb, J.A. The effect of nitrogen, phosphorus, and potassium fertilizers on prairie biomass yield, ethanol yield, and nutrient harvest. Bioenergy Res. 2015, 8, 279–291. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Nelson, K.; Palmer, M.A. Linking species diversity to the functioning of ecosystems: On the importance of environmental context. Oikos 2000, 91, 175–183. [Google Scholar] [CrossRef] [Green Version]
- Adler, P.R.; Sanderson, M.A.; Weimer, P.J.; Vogel, K.P. Plant species composition and biofuel yields of conservation grasslands. Ecol. Appl. 2009, 19, 2202–2209. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.V.; Kiniry, J.R.; Sanchez, H.; Polley, H.W.; Fay, P.A. Comparing biomass yields of low-input high-diversity communities with managed monocultures across the central United States. Bioenergy Res. 2010, 3, 353–361. [Google Scholar] [CrossRef] [Green Version]
- Griffith, A.P.; Epplin, F.M.; Fuhlendorf, S.D.; Gillen, R. A comparison of perennial polycultures and monocultures for producing biomass for biorefinery feedstock. Agron. J. 2011, 103, 617–627. [Google Scholar] [CrossRef] [Green Version]
- Tipu, S.U.; Hossain, K.L.; Islam, M.O.; Hossain, M.A. Effect of pruning height on shoot biomass yield of Leucaena leucocephala. Asian J. Plant Sci. 2006, 5, 1043–1046. [Google Scholar]
- Baatar, B.; Svavarsdóttir, K.; Aradóttir, Á.L.; Ægisdóttir, H.H. Effects of Cutting Height and Frequency on Yield in a Mongolian Rangeland; Land Restoration Training Programme: Reykjavík, Iceland, 2008. [Google Scholar]
- Schulte, L.A.; Niemi, J.; Helmers, M.J.; Liebman, M.; Arbuckle, J.D.; James, D.E.; Kolka, R.K.; O’Neal, M.E.; Tomer, M.D.; Tyndall, J.C.; et al. Prairie strips improve corn–soybean croplands. Proc. Natl. Acad. Sci. USA 2017, 114, 11247–11252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
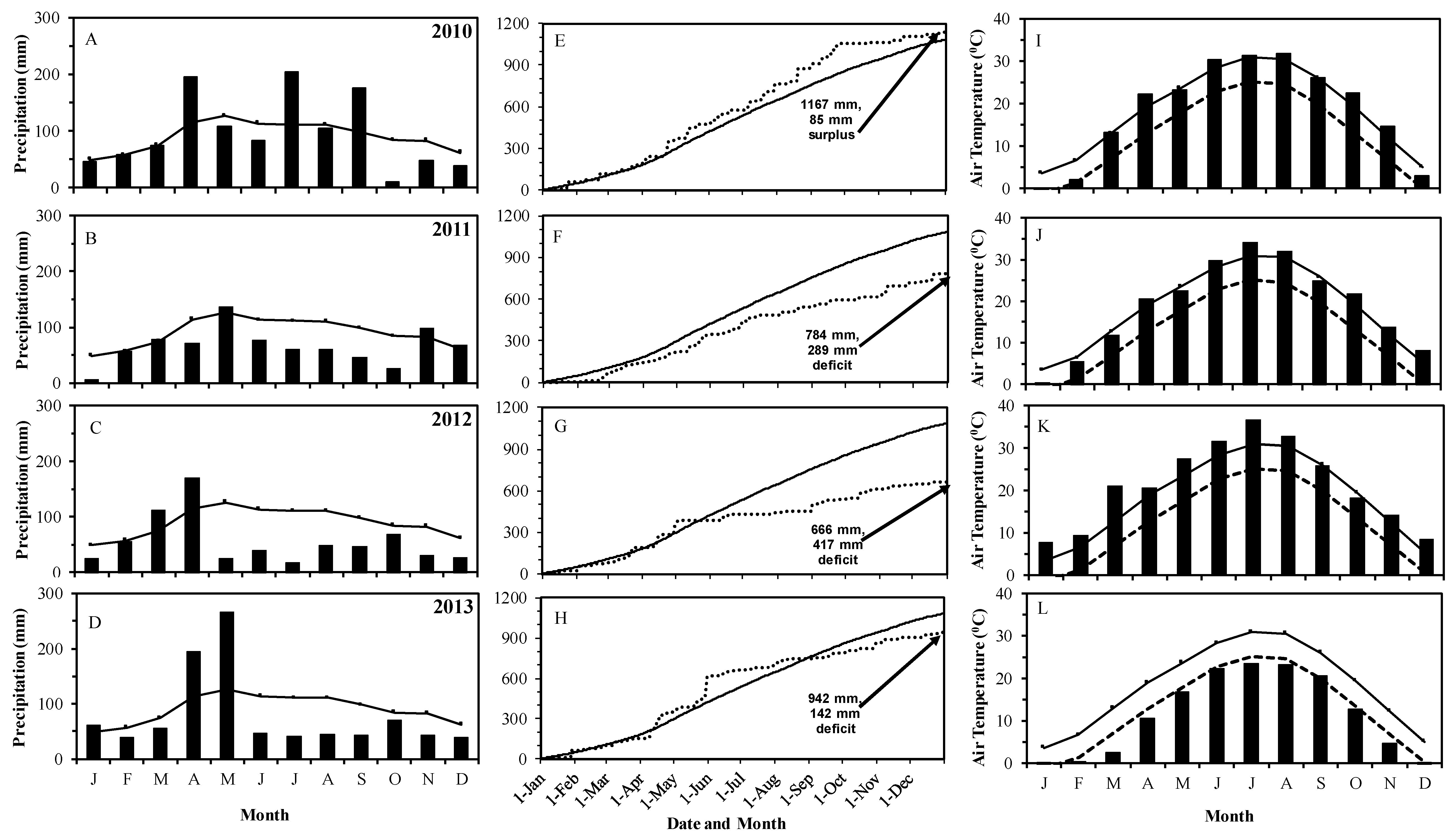
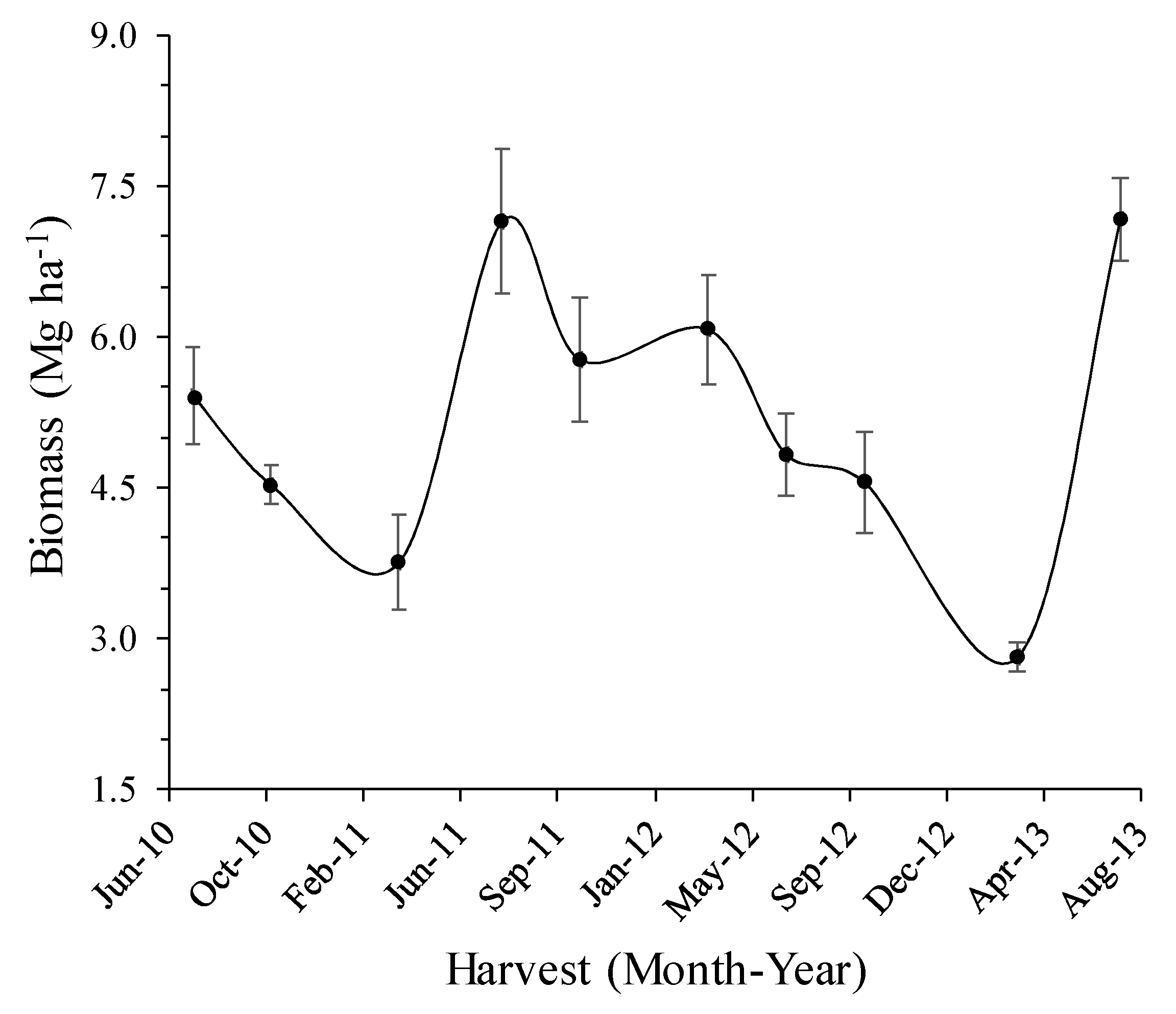
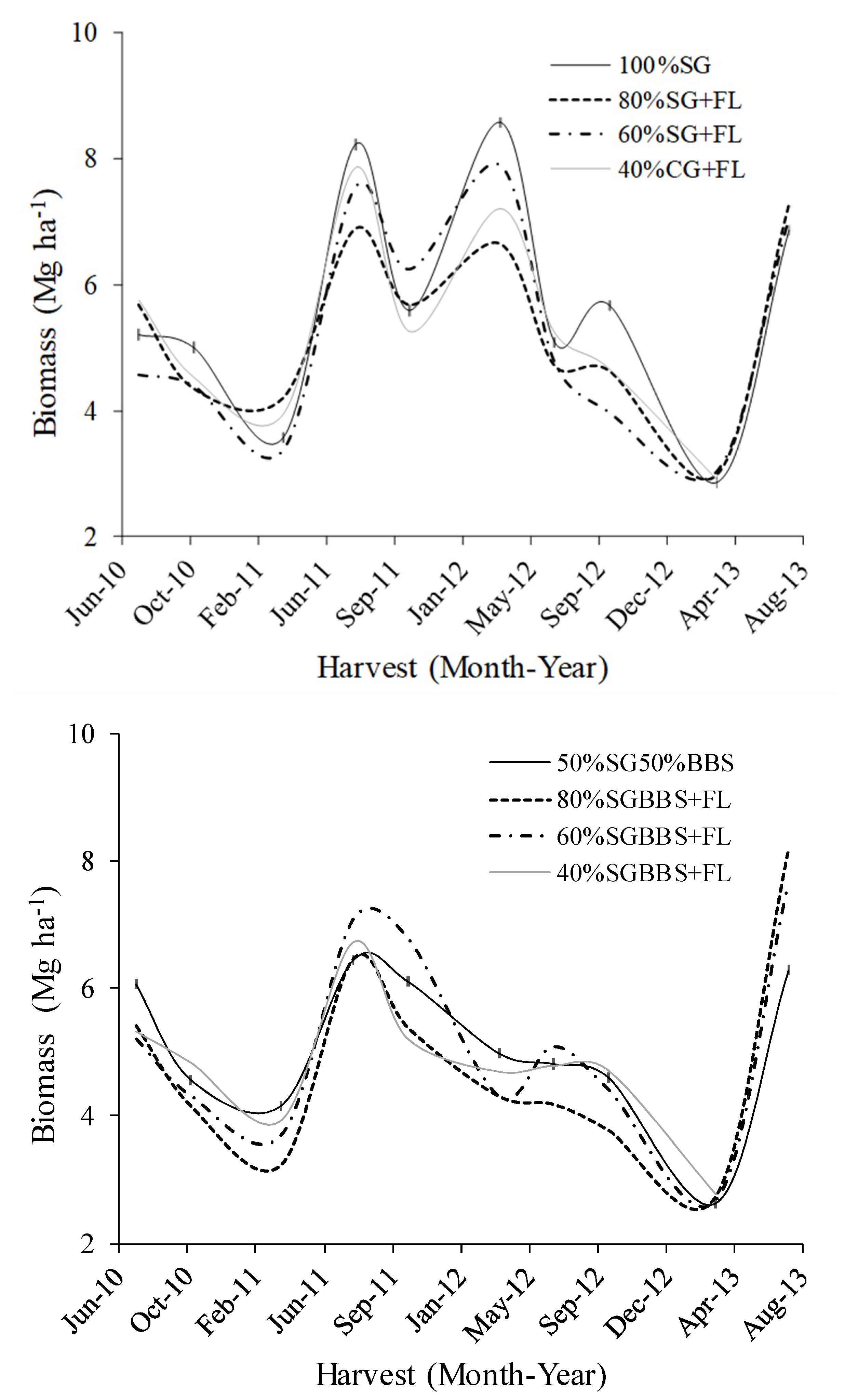
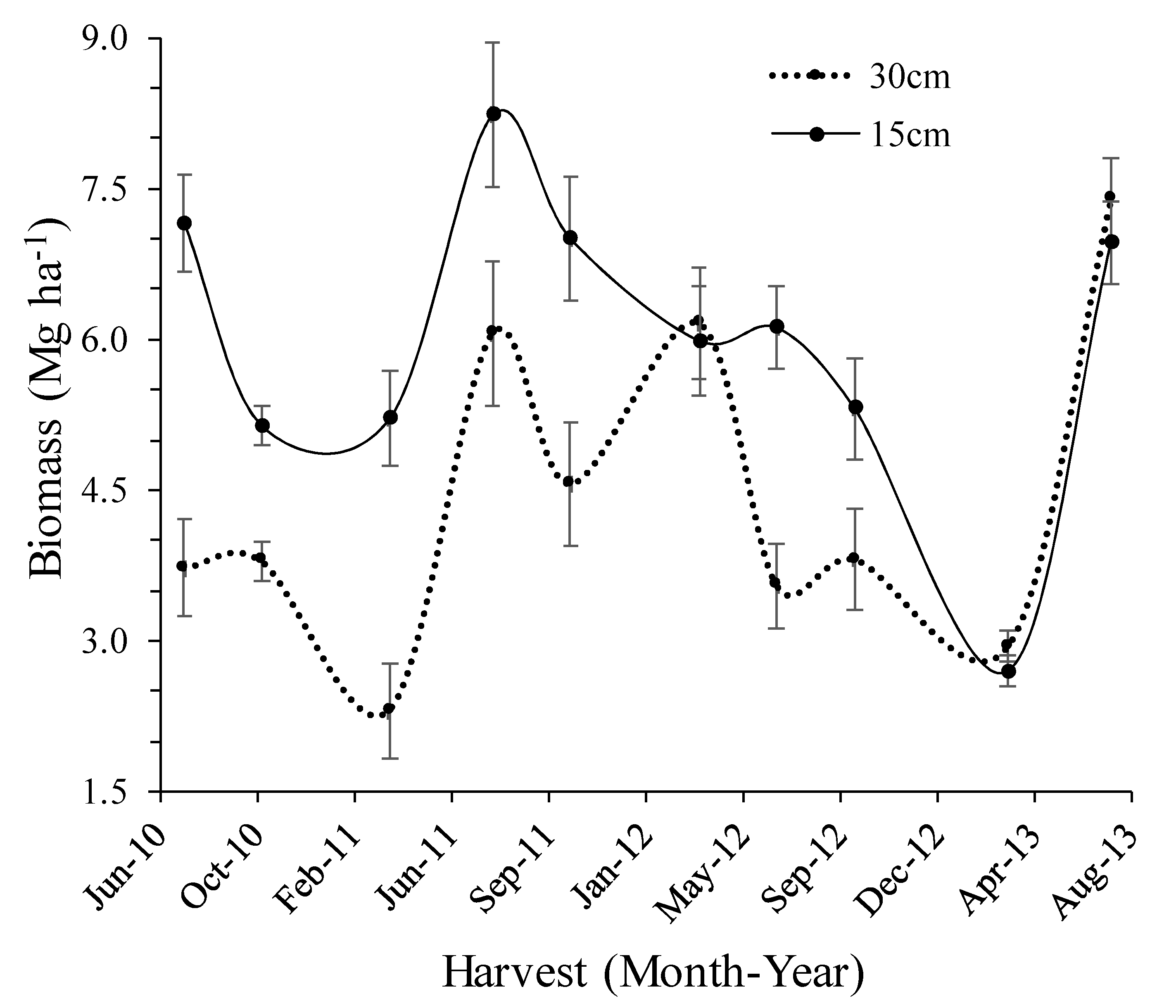
| Rainfall Category | Long-Term | 2010 | 2011 | 2012 | 2013 |
|---|---|---|---|---|---|
| Annual (mm) | 1083 | 1149 | 784 | 666 | 942 |
| Percent deviation from the normal precipitation | 6 | −28 | −38 | −13 | |
| 1 March to 31 October (mm) | 832 | 959 | 554 | 529 | 760 |
| Percent deviation from the normal precipitation | 15 | −33 | −36 | −9 | |
| 1 June to 1 September (mm) | 335 | 393 | 197 | 106 | 133 |
| Percent deviation from the normal precipitation | 17 | −41 | −68 | −60 |
| Harvest | 15 cm | 30 cm | |||||
|---|---|---|---|---|---|---|---|
| Harvest Time | Number | Yield | Change | Annual | Yield | Change | Annual |
| Mg ha−1 | % | Mg ha−1 | Mg ha−1 | % | Mg ha−1 | ||
| July 2010 | 1 | 7.16 | 3.72 | ||||
| October 2010 | 2 | 5.14 | −28 | 6.15 | 3.79 | 2 | 3.76 |
| March 2011 | 3 | 5.21 | 1 | 2.30 | −39 | ||
| July 2011 | 4 | 8.23 | 58 | 6.06 | 163 | ||
| October 2011 | 5 | 7.00 | −15 | 6.81 | 4.56 | −25 | 4.31 |
| March 2012 | 6 | 5.99 | −14 | 6.16 | 35 | ||
| July 2012 | 7 | 6.12 | 2 | 3.54 | −42 | ||
| October 2012 | 8 | 5.31 | −13 | 5.80 | 3.81 | 7 | 4.5 |
| March 2013 | 9 | 2.70 | −49 | 2.94 | −23 | ||
| July 2013 | 10 | 6.96 | 158 | 4.83 | 7.40 | 152 | 5.17 |
| Study period | 5.98 | 4.43 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Udawatta, R.P.; Gantzer, C.J.; Reinbott, T.M.; Wright, R.L.; Robert, P.A., II; Wehtje, W. Influence of Species Composition and Management on Biomass Production in Missouri. Agriculture 2020, 10, 75. https://doi.org/10.3390/agriculture10030075
Udawatta RP, Gantzer CJ, Reinbott TM, Wright RL, Robert PA II, Wehtje W. Influence of Species Composition and Management on Biomass Production in Missouri. Agriculture. 2020; 10(3):75. https://doi.org/10.3390/agriculture10030075
Chicago/Turabian StyleUdawatta, Ranjith P., Clark J. Gantzer, Timothy M. Reinbott, Ray L. Wright, Pierce A. Robert, II, and Walter Wehtje. 2020. "Influence of Species Composition and Management on Biomass Production in Missouri" Agriculture 10, no. 3: 75. https://doi.org/10.3390/agriculture10030075
APA StyleUdawatta, R. P., Gantzer, C. J., Reinbott, T. M., Wright, R. L., Robert, P. A., II, & Wehtje, W. (2020). Influence of Species Composition and Management on Biomass Production in Missouri. Agriculture, 10(3), 75. https://doi.org/10.3390/agriculture10030075




