Root Growth of Hordeum vulgare and Vicia faba in the Biopore Sheath
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Setup
2.2. Statistical Data Analysis
3. Results
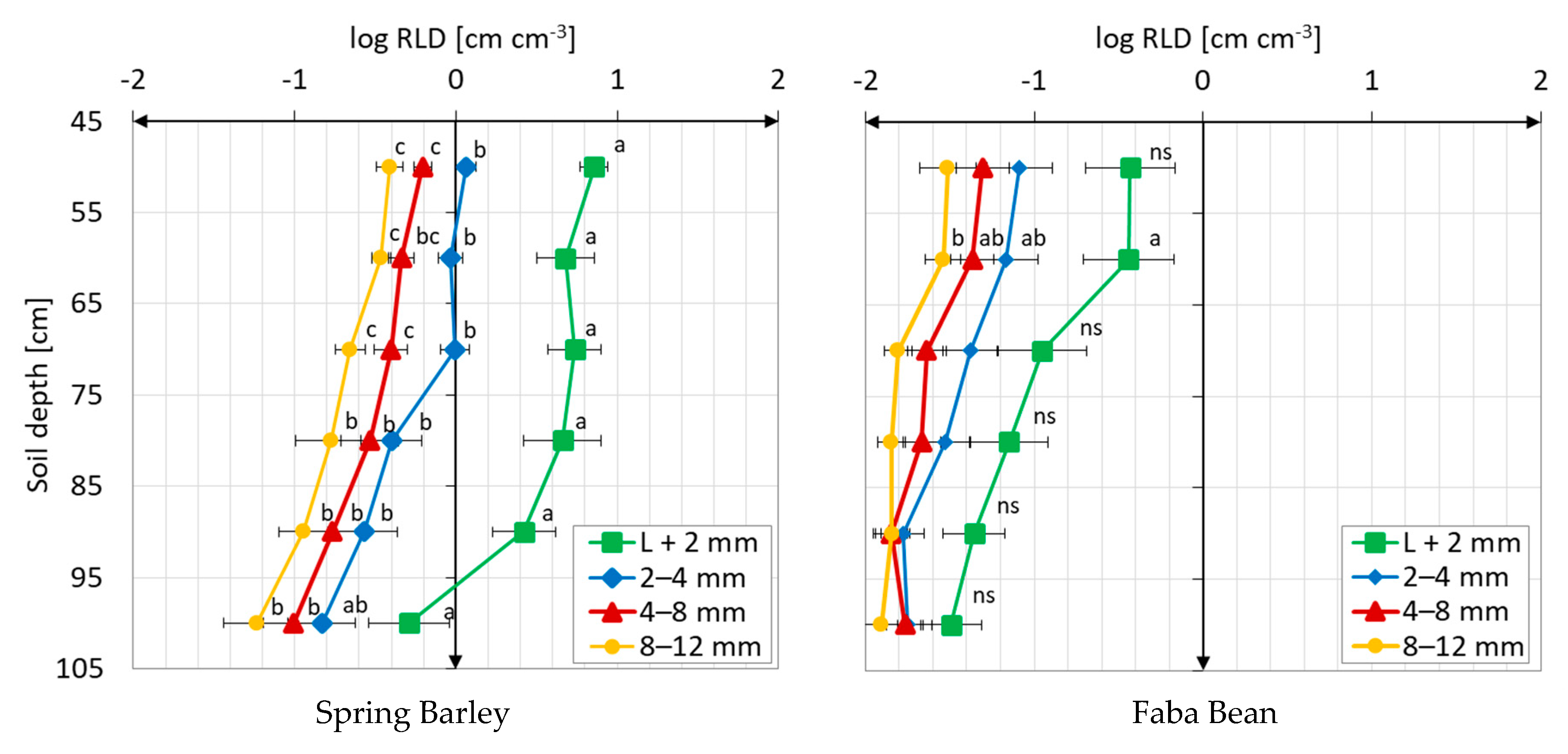
3.1. Root Distribution
3.2. Nt-, Ct-Content and CN-Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.; Crespi, M. Plant root growth, architecture and function. Plant Soil 2009, 321, 153–187. [Google Scholar] [CrossRef]
- Kutschera, L.; Lichtenegger, E.; Sobotik, M. Hordeum vulgare L., vielzeilige gerste. In Wurzelatlas der Kulturpflanzen Gemäßigter Gebiete Mit Arten des Feldgemüseanbaus; DLG-Verlags-GmbH: Frankfurt am Main, Germany, 2009; Volume 7, p. 212. [Google Scholar]
- Amelung, W.; Blume, H.-P.; Fleige, H.; Horn, R.; Kandeler, E.; Kögel-Knabner, I.; Kretzschmar, R.; Stahr, K.; Wilke, B.-M. Böden als pflanzenstandorte. In Scheffer/Schachtschabel Lehrbuch der Bodenkunde, 17th ed.; Springer-Verlag GmbH: Berlin/Heidelberg, Germany, 2018; pp. 491–581. [Google Scholar] [CrossRef]
- Gaiser, T.; Perkons, U.; Küpper, P.M.; Uteau Puschmann, D.; Peth, S.; Kautz, T.; Pfeifer, J.; Ewert, F.; Horn, R.; Köpke, U. Evidence of improved water uptake from subsoil by spring wheat following lucerne in a temperate humid climate. Field Crops Res. 2012, 126, 56–62. [Google Scholar] [CrossRef]
- Fleige, H.; Grimme, H.; Renger, M.; Strebel, O. Zur erfassung der nährstoffanlieferung durch diffusion im effektiven wurzelraum. Mitt. Dtsch. Bodenkd. Ges. 1983, 38, 381–386. [Google Scholar]
- Kuhlmann, H.; Baumgärtel, G. Potential importance of the subsoil for the P and Mg nutrition of wheat. Plant Soil 1991, 137, 259–266. [Google Scholar] [CrossRef]
- Ehlers, W.; Köpke, U.; Hesse, F.; Böhm, W. Penetration resistance and root growth of oats in tilled and untilled loess soil. Soil Tillage Res. 1983, 3, 261–275. [Google Scholar] [CrossRef]
- Kautz, T.; Amelung, W.; Ewert, F.; Gaiser, T.; Horn, R.; Jahn, R.; Javaux, M.; Kemna, A.; Kuzyakov, Y.; Munch, J.C.; et al. Nutrient acquisition from arable subsoils in temperate climates: A review. Soil Biol. Biochem. 2013, 57, 1003–1022. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial hotspots and hot moments in soil: Concept & review. Soil Biol. Biochem. 2015, 83, 184–199. [Google Scholar] [CrossRef]
- Tiunov, A.V.; Scheu, S. Microbial respiration, biomass, biovolume and nutrient status in burrow walls of Lumbricus terrestris L. (Lumbricidae). Soil Biol. Biochem. 1999, 31, 2039–2048. [Google Scholar] [CrossRef]
- Uksa, M.; Schloter, M.; Kautz, T.; Athmann, M.; Köpke, U.; Fischer, D. Spatial variability of hydrolytic and oxidative potential enzyme activities in different subsoil compartments. Biol. Fertil. Soils 2015, 51, 517–521. [Google Scholar] [CrossRef]
- Hagedorn, F.; Bundt, M. The age of preferential flow paths. Geoderma 2002, 108, 119–132. [Google Scholar] [CrossRef]
- Perkons, U.; Kautz, T.; Uteau, D.; Peth, S.; Geier, V.; Thomas, K.; Lütke Holz, K.; Athmann, M.; Pude, R.; Köpke, U. Root-length densities of various annual crops following crops with contrasting root systems. Soil Tillage Res. 2014, 137, 50–57. [Google Scholar] [CrossRef]
- Kautz, T.; Perkons, U.; Athmann, M.; Pude, R.; Köpke, U. Barley roots are not constrained to large-sized biopores in the subsoil of a deep Haplic Luvisol. Biol. Fertil. Soils 2013, 49, 959–963. [Google Scholar] [CrossRef]
- Stewart, J.B.; Moran, C.J.; Wood, J.T. Macropore sheath: Quantification of plant root and soil macropore association. Plant Soil 1999, 211, 59–67. [Google Scholar] [CrossRef]
- Pierret, A.; Moran, C.J.; Pankhurst, C.E. Differentiation of soil properties related to the spatial association of wheat roots and soil macropores. Plant Soil 1999, 211, 51–58. [Google Scholar] [CrossRef]
- White, R.G.; Kirkegaard, J.A. The distribution and abundance of wheat roots in a dense, structured subsoil – implications for water uptake. Plant Cell Environ. 2010, 33, 133–148. [Google Scholar] [CrossRef]
- Stirzaker, R.J.; Passioura, J.B.; Wilms, Y. Soil structure and plant growth: Impact of bulk density and biopores. Plant Soil 1996, 185, 151–162. [Google Scholar] [CrossRef]
- Passioura, J.B. Soil structure and plant growth. Aust. J. Soil Res. 1991, 29, 717–728. [Google Scholar] [CrossRef]
- Athmann, M.; Kautz, T.; Pude, R.; Köpke, U. Root growth in biopores—Evaluation with in situ endoscopy. Plant Soil 2013, 371, 179–190. [Google Scholar] [CrossRef]
- Bouché, M.B. Action de la faune sur les états de la matière organique dans les ecosystèmes. In Humification et Biodégradation; Kilbertius, G., Reisinger, O., Mourey, A., Cancela da Fonseca, J.A., Eds.; Pierron: Sarreguemines, France, 1975; pp. 157–168. [Google Scholar]
- Brown, G.G.; Baroisa, I.; Lavelle, P. Regulation of soil organic matter dynamics and microbial activity in the drilosphere and the role of interactions with other edaphic functional domains. Eur. J. Soil Biol. 2000, 36, 177–198. [Google Scholar] [CrossRef]
- Lavelle, P. Earthworm activities and the soil system. Biol. Fertil. Soils 1988, 6, 237–251. [Google Scholar] [CrossRef]
- Pankhurst, C.E.; Pierret, A.; Hawke, B.G.; Kirby, J.M. Microbiological and chemical properties of soil associated with macropores at different depths in a red-duplex soil in NSW Australia. Plant Soil 2002, 238, 11–20. [Google Scholar] [CrossRef]
- Athmann, M.; Kautz, T.; Banfield, C.; Bauke, S.; Hoang, D.T.T.; Lüsebrink, M.; Pausch, J.; Amelung, W.; Kuzyakov, Y.; Köpke, U. Six months of L. terrestris L. activity in root-formed biopores increases nutrient availability, microbial biomass and enzyme activity. Appl. Soil Ecol. 2017, 120, 135–142. [Google Scholar] [CrossRef]
- Don, A.; Steinberg, B.; Schöning, I.; Pritsch, K.; Joschko, M.; Gleixner, G.; Schulze, E.-D. Organic carbon sequestration in earthworm burrows. Soil Biol. Biochem. 2008, 40, 1803–1812. [Google Scholar] [CrossRef]
- Tiunov, A.V.; Bonkowski, M.; Alphei, J.; Scheu, S. Microflora, Protozoa and Nematoda in Lumbricus terrestris burrow walls: A laboratory experiment. Pedobiologia 2001, 45, 46–60. [Google Scholar] [CrossRef]
- Jégou, D.; Cluzeaua, D.; Hallaireb, V.; Balesdentc, J.; Tréhen, P. Burrowing activity of the earthworms Lumbricus terrestris and Aporrectodea giardi and consequences on C transfers in soil. Eur. J. Soil Biol. 2000, 36, 27–34. [Google Scholar] [CrossRef]
- Andriuzzi, W.; Bolger, T.; Schmidt, O. The drilosphere concept: Fine-scale incorporation of surface residue-derived N and C around natural Lumbricus terrestris burrows. Soil Biol. Biochem. 2013, 64, 136–138. [Google Scholar] [CrossRef]
- Bengough, A.G. Root Growth and function in relation to soil structure, composition, and strength. In Root Ecology; de Kroon, H., Visser, E.J.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 151–171. [Google Scholar]
- Uteau, D.; Pagenkemper, S.K.; Peth, S.; Horn, R. Root and time dependent soil structure formation and its influence on gas transport in the subsoil. Soil Tillage Res. 2013, 132, 69–76. [Google Scholar] [CrossRef]
- Lee, K.E. Earthworms: Their Ecology and Relationship with Soil and Land Use; Academic Press: Sydney, Australia, 1985. [Google Scholar]
- Hinsinger, P.; Bengough, A.G.; Vetterlein, D.; Young, I.M. Rhizosphere: Biophysics, biogeochemistry and ecological relevance. Plant Soil 2009, 321, 117–152. [Google Scholar] [CrossRef]
- Helliwell, J.R.; Sturrock, C.J.; Mairhofer, S.; Craigon, J.; Ashton, R.W.; Miller, A.J.; Whalley, W.R.; Mooney, S.J. The emergent rhizosphere: Imaging the development of the porous architecture at the root-soil interface. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Schrader, S.; Rogasik, H.; Onasch, I.; Jégou, D. Assessment of soil structural differentiation around earthworm burrows by means of X-ray computed tomography and scanning electron microscopy. Geoderma 2007, 137, 378–387. [Google Scholar] [CrossRef]
- Pagenkemper, S.K.; Athmann, M.; Uteau, D.; Kautz, T.; Peth, S.; Horn, R. The effect of earthworm activity on soil bioporosity—Investigated with X-ray computed tomography and endoscopy. Soil Tillage Res. 2015, 146, 79–88. [Google Scholar] [CrossRef]
- Bengough, A.G. Root elongation is restricted by axial but not by radial pressures: So what happens in field soil? Plant Soil 2012, 360, 15–18. [Google Scholar] [CrossRef]
- Kolb, E.; Hartmann, C.; Genet, P. Radial force development during root growth measured by photoelasticity. Plant Soil 2012, 360, 19–35. [Google Scholar] [CrossRef]
- Pätzold, S.; Vetterlein, D.; Jahn, R. DFG Research Unit 1320. Crop Sequence and the Nutrient Acquisition from the Subsoil. Description of the Reference Soil Profile. Available online: https://www.cka.uni-bonn.de/standort/bodenprofilbeschreibung-cka (accessed on 12 November 2020).
- Patterson, H.D.; Thompson, R. Recovery of inter-block information when block sizes are unequal. Biometrika 1971, 58, 545–554. [Google Scholar] [CrossRef]
- Littell, R.C.; Milliken, G.A.; Stroup, W.W.; Wolfinger, R.D.; Schabenberger, O. SAS for Mixed Model, 2nd ed.; SAS Institute Inc.: Cary, NC, USA, 2006. [Google Scholar]
- Kenward, M.G.; Roger, J.R. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics 1997, 53, 983–997. [Google Scholar] [CrossRef]
- Kackar, R.N.; Harville, D.A. Approximations for standard errors of estimators of fixed and random effects in mixed linear models. J. Am. Stat. Assoc. 1984, 388, 853–862. [Google Scholar]
- Piepho, H. A SAS macro for generating letter displays of pairwise mean comparisons. Commun. Biometry Crop Sci. 2012, 1, 4–13. [Google Scholar]
- Athmann, M.; Sondermann, J.; Kautz, T.; Köpke, U. Comparing macropore exploration by Faba bean, wheat, barley and oilseed rape roots using in situ endoscopy. J. Soil Sci. Plant Nutr. 2019, 19, 689–700. [Google Scholar] [CrossRef]
- Popova, L.; Van Dusschoten, D.; Nagel, K.A.; Fiorani, F.; Mazzolai, B. Plant root tortuosity: An indicator of root path formation in soil with different composition and density. Ann. Bot. 2016, 118, 685–698. [Google Scholar] [CrossRef]
- Lynch, J.; Marschner, P.; Rengel, Z. Effect of internal and external factors on root growth and development. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 331–346. [Google Scholar]
- Perkons, U. Bioporengenese Durch Homo- und Allorhize Kulturpflanzen: Einfluss auf das Wurzelwachstum der Nachfrüchte. Ph.D. Thesis, University of Bonn, Bonn, Germany, 2018. [Google Scholar]
- Han, E.; Kautz, T.; Köpke, U. Precrop root system determines root diameter of subsequent crop. Biol. Fertil. Soils 2016, 52, 113–118. [Google Scholar] [CrossRef]
- Atkinson, J.A.; Hawkesford, M.J.; Whalley, W.R.; Zhou, H.; Mooney, S.J. Soil strength influences wheat root interactions with soil macropores. Plant Cell Environ. 2020, 43, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Materechera, S.A.; Dexter, A.R.; Alston, A.M. Penetration of very strong soils by seedling roots of different species. Plant Soil 1991, 135, 31–41. [Google Scholar] [CrossRef]
- Li, H.B.; Ma, Q.H.; Li, H.G.; Zhan, F.S.; Rengel, Z.; Shen, J.B. Root morphological responses to localized nutrient supply differ among crop species with contrasting root traits. Plant Soil 2014, 376, 151–163. [Google Scholar] [CrossRef]
- Zhan, A.; Lynch, J.P. Reduced frequency of lateral root branching improves N capture from low-N soils in maize. J. Exp. Bot. 2015, 66, 2055–2065. [Google Scholar] [CrossRef] [PubMed]
- Colombi, T.; Walter, A. Genetic diversity under soil compaction in wheat: Root number as a promising trait for early plant vigor. Front. Plant Sci. 2017, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Correa, J.; Postma, J.A.; Watt, M.; Wojciechowski, T. Soil compaction and the architectural plasticity of root systems. J. Exp. Bot. 2019, 70, 6019–6034. [Google Scholar] [CrossRef]
- Watt, M.; Hugenholtz, P.; White, R.; Vinall, K. Numbers and locations of native bacteria on field-grown wheat roots quantified by fluorescence in situ hybridization (FISH). Environ. Microbiol. 2006, 8, 871–884. [Google Scholar] [CrossRef]
- Watt, M.; Silk, W.K.; Passioura, J.B. Rates of root and organism growth, soil conditions, and temporal and spatial development of the rhizosphere. Ann. Bot. 2006, 97, 839–855. [Google Scholar] [CrossRef]
- Jones, D.L.; Magthab, E.A.; Gleeson, D.B.; Hill, P.W.; Sánchez-Rodríguez, A.R.; Roberts, P.; Ge, T.; Murphy, D.V. Microbial competition for nitrogen and carbon is as intense in the subsoil as in the topsoil. Soil Biol. Biochem. 2018, 117, 72–82. [Google Scholar] [CrossRef]
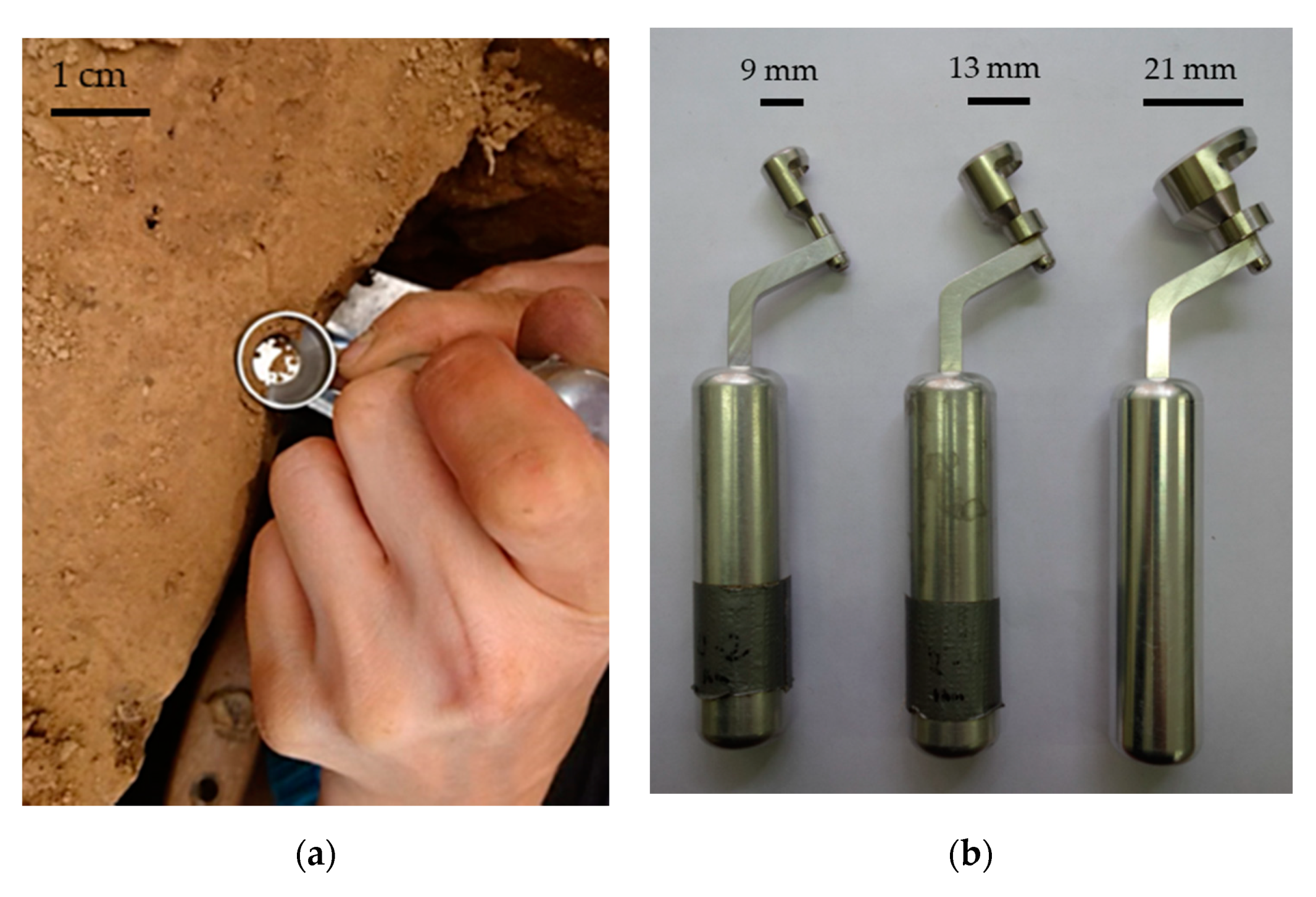
| Soil Type | Crop | Share of Roots [%] | Reference |
|---|---|---|---|
| Haplic Luvisol (silty loam) | Winter barley, Oilseed rape | 21 | Perkons et al. [13] |
| Haplic Luvisol (silty loam) | Winter barley | <25 | Kautz et al. [14] |
| Black Vertosol | Pasture, dominated by Queens-land blue grass and Tall oat grass | 11–26 | Stewart et al. [15] |
| Typic and Haplic Palexeralf (hard setting clay) | Wheat | 80 * | Pierret et al. [16] |
| Red Kondosol (acidic loam) | Wheat | 44–95 ** | White and Kirkegaard [17] |
| Fine | Small | Medium | Coarse | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Lateral | 0–0.2 mm | 0.2–0.4 mm | 0.4–0.6 mm | 0.6–5 mm | |||||
| Distance | (%) | (%) | (%) | (%) | |||||
| (mm) | Mean | SE | Mean | SE | Mean | SE | Mean | SE | |
| Spring Barley | L + 2 | 43.7 b | ±4.1 | 34.9 a | ±2.9 | 13.4 a | ±2.0 | 7.9 a | ±1.7 |
| 2–4 | 53.1 a | ±5.3 | 38.2 a | ±4.2 | 7.0 b | ±2.1 | 1.8 b | ±1.0 | |
| 4–8 | 51.3 a | ±4.9 | 41.8 a | ±4.3 | 5.8 b | ±1.5 | 1.1 b | ±0.6 | |
| 8–12 | 52.5 a | ±4.6 | 39.5 a | ±3.8 | 7.0 b | ±1.6 | 1.0 b | ±0.5 | |
| Faba Bean | L + 2 | 43.9 a | ±11.9 | 31.3 a | ±7.7 | 11.8 a | ±4.6 | 13.0 a | ±5.9 |
| 2–4 | 62.8 a | ±12.3 | 26.6 a | ±8.3 | 6.6 a | ±4.7 | 4.0 ab | ±3.1 | |
| 4–8 | 56.0 a | ±10.9 | 32.7 a | ±9.1 | 6.2 a | ±2.9 | 5.1 ab | ±4.0 | |
| 8–12 | 53.1 a | ±13.5 | 38.6 a | ±11.7 | 6.1 a | ±3.9 | 2.2 b | ±1.5 | |
| Spring Barley | Faba Bean | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil Depth | Lateral Distance | Nt (%) | Ct (%) | C/N | Nt (%) | Ct (%) | C/N | ||||||
| (cm) | (mm) | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE | Mean | SE |
| 45–55 | 0–2 | 0.070 a | ±0.003 | 0.65 a | ±0.02 | 9.3 a | ±0.2 | 0.062 a | ±0.003 | 0.55 a | ±0.03 | 8.8 a | ±0.2 |
| 2–4 | 0.060 b | ±0.003 | 0.52 b | ±0.02 | 8.8 ab | ±0.2 | 0.051 b | ±0.001 | 0.43 ab | ±0.01 | 8.4 ab | ±0.2 | |
| 4–8 | 0.056 c | ±0.002 | 0.47 b | ±0.01 | 8.5 b | ±0.3 | 0.047 b | ±0.001 | 0.39 b | ±0.01 | 8.2 b | ±0.2 | |
| 8–12 | 0.055 c | ±0.002 | 0.46 b | ±0.01 | 8.5 b | ±0.3 | 0.046 b | ±0.001 | 0.38 b | ±0.01 | 8.1 b | ±0.1 | |
| 55–65 | 0–2 | 0.068 a | ±0.003 | 0.61 a | ±0.02 | 8.9 a | ±0.2 | 0.060 a | ±0.002 | 0.52 a | ±0.03 | 8.6 a | ±0.2 |
| 2–4 | 0.058 b | ±0.002 | 0.50 b | ±0.02 | 8.5 ab | ±0.3 | 0.052 b | ±0.002 | 0.44 ab | ±0.03 | 8.5 ab | ±0.4 | |
| 4–8 | 0.056 c | ±0.001 | 0.46 b | ±0.01 | 8.2 b | ±0.3 | 0.049 b | ±0.001 | 0.39 b | ±0.01 | 8.0 b | ±0.1 | |
| 8–12 | 0.054 c | ±0.001 | 0.44 b | ±0.01 | 8.2 b | ±0.3 | 0.048 b | ±0.001 | 0.38 b | ±0.01 | 7.9 b | ±0.1 | |
| 65–75 | 0–2 | 0.068 a | ±0.002 | 0.61 a | ±0.02 | 9.1 a | ±0.2 | 0.059 a | ±0.002 | 0.50 a | ±0.03 | 8.4 a | ±0.2 |
| 2–4 | 0.056 b | ±0.001 | 0.45 b | ±0.01 | 8.2 b | ±0.2 | 0.053 ab | ±0.002 | 0.43 ab | ±0.02 | 8.1 a | ±0.2 | |
| 4–8 | 0.052 bc | ±0.001 | 0.40 c | ±0.01 | 7.7 bc | ±0.2 | 0.050 b | ±0.001 | 0.38 b | ±0.01 | 7.8 a | ±0.2 | |
| 8–12 | 0.050 c | ±0.001 | 0.38 c | ±0.01 | 7.6 c | ±0.2 | 0.048 b | ±0.002 | 0.37 b | ±0.01 | 7.5 a | ±0.2 | |
| 75–85 | 0–2 | 0.068 a | ±0.002 | 0.59 a | ±0.03 | 8.6 a | ±0.2 | 0.058 a | ±0.002 | 0.50 a | ±0.03 | 8.5 a | ±0.2 |
| 2–4 | 0.054 b | ±0.001 | 0.41 b | ±0.01 | 7.7 b | ±0.1 | 0.051 ab | ±0.002 | 0.41 ab | ±0.02 | 8.0 ab | ±0.3 | |
| 4–8 | 0.051 c | ±0.001 | 0.38 b | ±0.01 | 7.4 b | ±0.2 | 0.048 bc | ±0.001 | 0.37 b | ±0.01 | 7.7 b | ±0.3 | |
| 8–12 | 0.050 c | ±0.001 | 0.37 b | ±0.01 | 7.3 b | ±0.2 | 0.046 c | ±0.001 | 0.35 b | ±0.01 | 7.7 b | ±0.3 | |
| 85–95 | 0–2 | 0.064 a | ±0.002 | 0.57 a | ±0.03 | 9.0 a | ±0.5 | 0.056 a | ±0.002 | 0.48 a | ±0.03 | 8.4 a | ±0.3 |
| 2–4 | 0.051 b | ±0.001 | 0.43 ab | ±0.03 | 8.3 b | ±0.6 | 0.048 ab | ±0.001 | 0.38 ab | ±0.02 | 7.9 b | ±0.3 | |
| 4–8 | 0.048 b | ±0.001 | 0.37 b | ±0.02 | 7.6 b | ±0.4 | 0.045 bc | ±0.001 | 0.35 b | ±0.01 | 7.7 b | ±0.2 | |
| 8–12 | 0.045 c | ±0.001 | 0.35 b | ±0.02 | 7.7 b | ±0.4 | 0.044 c | ±0.001 | 0.33 b | ±0.01 | 7.6 b | ±0.3 | |
| 95–105 | 0–2 | 0.060 a | ±0.002 | 0.53 a | ±0.04 | 8.7 a | ±0.3 | 0.056 a | ±0.002 | 0.48 a | ±0.03 | 8.6 a | ±0.3 |
| 2–4 | 0.047 b | ±0.002 | 0.39 ab | ±0.02 | 8.1 b | ±0.4 | 0.047 b | ±0.001 | 0.37 ab | ±0.01 | 7.8 b | ±0.3 | |
| 4–8 | 0.042 c | ±0.002 | 0.33 b | ±0.01 | 7.2 c | ±0.1 | 0.045 b | ±0.001 | 0.34 b | ±0.01 | 7.6 bc | ±0.3 | |
| 8–12 | 0.040 c | ±0.002 | 0.32 b | ±0.01 | 7.3 c | ±0.2 | 0.044 b | ±0.001 | 0.32 b | ±0.01 | 7.5 c | ±0.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petzoldt, L.; Athmann, M.; Buechse, A.; Kautz, T. Root Growth of Hordeum vulgare and Vicia faba in the Biopore Sheath. Agriculture 2020, 10, 650. https://doi.org/10.3390/agriculture10120650
Petzoldt L, Athmann M, Buechse A, Kautz T. Root Growth of Hordeum vulgare and Vicia faba in the Biopore Sheath. Agriculture. 2020; 10(12):650. https://doi.org/10.3390/agriculture10120650
Chicago/Turabian StylePetzoldt, Lisa, Miriam Athmann, Andreas Buechse, and Timo Kautz. 2020. "Root Growth of Hordeum vulgare and Vicia faba in the Biopore Sheath" Agriculture 10, no. 12: 650. https://doi.org/10.3390/agriculture10120650
APA StylePetzoldt, L., Athmann, M., Buechse, A., & Kautz, T. (2020). Root Growth of Hordeum vulgare and Vicia faba in the Biopore Sheath. Agriculture, 10(12), 650. https://doi.org/10.3390/agriculture10120650






