Seasonal Phenology of the Major Insect Pests of Quinoa (Chenopodium quinoa Willd.) and Their Natural Enemies in a Traditional Zone and Two New Production Zones of Peru
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Sites
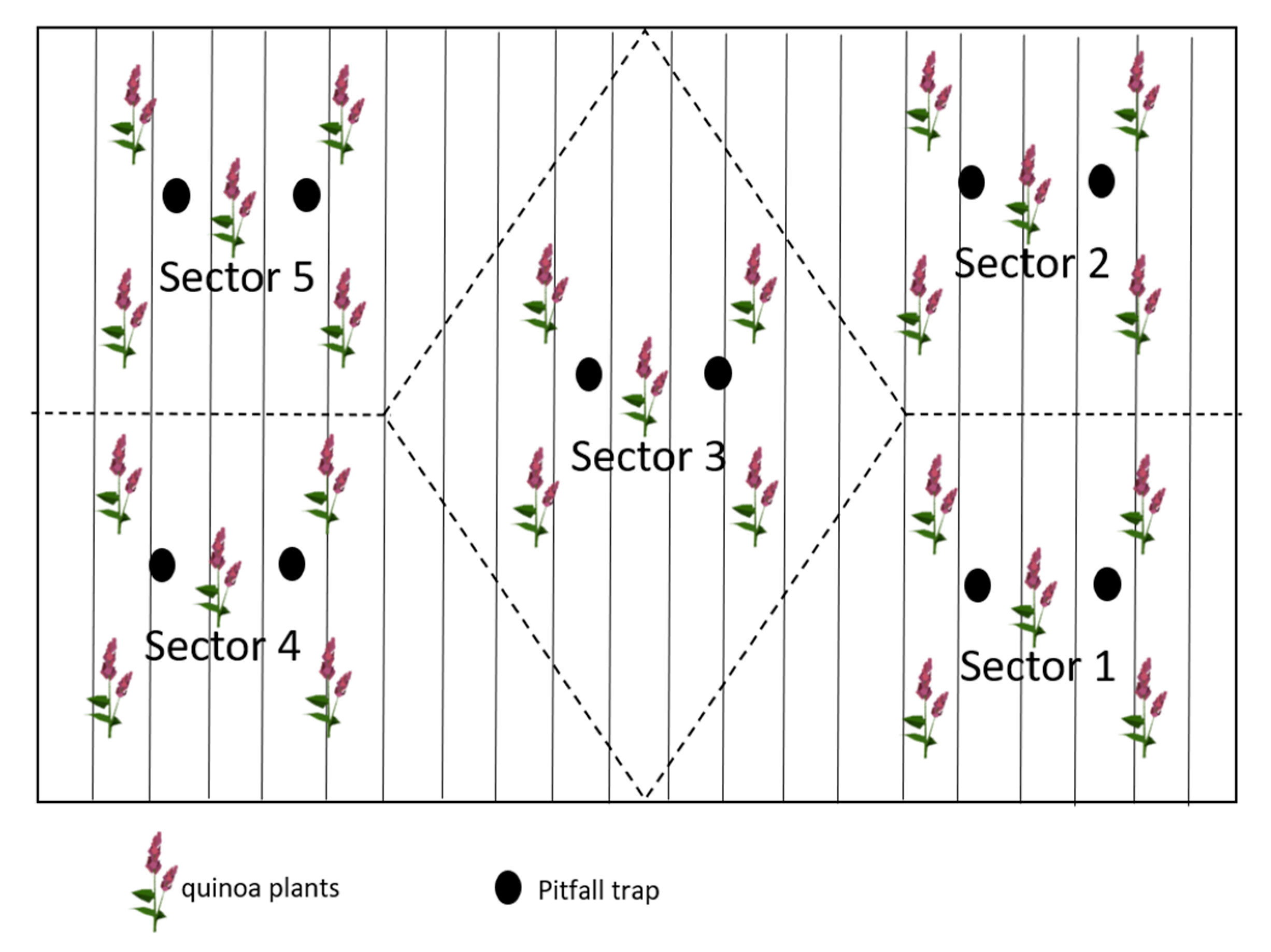
2.2. Sampling Procedure
2.3. Sample Processing and Identification
2.4. Data Analysis
3. Results
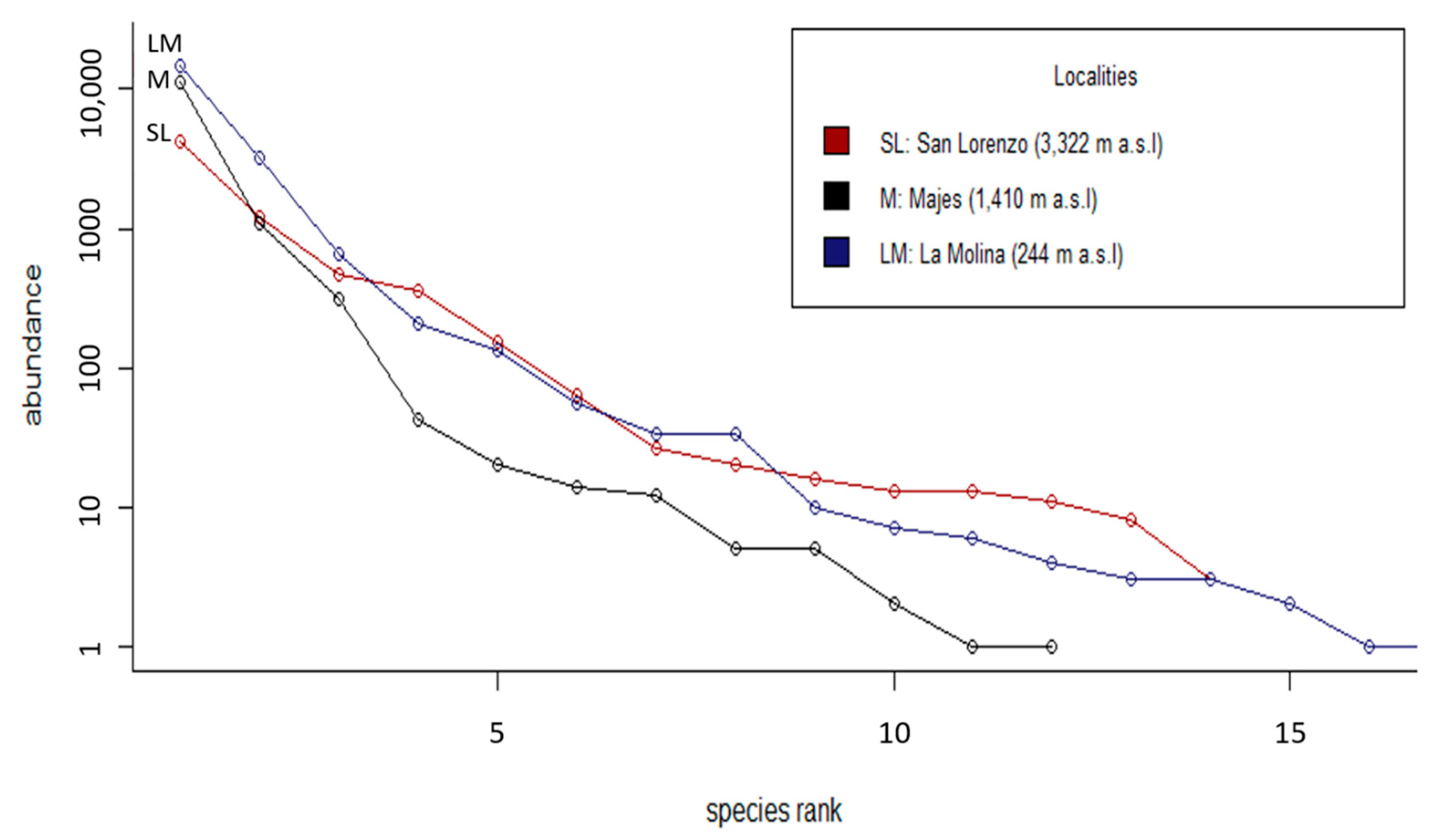
3.1. Abundance and Diversity of Phytophagous Insects
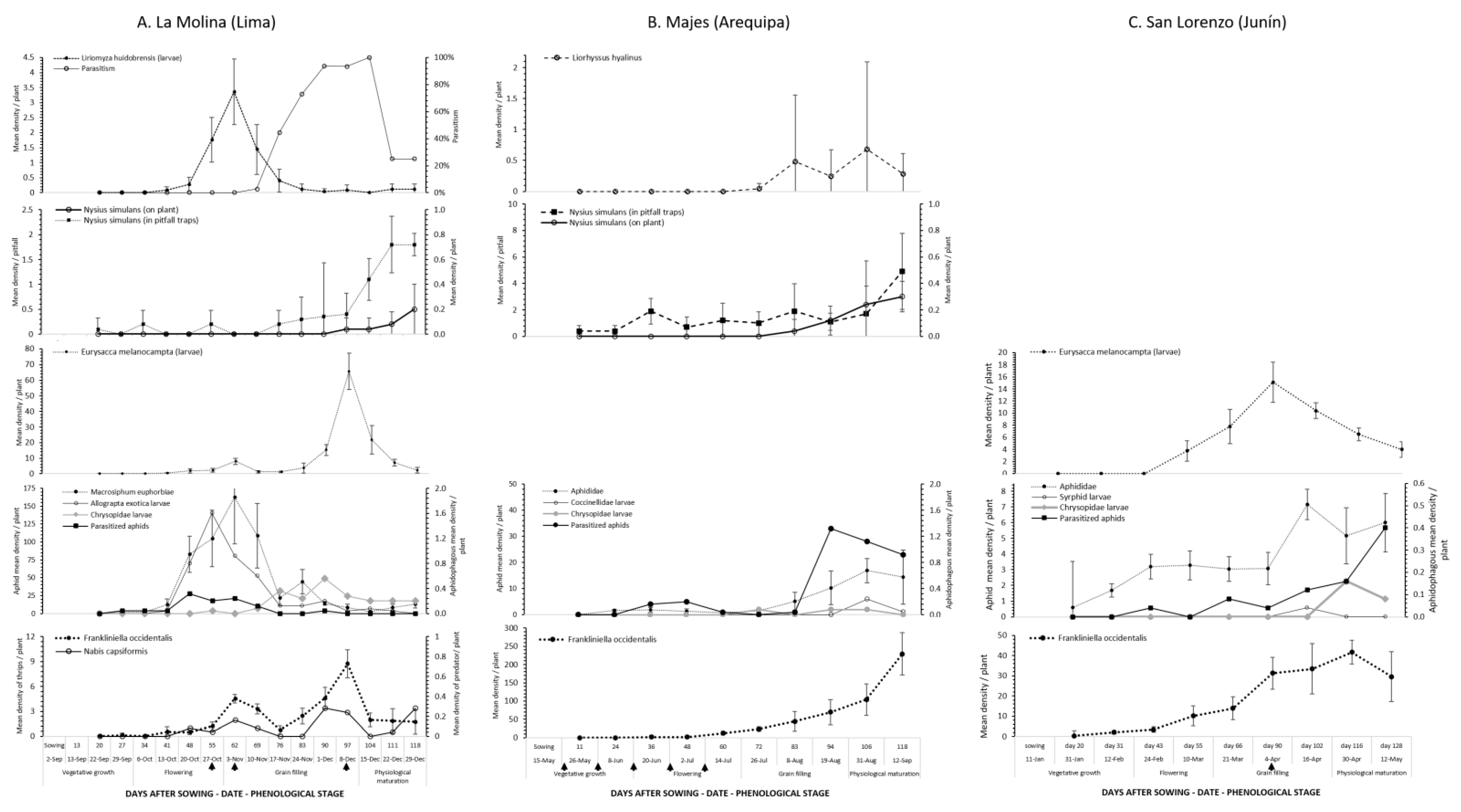
3.2. Phenology of Phytophagous Insects of Economic Importance
3.2.1. Quinoa Moth
3.2.2. Aphid–Natural Enemy Complex
3.2.3. Leafminer Flies and Natural Enemy Complex
3.2.4. Hemipteran Pests
3.2.5. Western Flower Thrips
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gómez-Pando, L.; Mujica, A.; Chura, E.; Canahua, A.; Pérez, A.; Tejada, T.; Villantoy, A.; Pocco, M.; Gonzáles, V.; Ccoñas, W. Perú: Capítulo número 5.2. In Estado del Arte de la Quinua en el Mundo en 2013; Bazile, D., Bertero, D., Nieto, C., Eds.; FAO (Santiago de Chile) y CIRAD: Montpellier, France, 2014; pp. 450–461. [Google Scholar]
- Gamboa, C.; Van den Broeck, G.; Maertens, M. Smallholders’ preferences for improved quinoa varieties in the Peruvian Andes. Sustainability 2018, 10, 3735. [Google Scholar] [CrossRef] [Green Version]
- Mercado, W.; Ubillus, K. Characterization of producers and quinoa supply chains in the Peruvian regions of Puno and Junín. Sci. Agrope. 2017, 8, 251–265. [Google Scholar] [CrossRef] [Green Version]
- Ministerio de Agricultura y Riego. Available online: file:///C:/Users/User/Downloads/boletin-quinua%20.pdf (accessed on 1 May 2020).
- Bedoya-Perales, N.; Pumi, G.; Mujica, A.; Talamini, E.; Domingos Padula, A. Quinoa expansion in Peru and its implications for land use management. Sustainability 2018, 10, 532. [Google Scholar] [CrossRef] [Green Version]
- Bedoya-Perales, N.; Pumi, G.; Talamini, E.; Domingos Padula, A. The quinoa boom in Peru: Will land competition threaten sustainability in one of the cradles of agriculture? Land Use Policy 2018, 79, 475–480. [Google Scholar] [CrossRef]
- Cruces, L.; Callohuari, Y.; Carrera, C. Quinua: Manejo Integrado de Plagas. Estrategias en el Cultivo de Quinua Para Fortalecer el Sistema Agroalimentario en la Zona Andina; Organización de las Naciones Unidas para la Alimentación y la Agricultura: Santiago, Chile, 2016; pp. 2–71. [Google Scholar]
- Latorre, J. Is Quinoa Cultivation on the Coastal Desert of Peru Sustainable? A Case Study from Majes, Arequipa. Master’s Thesis, Aarhus University, Aarhus, Denmark, 2017. [Google Scholar]
- Cruces, L.; de la Peña, E.; De Clercq, P. Insect diversity associated with quinoa (Chenopodium quinoa Willd.) in three altitudinal production zones of Peru. Int. J. Trop. Insect Sci. 2020, 40, 955–968. [Google Scholar] [CrossRef]
- Saravia, R.; Plata, G.; Gandarillas, A. Plagas y Enfermedades del Cultivo de Quinua; Fundación PROINPA: Cochabamba, Bolivia, 2014; pp. 9–76. [Google Scholar]
- Valoy, M.; Reguilón, C.; Podazza, G. The potential of using natural enemies and chemical compounds in quinoa for biological control of insect pests. In Quinoa: Improvement and Sustainable Production; Murphy, K.S., Matanguihan, J., Eds.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 63–86. [Google Scholar]
- Blackman, R.; Eastop, V. Aphids on the World’s Crops: An Identification and Information Guide, 2nd ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2000; pp. 1–466. [Google Scholar]
- Blackman, R.; Eastop, V. Aphids on the World’s Herbaceous Plants and Shrubs; John Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 1, pp. 1–1020. [Google Scholar]
- Stary, P. A review of the Aphidius species (Hymenoptera: Aphidiidae) of Europe. Annot. Zool. Bot. Bratisl. 1973, 85, 1–85. [Google Scholar]
- Carver, M.; Franzmann, B. Lysiphlebus Foerster (Hymenoptera: Braconidae: Aphidiinae) in Australia. Aust. J. Entomol. 2001, 40, 198–201. [Google Scholar] [CrossRef]
- Kavallieratos, N.; Tomanović, Ň.; Starý, P.; ňikić, V.; Petrović-Obradović, O. Parasitoids (Hymenoptera: Braconidae: Aphidiinae) attacking aphids feeding on Solanaceae and Cucurbitaceae crops in Southeastern Europe: Aphidiine-aphid-plant associations and key. Ann. Entomol. Soc. Am. 2010, 103, 153–164. [Google Scholar] [CrossRef]
- Kavallieratos, N.; Tomanović, Ž.; Petrović, A.; Janković, M.; Starý, P.; Yovkova, M.; Athanassiou, C. Review and key for the identification of parasitoids (Hymenoptera: Braconidae: Aphidiinae) of aphids infesting herbaceous and shrubby ornamental plants in Southeastern Europe. Ann. Entomol. Soc. Am. 2013, 106, 294–309. [Google Scholar] [CrossRef]
- Castro, V.; Araya, J. Clave de identificación de huevos, larvas y pupas de Allograpta (Diptera: Syrphidae) comunes en la zona central de Chile. Bol. Sanid. Veg. Plagas 2012, 38, 83–94. [Google Scholar]
- Bousquet, Y. Illustrated Identification Guide to Adults and Larvae of Northeastern North American Ground Beetles (Coleoptera, Carabidae); Pensoft: Sofia, Bulgaria, 2010; pp. 58–306. [Google Scholar]
- Moret, P. Contribution à la connaissance du genre néotropical Blennidus Motschulsky, 1865. Bull. Société Entomol. Fr. 1995, 100, 489–500. [Google Scholar]
- Moret, P. Clave de identificación para los géneros de Carabidae (Coleoptera) presentes en los páramos del Ecuador y del sur de Colombia. Rev. Colomb. Entomol. 2003, 29, 185–190. [Google Scholar]
- Straneo, S. Sul genere Blennidus Motschulsky 1865 (Col. Carabidae, Pterostichini). Boll. Mus. Reg. Sci. Nat. Torino 1986, 4, 369–393. [Google Scholar]
- Krysan, J.; Branson, T.; Schroeder, R.; Steiner, W., Jr. Elevation of Diabrotica sicuanica (Coleoptera: Chrysomelidae) to the species level with notes on the altitudinal distribution of Diabrotica species in the Cuzco department of Peru. Entomol. News 1984, 95, 91–98. [Google Scholar]
- Biondi, M.; D’Alessandro, P. Afrotropical flea beetle genera: A key to their identification, updated catalogue and biogeographical analysis (Coleoptera, Chrysomelidae, Galerucinae, Alticini). Zookeys 2012, 1–158. [Google Scholar] [CrossRef] [Green Version]
- Key to the World Genera of Eulophidae Parasitoids (Hymenoptera) of Leafmining Agromyzidae (Diptera). Available online: https://keys.lucidcentral.org/keys/v3/eulophidae_parasitoids/ (accessed on 10 October 2018).
- Povolny, D. Gnorimoschemini of Southern South America. II the genus Eurysacca (Lepidoptera Gelechiidae). Steenstrupia 1986, 12, 1–47. [Google Scholar]
- Henry, T.; Dellapé, P.; de Paula, A. The big-eyed bugs, chinch bugs, and seed bugs (Lygaeoidea). In True Bugs (Heteroptera) of the Neotropics; Panizzi, A., Grazia, J., Eds.; Springer: Dordrecht, The Netherlands, 2015; pp. 459–514. [Google Scholar]
- Bouček, Z.; Rasplus, J. Illustrated Key to West-Palearctic Genera of Pteromalidae (Hymenoptera: Chalcidoidea); Institut National de la Recherche Agronomique (INRA): Versailles, Paris, France, 1991; pp. 22–113. [Google Scholar]
- Horton, D.; Miliczky, E.; Lewis, T.; Cooper, W.; Waters, T.; Wohleb, C.; Zack, R.; Johnson, D.; Jensen, A. New North American records for the old world psyllid Heterotrioza chenopodii (Reuter) (Hemiptera: Psylloidea: Triozidae) with biological observations. Proc. Entomol. Soc. Wash. 2018, 120, 134–152. [Google Scholar] [CrossRef]
- Göllner-Scheiding, U. Revision der gattung Liorhyssus Stål, 1870 (Heteroptera, Rhopalidae). Dtsch. Entomol. Z. 1976, 23, 181–206. [Google Scholar] [CrossRef]
- Cornelis, M.; Quiran, E.; Coscaron, M. The scentless plant bug, Liorhyssus hyalinus (Fabricius) (Hemiptera: Heteroptera: Rhopalidae): Description of immature stages and notes on its life history. Zootaxa 2012, 3525, 83–88. [Google Scholar] [CrossRef]
- Spencer, K. Agromyzidae (Diptera) of Economic Importance; Springer Science & Business Media: Dordrecht, The Netherlands, 1973; Volume 9, pp. 215–219. [Google Scholar]
- Korytkowski, C. Contribución al conocimiento de los Agromyzidae (Diptera: Muscomorpha) en el Perú. Rev. Peru. Entomol. 2014, 49, 1–106. [Google Scholar]
- Cornelis, M.; Coscarón, M. The Nabidae (Insecta, Hemiptera, Heteroptera) of Argentina. ZooKeys 2013, 333, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Pall, J.; Kihn, R.; Diez, F. A review of genus Nysius Dallas in Argentina (Hemiptera: Heteroptera: Orsillidae). Zootaxa 2016, 4132, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Burckhardt, D. Jumping plant lice (Homoptera: Psylloidea) of the temperate neotropical region. Part 1: Psyllidae (Subfamilies Aphalarinae, Rhinocolinae and Aphalaroidinae). Zool. J. Linn. Soc. 1987, 89, 299–392. [Google Scholar] [CrossRef]
- Hodkinson, I.; White, I. Handbooks for the Identification of British Insects: Homoptera: Psylloidea; Royal Entomological Society of London: Queen’s Gate, London, UK, 1979; Volume 2, pp. 1–76. [Google Scholar]
- Derocles, S.; Le Ralec, A.; Plantegenest, M.; Chaubet, B.; Cruaud, C.; Cruaud, A.; Rasplus, J. Identification of molecular markers for DNA barcoding in the Aphidiinae (Hym. Braconidae). Mol. Ecol. Resour. 2012, 12, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Masuda, T.; Mochizuki, A.; Konishi, K.; Tokumaru, S.; Ueno, K.; Yamaguchi, T. Primer design for identifying economically important Liriomyza species (Diptera: Agromyzidae) by multiplex PCR. Mol. Ecol. Resour. 2013, 13, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Shufran, K.; Puterka, G. DNA barcoding to identify all life stages of holocyclic cereal aphids (Hemiptera: Aphididae) on wheat and other Poaceae. Ann. Entomol. Soc. Am. 2011, 104, 39–42. [Google Scholar] [CrossRef] [Green Version]
- Harbhajan, K.; Kaur, S. DNA barcoding of six species of family Rhopalidae (Insecta: Hemiptera: Heteroptera) from India. Int. J. Life Sci. 2017, 5, 517–526. [Google Scholar]
- Ding, T.; Chi, H.; Gökçe, A.; Gao, Y.; Zhang, B. Demographic analysis of arrhenotokous parthenogenesis and bisexual reproduction of Frankliniella occidentalis (Pergande) (Thysanoptera: Thripidae). Sci. Rep. 2018, 3346, 1–10. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2017; Available online: https://www.r-project.org/ (accessed on 30 June 2019).
- Oksanen, J.; Blanchet, F.; Michael, F.; Kindt, R.; Legendre, P.; Dan McGlinn, P.; Minchin, P.; O’hara, R.; Simpson, G.; Solymos, P.; et al. Package ‘Vegan’. Community Ecology Package, Version 2.5. 2019. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 10 May 2020).
- De Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research. R Package Version, 1.3. 2020. Available online: https://cran.r-project.org/web/packages/agricolae/agricolae.pdf (accessed on 20 July 2020).
- Ripley, B.; Venables, B.; Bates, D.; Hornik, K.; Gebhardt, A.; Firth, D.; Ripley, M. Package ‘Mass’. CRAN Repos. Httpcran R-Proj. 2020. Available online: https://cran.r-project.org/web/packages/MASS/MASS.pdf (accessed on 10 October 2020).
- Mujica, N.; Kroschel, J. Leafminer fly (Diptera: Agromyzidae) occurrence, distribution, and parasitoid associations in field and vegetable crops along the Peruvian Coast. Environ. Entomol. 2011, 40, 217–230. [Google Scholar] [CrossRef] [Green Version]
- Yábar, E.; Gianoli, E.; Echegaray, E. Insect pests and natural enemies in two varieties of quinua (Chenopodium Quinoa) at Cusco, Peru. J. Appl. Entomol. 2002, 126, 275–280. [Google Scholar] [CrossRef] [Green Version]
- Rasmussen, C.; Lagnaoui, A.; Esbjerg, P. Advances in the knowledge of quinoa pests. Food Rev. Int. 2003, 19, 61–75. [Google Scholar] [CrossRef] [Green Version]
- Quispe, R.; Saravia, R.; Villca, M.; Lino, V. Complejo polilla. In Plagas y Enfermedades del Cultivo de Quinua; Saravia, R., Plata, G., Gandarillas, G., Eds.; Fundación PROINPA: Cochabamba, Bolivia, 2014; pp. 49–62. [Google Scholar]
- Blanco, A. Umbral Económico de Kcona Kcona, Eurysacca melanocampta (Lepidoptera Gelechiidae) en Quinua (Chenopodium quinoa Willd). Licentiate Thesis, Universidad Nacional del Altiplano, Puno, Perú, 1994. [Google Scholar]
- Villanueva, S. Determinación del “Umbral Económico” y “Nivel Crítico” de “Kcona Kcona” (Scrobipalpula sp.) en Quinua (Chenopodium quinoa Willd). Licentiate Thesis, Universidad Nacional del Altiplano, Puno, Peru, 1978. [Google Scholar]
- Crespo, L.; Saravia, R. Insectos plaga ocasionales en el cultivo de quinua. In Plagas y Enfermedades del Cultivo de Quinua; Saravia, R., Plata, G., Gandarillas, G., Eds.; Fundación PROINPA: Cochabamba, Bolivia, 2014; pp. 63–81. [Google Scholar]
- Bale, J.; Harrington, R.; Clough, M. Low temperature mortality of the peach-potato aphid Myzus persicae. Ecol. Entomol. 1988, 13, 121–129. [Google Scholar] [CrossRef]
- De Conti, B.; Bueno, V.; Sampaio, M.; Van Lenteren, J. Development and survival of Aulacorthum solani, Macrosiphum euphorbiae and Uroleucon ambrosiae at six temperatures. Bull. Insectol. 2011, 64, 63–68. [Google Scholar]
- Sabahi, Q.; Rasekh, A.; Michaud, J. Toxicity of three insecticides to Lysiphlebus Fabarum, a parasitoid of the black bean aphid, Aphis Fabae. J. Insect Sci. 2011, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Thompson, F.; Rotheray, G.; Zumbado, M. Syrphidae (Flower flies). In Manual of Central American Diptera; Brown, B., Borkent, A., Cumming, J., Wood, D., Woodley, N., Zumbado, M., Eds.; NRC Research Press: Ottawa, ON, Canada, 2010; Volume 2, pp. 7637–7690. [Google Scholar]
- Campos, D.; Sharkey, M. Familia Braconidae. In Introducción a Los Hymenoptera de La Región Neotropical; Fernández, F., Sharkey, M., Eds.; Sociedad Colombiana de Entomología y Universidad Nacional de Colombia: Bogotá, DC, USA, 2006; pp. 3313–3365. [Google Scholar]
- Heckman, C. Neuroptera (Including Megaloptera); Springer: Cham, Zug, Switzerland, 2017; pp. 2–72. [Google Scholar]
- Horn, D. Selective mortality of parasitoids and predators of Myzus persicae on collards treated with malathion, carbaryl, or Bacillus thuringiensis. Entomol. Exp. Appl. 1983, 34, 208–211. [Google Scholar] [CrossRef]
- Drescher, W.; Geusen-Pfister, H. Comparative testing of the oral toxicity of acephate, dimethoate and methomyl to honeybees, bumblebees and Syrphidae. Acta Hortic. 1991, 288, 133–138. [Google Scholar] [CrossRef]
- Cranshaw, W.; Kondratieff, B.; Qian, T. Insects associated with quinoa, Chenopodium quinoa, in Colorado. J. Kans. Entomol. Soc. 1990, 63, 195–199. [Google Scholar]
- Jensen, S. Insecticide resistance in the Western Flower Thrips, Frankliniella occidentalis. Integr. Pest Manag. Rev. 2000, 5, 131–146. [Google Scholar] [CrossRef]
- Mujica, N.; Sporleder, M.; Carhuapoma, P.; Kroschel, J. A Temperature-dependent phenology model for Liriomyza huidobrensis (Diptera: Agromyzidae). J. Econ. Entomol. 2017, 110, 1333–1344. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, G.; Redolfi de Huiza, I. Liriomyza huidobrensis y sus parasitoides en papa cultivada en Rímac y Cañete, 1986. Rev. Peru. Entomol. 1988, 31, 110–112. [Google Scholar]
- Burgos, A. Efecto de La Temperature en la Biología y Comportamiento de Diglyphus websteri (Crawford) (Hymenoptera: Eulophidae). Master’s Thesis, Universidad Nacional Agraria La Molina, Lima, Peru, 2013. [Google Scholar]
- Mujica, N.; Valencia, C.; Ramirez, L.; Prudencio, C.; Kroschel, J. Temperature-Dependent Development of Three Parasitoids of the Leafminer Fly Liriomyza huidobrensis. Tropical roots and tubers in a changing climate: A convenient opportunity for the world. In Proceedings of the Fifteenth Triennial Symposium of the International Society for Tropical Root Crops, Lima, Peru, 2–6 November 2009; pp. 1711–1777. [Google Scholar]
- Cisneros, F. Control Químico de Las Plagas Agrícolas; Sociedad Entomológica del Perú: Lima, Peru, 2012; pp. 7–82. [Google Scholar]
- Sparks, T.; Nauen, R. IRAC: Mode of action classification and insecticide resistance management. Pestic. Biochem. Physiol. 2015, 121, 122–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hénault-Ethier, L. Health and Environmental Impacts of Pyrethroid Insecticides: What We Know, What We Don’t Know and What We Should Do about It. Executive Summary and Scientific Literature Review. Prepared for Équiterre. Montreal 2015. Available online: https://www.equiterre.org/sites/fichiers/health_and_environmental_impacts_of_pyrethroid_insecticides_full_report_en.pdf (accessed on 20 May 2020).
- Croft, B.; Whalon, M. Selective toxicity of pyrethroid insecticides to arthropod natural enemies and pests of agricultural crops. Entomophaga 1982, 27, 3–21. [Google Scholar] [CrossRef]
| Localities | |||
|---|---|---|---|
| La Molina District, Province of Lima, Department of Lima | Majes District, Province of Caylloma, Department of Arequipa | San Lorenzo District, Province of Jauja, Department of Junín | |
| Mean monthly temp. (minimum–maximum) | 16.67–22.97 °C | 10.52–25.52 °C | 6.96–20.06 °C |
| Mean monthly RH (minimum–maximum) | 74.65–96.25% | 31.2–60.2% | 65.51–75.75% |
| Total precipitation during the sampling period | 5.9 mm | 0 mm | 276.2 mm |
| Sowing–harvest | 2 September 2015–10 January 2016 | 15 May 2016–20 September 2016 | 11 January 2016–20 May 2016 |
| Field dimensions | 85 m × 96.3 m (0.66 ha) | 93.5 m × 96.3 m (0.9 ha) | 102 m × 96 m (0.98 ha) |
| Variety | Pasancalla | Inia Salcedo | Pasancalla |
| Irrigation | Surface irrigation 100 irrigation furrows of 85 cm width, 10 irrigation blocks | Drip irrigation 110 irrigation furrows of 85 cm width, 4 irrigation blocks | Rain-fed 120 furrows of 85 cm width, 12 irrigation blocks |
| Soil type | Clay loam | Loamy sand | Loam |
| Neighbouring crops | Quinoa (Chenopodium quinoa); barley (Hordeum vulgare); kiwicha (Amaranthus caudatus); wheat (Triticum spp.) | Quinoa (Chenopodium quinoa); artichoke (Cynara scolymus) | Quinoa (Chenopodium quinoa); corn (Zea mays); potato (Solanum tuberosum) |
| Fungicides | 1° benomyl (15 September 2015); 2° metalaxyl + mancozeb (4 October 2015); 3° dimethomorph (20 October 2015); 4° propamocarb + fluopicolide (3 November 2015) | 1° benomyl (22 May 2016); 2° metalaxyl + mancozeb (12 June 2016); 3° dimethomorph (26 June 2016); 4° propamocarb + fluopicolide (10 July 2016) | 1° benomyl (25 January 2016); 2° metalaxyl + mancozeb (14 February 2016); 3° dimethomorph (28 February 2016); 4° propamocarb + fluopicolide (15 March 2016) |
| Insecticides | 1° Bacillus thuringiensis (27 October 2015); 2° dimethoate + methomyl (3 November 2015); 3° emamectin benzoate + methomyl (8 December 2015) | 1° alpha-cypermethrin (22 May 2016); 2° emamectin benzoate (29 May 2016); 3° zeta-cypermethrin (12 June 2016); 4° alpha-cypermethrin (26 June 2016); 5° alpha-cypermethrin + emamectin benzoate (10 July 2016) | 1° Bacillus thuringiensis + emamectin benzoate (4 April 2016) |
| Weed management | Manual control | Manual control | Manual control |
| Previous crop | Wheat | Corn | Fallow period of 6 months |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruces, L.; Peña, E.d.l.; De Clercq, P. Seasonal Phenology of the Major Insect Pests of Quinoa (Chenopodium quinoa Willd.) and Their Natural Enemies in a Traditional Zone and Two New Production Zones of Peru. Agriculture 2020, 10, 644. https://doi.org/10.3390/agriculture10120644
Cruces L, Peña Edl, De Clercq P. Seasonal Phenology of the Major Insect Pests of Quinoa (Chenopodium quinoa Willd.) and Their Natural Enemies in a Traditional Zone and Two New Production Zones of Peru. Agriculture. 2020; 10(12):644. https://doi.org/10.3390/agriculture10120644
Chicago/Turabian StyleCruces, Luis, Eduardo de la Peña, and Patrick De Clercq. 2020. "Seasonal Phenology of the Major Insect Pests of Quinoa (Chenopodium quinoa Willd.) and Their Natural Enemies in a Traditional Zone and Two New Production Zones of Peru" Agriculture 10, no. 12: 644. https://doi.org/10.3390/agriculture10120644
APA StyleCruces, L., Peña, E. d. l., & De Clercq, P. (2020). Seasonal Phenology of the Major Insect Pests of Quinoa (Chenopodium quinoa Willd.) and Their Natural Enemies in a Traditional Zone and Two New Production Zones of Peru. Agriculture, 10(12), 644. https://doi.org/10.3390/agriculture10120644







