Effect of Vineyard Floor Management on Seasonal Changes of Cultivable Fungal Diversity in the Rhizosphere
Abstract
1. Introduction
2. Materials and Methods
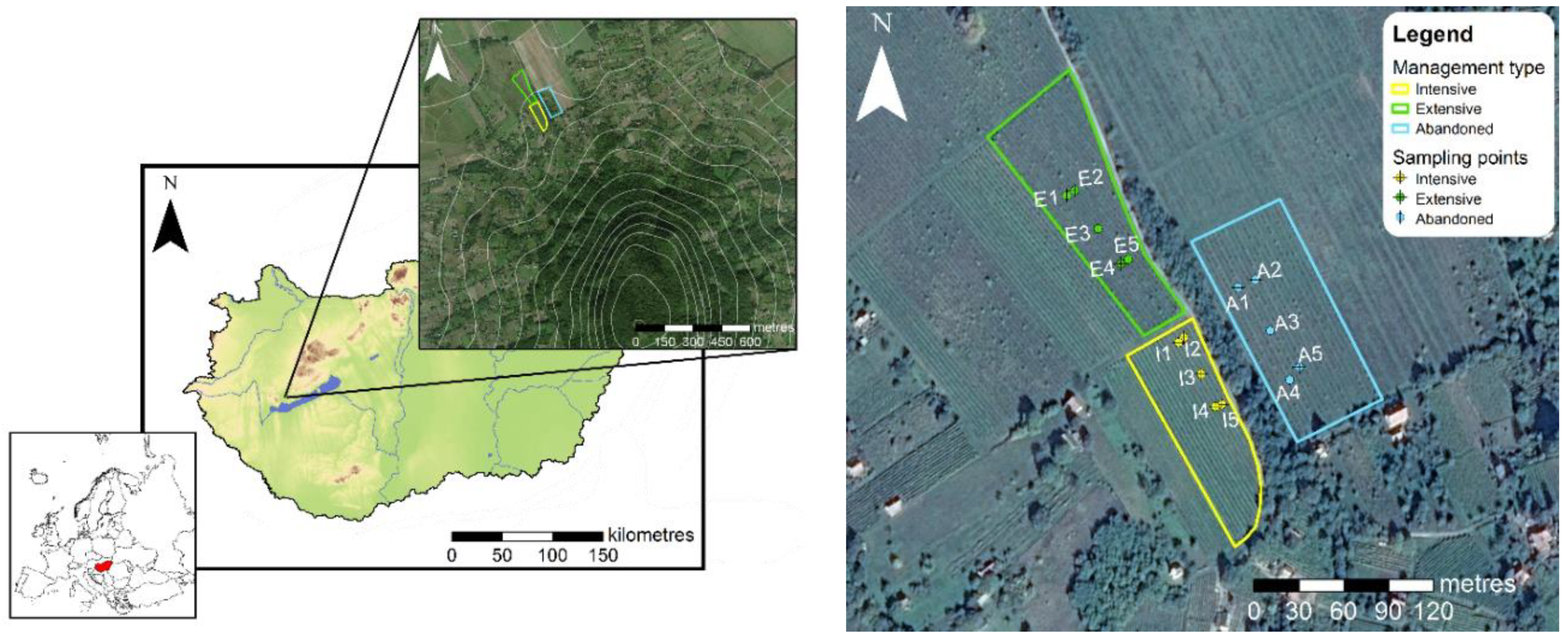
2.1. Sampling
2.2. Cultivation Methods and Isolation
2.3. Taxonomic Identification of the Fungi Cultivated
2.4. Physical Parameters
2.5. Calculation of Diversity Indices
- → number of species.
- → exponential of Shannon index.
- → reciprocal of Simpson index.
3. Results
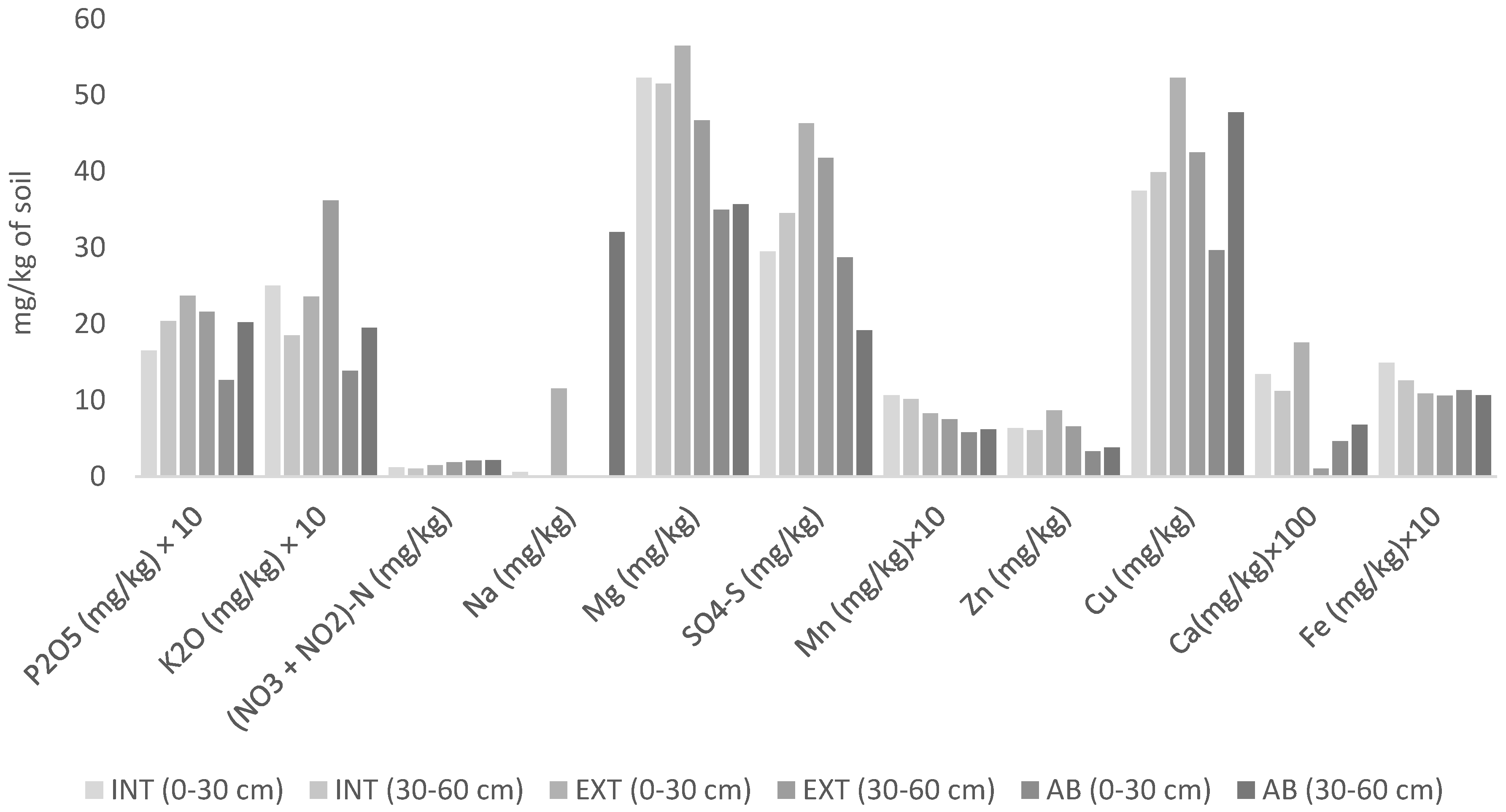
3.1. Results of Soil Analysis
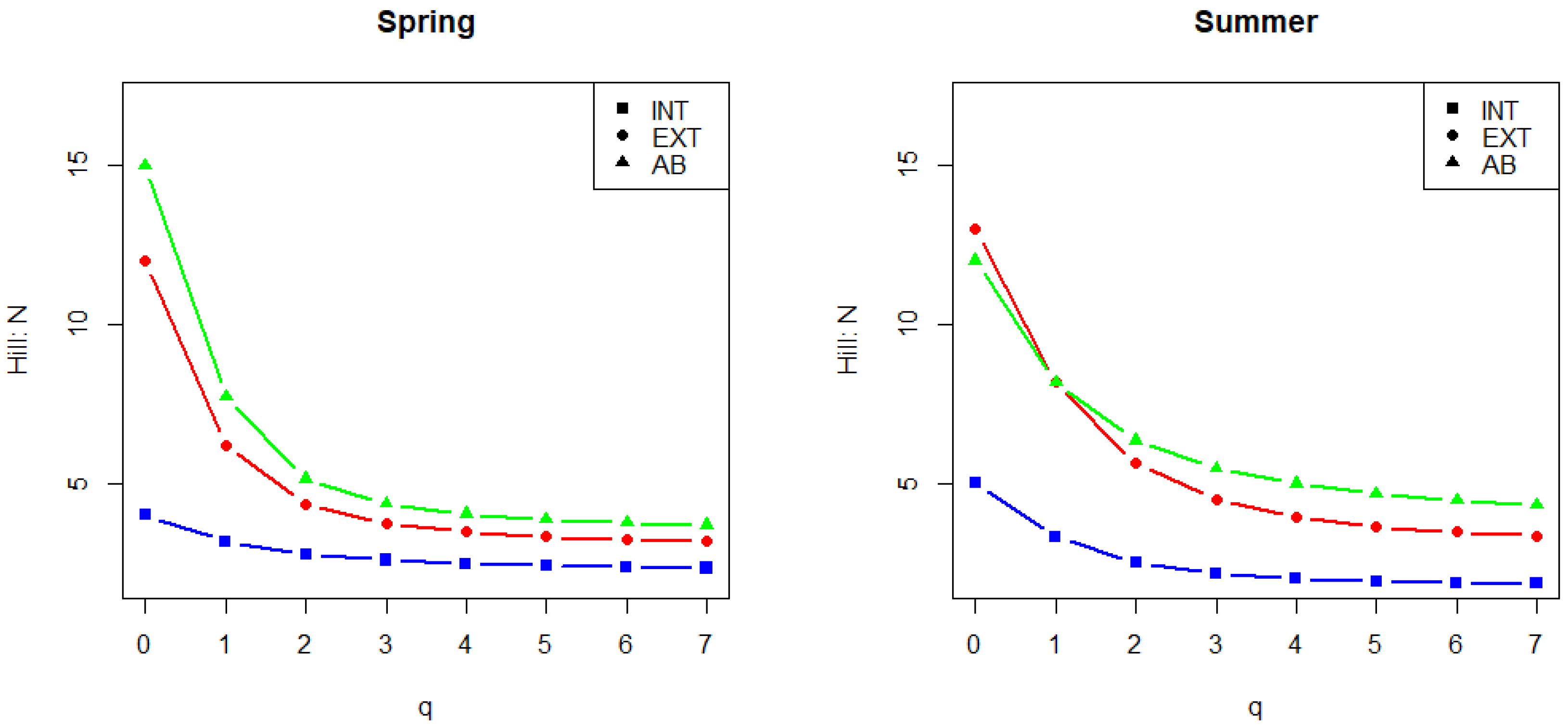
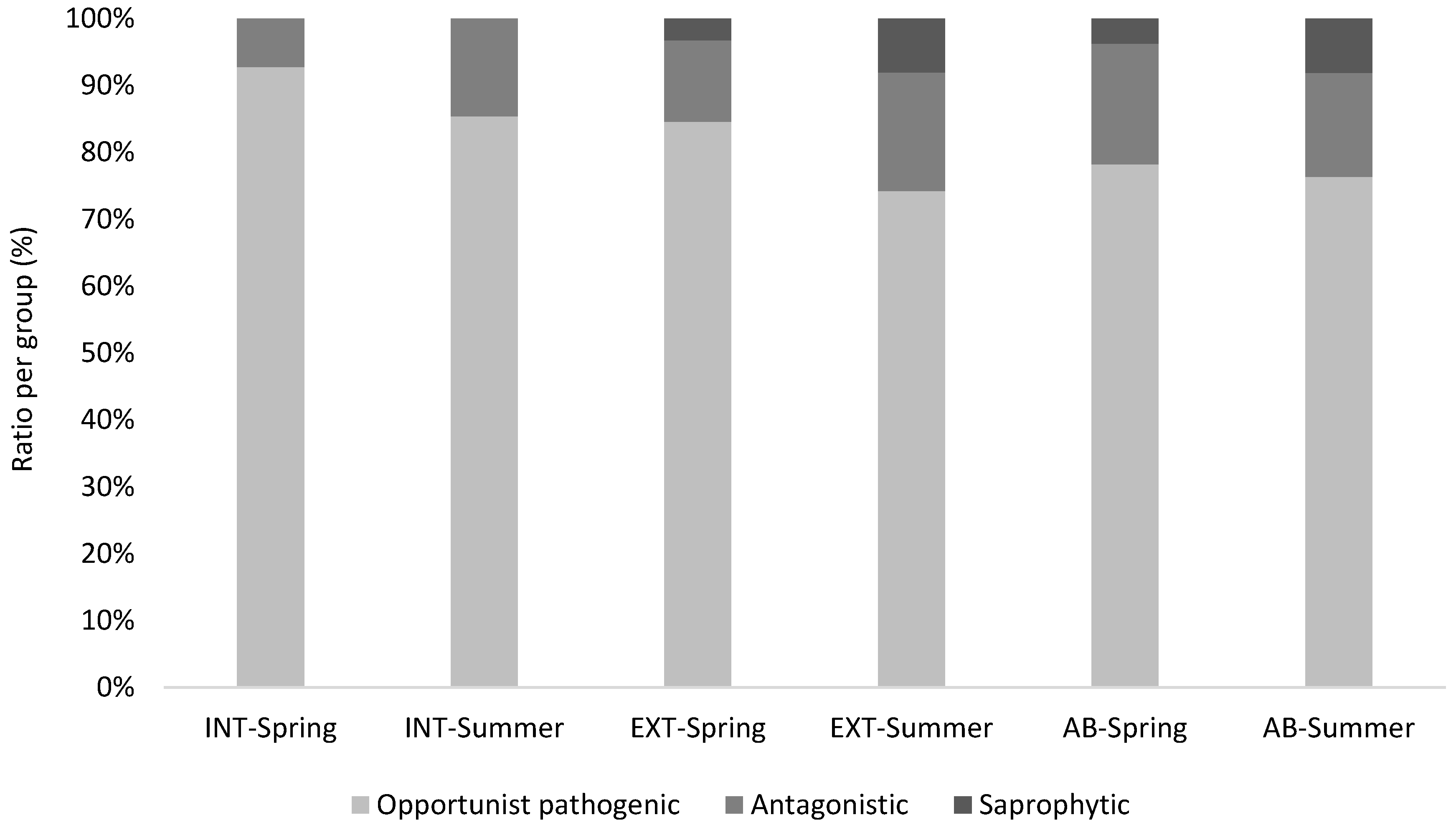
3.2. Mycological Results in Connection with Vineyard Floor Management
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stankovics, P.; Montanarella, L.; Kassai, P.; Tóth, G.; Tóth, Z. The interrelations of land ownership, soil protection and privileges of capital in the aspect of land take. Land Use Policy 2020, 99, 105071. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. Fems Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef] [PubMed]
- Döring, J.; Collins, C.; Frisch, M.; Kauer, R. Organic and Biodynamic Viticulture Affect Biodiversity and Properties of Vine and Wine: A Systematic Quantitative Review. Am. J. Enol. Vitic. 2019, 70, 221–242. [Google Scholar] [CrossRef]
- Huber, L.; Hoffmann, M.; Rühl, E.H.; Kirchmair, M. Disease suppressiveness of vineyard soils infested with grape phylloxera. Acta Hortic. 2009, 41–52. [Google Scholar] [CrossRef]
- Pancher, M.; Ceol, M.; Corneo, P.E.; Longa, C.M.O.; Yousaf, S.; Pertot, I.; Campisano, A. Fungal Endophytic Communities in Grapevines (Vitis vinifera L.) Respond to Crop Management. Appl. Environ. Microbiol. 2012, 78, 4308–4317. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, M.M.; Menéndez, C.M. Influence of seasonality and management practices on diversity and composition of fungal communities in vineyard soils. Appl. Soil Ecol. 2019, 135, 113–119. [Google Scholar] [CrossRef]
- Feliciano, A.J.; Gubler, W.D. Histological investigations on infection of grape roots and shoots by Phaeoacremonium spp. Phytopathol. Mediterr. 2001, 40, S387–S393. [Google Scholar]
- Willsey, T.; Chatterton, S.; Cárcamo, H. Interactions of Root-Feeding Insects with Fungal and Oomycete Plant Pathogens. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Benheim, D.; Rochfort, S.; Robertson, E.; Potter, I.D.; Powell, K.S. Grape phylloxera (Daktulosphaira vitifoliae)—A review of potential detection and alternative management options. Ann. Appl. Biol. 2012, 161, 91–115. [Google Scholar] [CrossRef]
- Granett, J.; Omer, A.D.; Pessereau, P.; Walker, M.A. Fungal infections of grapevine roots in phylloxera-infested vineyards. Vitis 1998, 37, 39–42. [Google Scholar]
- Edwards, J.; Norng, S.; Powell, K.S.; Granett, J. Relationships between grape phylloxera abundance, fungal interactions and grapevine decline. Acta Hortic. 2007, 151–157. [Google Scholar] [CrossRef]
- Vincent, C.; Hallman, G.; Panneton, B.; Fleurat-Lessard, F. Management of Agricultural Insects with Physical Control Methods. Ann. Rev. Entomol. 2003, 48, 261–281. [Google Scholar] [CrossRef]
- Poli, A.; Lazzari, A.; Prigione, V.; Voyron, S.; Spadaro, D.; Varese, G.C. Influence of plant genotype on the cultivable fungi associated to tomato rhizosphere and roots in different soils. Fungal Biol. 2016, 120, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Gilinsky, A.; Newton, S.K.; Vega, R.F. Sustainability in the global wine industry: Concepts and cases. Agric. Agric. Sci. Procedia 2016, 8, 37–49. [Google Scholar] [CrossRef]
- Vukicevich, E.; Thomas Lowery, D.; Úrbez-Torres, J.R.; Bowen, P.; Hart, M. Groundcover management changes grapevine root fungal communities and plant-soil feedback. Plant Soil 2018, 424, 419–433. [Google Scholar] [CrossRef]
- Xia, Y.; Sahib, M.R.; Amna, A.; Opiyo, S.O.; Zhao, Z.; Gao, Y.G. Culturable endophytic fungal communities associated with plants in organic and conventional farming systems and their effects on plant growth. Sci. Rep. 2019, 9, 1669. [Google Scholar] [CrossRef] [PubMed]
- Torres, R.L.; Lloreda, M.D.L.F.; Gonzalez, P.J.; García-Gutierrez, J.R.L.; Trujillo, P.B. Effect of soil management strategies on the characteristics of the grapevine root system in irrigated vineyards under semi-arid conditions. Aust. J. Grape Wine Res. 2018, 24, 439–449. [Google Scholar] [CrossRef]
- Birkas, M.; Biro, B.; Kisić, I.; Stipešević, B. The importance of the soil microbial status–A review of research and practical experience in the Pannonian region. In Soil Tillage and Microbial Activities; Research Signpost: Thiruvananthapuram, India, 2011; pp. 19–36. [Google Scholar]
- Radić, T.; Likar, M.; Hančević, K.; Bogdanović, I.; Pasković, I. Occurrence of root endophytic fungi in organic versus conventional vineyards on the Croatian coast. Agric. Ecosyst. Environ. 2014, 192, 115–121. [Google Scholar] [CrossRef]
- Hendgen, M.; Hoppe, B.; Döring, J.; Friedel, M.; Kauer, R.; Frisch, M.; Dahl, A.; Kellner, H. Effects of different management regimes on microbial biodiversity in vineyard soils. Sci. Rep. 2018, 8, 9393. [Google Scholar] [CrossRef]
- Maeder, P.; Fliessbach, A.; Dubois, D.; Gunst, L.; Fried, P.; Niggli, U. Soil Fertility and Biodiversity in Organic Farming. Science 2002, 296, 1694–1697. [Google Scholar] [CrossRef]
- van Elsas, J.D.; Garbeva, P.; Salles, J. Effects of agronomical measures on the microbial diversity of soils as related to the suppression of soil-borne plant pathogens. Biodegradation 2002, 13, 29–40. [Google Scholar] [CrossRef]
- Alabouvette, C.; Steinberg, C. The soil as a reservoir for antagonists to plant diseases. In An Ecological and Societal Approach to Biological Control; Eilenberg, J., Hokkanen, H.M.T., Eds.; Progress in Biological Control; Springer: Dordrecht, The Netherlands, 2006; pp. 123–144. ISBN 978-1-4020-4401-4. [Google Scholar]
- Stirling, G.R.; Smith, M.K.; Smith, J.P.; Stirling, A.M.; Hamill, S.D. Organic inputs, tillage and rotation practices influence soil health and suppressiveness to soilborne pests and pathogens of ginger. Australas. Plant Pathol. 2012, 41, 99–112. [Google Scholar] [CrossRef]
- Jahn, R.; Blume, H.P.; Asio, V.B.; Spaargaren, O.; Schad, P. Guidelines for Soil Description, 4th ed.; FAO: Rome, Italy, 2006; ISBN 978-92-5-105521-2. [Google Scholar]
- von Arx, J.A. Plant Pathogenic Fungi; Lubrecht & Cramer Ltd.: Berlin, Germany, 1987; Volume 87. [Google Scholar]
- Kiffer, E.; Morelet, M. The Deuteromycetes—Mitosporic Fungi: Classification and Generic Keys; CRC Press: Boca Raton, FL, USA, 2011; ISBN 978-1-4822-9419-4. [Google Scholar]
- Leslie, J.; Summerell, B. The Fusarium Laboratory Manual; Blackwell Publishing: Hoboken, NJ, USA, 2006. [Google Scholar]
- Samson, R.A.; Houbraken, J.; Thrane, U. Food and Indoor Fungi; CBS KNAW Biodiversity Center Utrecht: Utrecht, The Netherlands, 2010. [Google Scholar]
- Deak, T. Handbook of Food Spoilage Yeasts; CRC Press: Boca Raton, FL, USA, 2007; ISBN 978-1-4200-4494-2. [Google Scholar]
- Magurran, E. Measuring Biological Diversity; Malden Blackwell Science Ltd.: Malden, MA, USA, 2004. [Google Scholar]
- Chao, A.; Chiu, C.-H.; Jost, L. Unifying Species Diversity, Phylogenetic Diversity, Functional Diversity, and Related Similarity and Differentiation Measures Through Hill Numbers. Annu. Rev. Ecol. Evol. Syst. 2014, 45, 297–324. [Google Scholar] [CrossRef]
- Fernández-Calviño, D.; Martín, A.; Arias-Estévez, M.; Bååth, E.; Díaz-Raviña, M. Microbial community structure of vineyard soils with different pH and copper content. Appl. Soil Ecol. 2010, 46, 276–282. [Google Scholar] [CrossRef]
- Lőrincz, A.; Sz Nagy, L.; Zanathy, G. Viticulture (Szőlőtermesztés); Mezőgazda Kiadó: Budapest, Hungary, 2015; ISBN 978-963-286-712-0. [Google Scholar]
- Omer, A.D.; Granett, J.; Wakeman, R.J. Pathogenicity of Fusarium oxysporum on Different Vitis Rootstocks. J. Phytopathol. 1999, 147, 433–436. [Google Scholar] [CrossRef]
- Omer, A.D.; Granett, J. Relationship between grape phylloxera and fungal infections in grapevine roots / Beziehungen zwischen der Reblaus und Pilzinfektionen an Wurzeln der Weinrebe. Z. Pflanzenkrankh. Pflanzenschutz/J. Plant Dis. Prot. 2000, 107, 285–294. [Google Scholar]
- Fourie, P.H.; Halleen, F.; van der Vyver, J.; Schreuder, W. Effect of Trichoderma treatments on the occurrence of decline pathogens in the roots and rootstocks of nursery grapevines. Phytopathol. Mediterr. 2001, 40, S473–S478. [Google Scholar]
- Halleen, F.; Crous, R.W.; Petrin, O. Fungi associated with healthy grapevine cuttings in nurseries, with special reference to pathogens involved in the decline of young vines. Australas. Plant Pathol. 2003, 32, 47–52. [Google Scholar] [CrossRef]
- Halleen, F.; Mostert, L.; Crous, P.W. Pathogenicity testing of lesser-known vascular fungi of grapevines. Australas. Plant Pathol. 2007, 36, 277–285. [Google Scholar] [CrossRef]
- Fătu, A.-C.; Dinu, M.M.; Oprea, M.; Andrei, A.-M. Microflora asociated to rhizosphere and rhizoplane of grapevines in some Romanian wine-growing areas. Rom. J. Plant Prot. 2014, 7, 103–110. [Google Scholar]
- Musetti, R.; Vecchione, A.; Stringher, L.; Borselli, S.; Zulini, L.; Marzani, C.; D’Ambrosio, M.; di Toppi, L.S.; Pertot, I. Inhibition of Sporulation and Ultrastructural Alterations of Grapevine Downy Mildew by the Endophytic Fungus Alternaria alternata. Phytopathol. 2006, 96, 689–698. [Google Scholar] [CrossRef]
- Armijo, G.; Schlechter, R.; Agurto, M.; Muñoz, D.; Nuñez, C.; Arce-Johnson, P. Grapevine Pathogenic Microorganisms: Understanding Infection Strategies and Host Response Scenarios. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Komárek, M.; Čadková, E.; Chrastný, V.; Bordas, F.; Bollinger, J.-C. Contamination of vineyard soils with fungicides: A review of environmental and toxicological aspects. Environ. Int. 2010, 36, 138–151. [Google Scholar] [CrossRef]
- Fleurat-Lessard, P.; Dédaldéchamp, F.; Thibault, F.; Béré, E.; Roblin, G. Antifungal effects of iron sulfate on grapevine fungal pathogens. Sci. Hortic. 2011, 130, 517–523. [Google Scholar] [CrossRef]
- Ge, C.-R.; Zhang, Q.-C. Microbial Community Structure and Enzyme Activities in a Sequence of Copper-Polluted Soils. Pedosphere 2011, 21, 164–169. [Google Scholar] [CrossRef]
- Cavani, L.; Manici, L.M.; Caputo, F.; Peruzzi, E.; Ciavatta, C. Ecological restoration of a copper polluted vineyard: Long-term impact of farmland abandonment on soil bio-chemical properties and microbial communities. J. Environ. Manag. 2016, 182, 37–47. [Google Scholar] [CrossRef]
- Keiblinger, K.M.; Schneider, M.; Gorfer, M.; Paumann, M.; Deltedesco, E.; Berger, H.; Jöchlinger, L.; Mentler, A.; Zechmeister-Boltenstern, S.; Soja, G.; et al. Assessment of Cu applications in two contrasting soils—Effects on soil microbial activity and the fungal community structure. Ecotoxicology 2018, 27, 217–233. [Google Scholar] [CrossRef]
- Holland, T.C.; Bowen, P.A.; Bogdanoff, C.P.; Lowery, T.D.; Shaposhnikova, O.; Smith, S.; Hart, M.M. Evaluating the diversity of soil microbial communities in vineyards relative to adjacent native ecosystems. Appl. Soil Ecol. 2016, 100, 91–103. [Google Scholar] [CrossRef]
- Bertsch, C.; Ramírez-Suero, M.; Magnin-Robert, M.; Larignon, P.; Chong, J.; Abou-Mansour, E.; Spagnolo, A.; Clément, C.; Fontaine, F. Grapevine trunk diseases: Complex and still poorly understood. Plant Pathol. 2013, 62, 243–265. [Google Scholar] [CrossRef]
- Van Coller, G.J.; Denman, S.; Crous, P.W.; Lamprecht, S.C. New perspective on soilborne diseases of grapevines in nurseries. Wineland 2005, 11, 102–105. [Google Scholar]
- Varanda, C.M.R.; Oliveira, M.; Materatski, P.; Landum, M.; Clara, M.I.E.; do Rosário Félix, M. Fungal endophytic communities associated to the phyllosphere of grapevine cultivars under different types of management. Fungal Biol. 2016, 120, 1525–1536. [Google Scholar] [CrossRef]
- Winter, S.; Bauer, T.; Strauss, P.; Kratschmer, S.; Paredes, D.; Popescu, D.; Landa, B.; Guzmán, G.; Gómez, J.A.; Guernion, M.; et al. Effects of vegetation management intensity on biodiversity and ecosystem services in vineyards: A meta-analysis. J. Appl. Ecol. 2018, 55, 2484–2495. [Google Scholar] [CrossRef]
- Hagn, A.; Pritsch, K.; Schloter, M.; Munch, J.C. Fungal diversity in agricultural soil under different farming management systems, with special reference to biocontrol strains of Trichoderma spp. Biol. Fertil. Soils 2003, 38, 236–244. [Google Scholar] [CrossRef]
| INT | INT | EXT | EXT | AB | AB | |
|---|---|---|---|---|---|---|
| (0–30 cm) | (30–60 cm) | (0–30 cm) | (30–60 cm) | (0–30 cm) | (30–60 cm) | |
| pH (H2O) * | 6.9 | 7 | 6.73 | 6.75 | 6.04 | 6.11 |
| pH (KCl) * | 6.51 | 6.59 | 6.41 | 6.49 | 5.33 | 5.67 |
| CaCO3 (g kg−1) ** | 2.5 | 2.5 | 1.6 | 4.1 | 2.5 | 3.3 |
| Total organic matter (g kg−1) ** | 6.6 | 6.6 | 7.1 | 7.6 | 7.5 | 9.0 |
| CORG (g kg−1) ** | 3.8 | 3.8 | 4.1 | 4.4 | 4.4 | 5.2 |
| INT | EXT | AB | |||
|---|---|---|---|---|---|
| Spring | Summer | Spring | Summer | Spring | Summer |
| 47.8 | 73.1 | 62.5 | 92.4 | 54.8 | 78.9 |
| Morphotype | Classes of Behavior | INT | EXT | AB | |||
|---|---|---|---|---|---|---|---|
| Spring | Summer | Spring | Summer | Spring | Summer | ||
| Acremonium sp. | Antagonistic | 0 | 0 | 1.63 | 0 | 3.76 | 2.03 |
| Alternaria sp. | Pathogenic | 0 | 0 | 8.94 | 2.42 | 2.26 | 0 |
| Aspergillus sp. | Pathogenic | 0 | 0 | 1.63 | 1.61 | 2.26 | 0 |
| Coniothyrium sp. | Antagonistic | 0 | 3.08 | 0 | 0 | 0 | 0 |
| Cylindrocarpon sp. | Pathogenic | 34.06 | 13.08 | 27.64 | 12.90 | 27.82 | 13.51 |
| Doratomyces sp. | Saprophytic | 0 | 0 | 0 | 0 | 1.50 | 0 |
| Fusarium spp. | Pathogenic | 47.83 | 59.23 | 36.59 | 35.48 | 30.83 | 28.38 |
| F. oxysporum | 30.43 | 50 | 13.01 | 18.55 | 10.53 | 15.54 | |
| F. solani | 17.39 | 9.23 | 23.58 | 16.94 | 20.30 | 12.84 | |
| Gliomastix sp. | Antagonistic | 0 | 0 | 1.63 | 4.03 | 2.26 | 3.38 |
| Myrothecium sp. | Antagonistic | 0 | 0 | 0 | 4.03 | 1.50 | 0 |
| Mortierella sp. | Saprophytic | 0 | 0 | 1.63 | 1.61 | 0 | 2.70 |
| Mucor sp. | Saprophytic | 0 | 0 | 0 | 2.42 | 0 | 2.03 |
| Oidiodendron sp. | Antagonistic | 0 | 0 | 0 | 0 | 2.26 | 2.70 |
| Paecilomyces sp. | Antagonistic | 0 | 0 | 1.63 | 0 | 3.01 | 0 |
| Penicillium sp. | Saprophytic | 0 | 0 | 1.63 | 4.03 | 0 | 3.38 |
| Phaeoacremonium sp. | Pathogenic | 10.87 | 13.08 | 5.69 | 10.48 | 11.28 | 17.57 |
| Pythium sp. | Pathogenic | 0 | 0 | 4.07 | 8.87 | 3.01 | 12.84 |
| Torula sp. | Saprophytic | 0 | 0 | 0 | 0 | 2.26 | 0 |
| Trichoderma sp. | Antagonistic | 7.25 | 11.54 | 7.32 | 9.68 | 5.26 | 7.43 |
| Verticillium sp. | Saprophytic | 0 | 0 | 0 | 2.42 | 0.75 | 4.05 |
| TOTAL | 100 | 100 | 100 | 100 | 100 | 100 | |
| Index | INT | EXT | AB | |||
|---|---|---|---|---|---|---|
| Spring | Summer | Spring | Summer | Spring | Summer | |
| Number of morphotypes | 4 | 5 | 12 | 13 | 15 | 12 |
| Number of colonies | 138 | 130 | 123 | 124 | 133 | 148 |
| Shannon diversity (H’) | 1.15 | 1.2 | 1.83 | 2.10 | 2.04 | 2.10 |
| Shannon evenness (J’) | 0.83 | 0.74 | 0.73 | 0.82 | 0.75 | 0.85 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovács, B.; Dobolyi, C.; Sebők, F.; Kocsis, L.; Tóth, Z. Effect of Vineyard Floor Management on Seasonal Changes of Cultivable Fungal Diversity in the Rhizosphere. Agriculture 2020, 10, 534. https://doi.org/10.3390/agriculture10110534
Kovács B, Dobolyi C, Sebők F, Kocsis L, Tóth Z. Effect of Vineyard Floor Management on Seasonal Changes of Cultivable Fungal Diversity in the Rhizosphere. Agriculture. 2020; 10(11):534. https://doi.org/10.3390/agriculture10110534
Chicago/Turabian StyleKovács, Barnabás, Csaba Dobolyi, Flóra Sebők, László Kocsis, and Zoltán Tóth. 2020. "Effect of Vineyard Floor Management on Seasonal Changes of Cultivable Fungal Diversity in the Rhizosphere" Agriculture 10, no. 11: 534. https://doi.org/10.3390/agriculture10110534
APA StyleKovács, B., Dobolyi, C., Sebők, F., Kocsis, L., & Tóth, Z. (2020). Effect of Vineyard Floor Management on Seasonal Changes of Cultivable Fungal Diversity in the Rhizosphere. Agriculture, 10(11), 534. https://doi.org/10.3390/agriculture10110534





