The Thin Line between Pathogenicity and Endophytism: The Case of Lasiodiplodia theobromae
Abstract
1. Introduction
2. Taxonomic and Phylogenetic Aspects
3. Endophytic Occurrence of Lasiodiplodia theobromae
4. Biological and Ecological Traits
5. Bioactivities of Endophytic Isolates of Lasiodiplodia theobromae
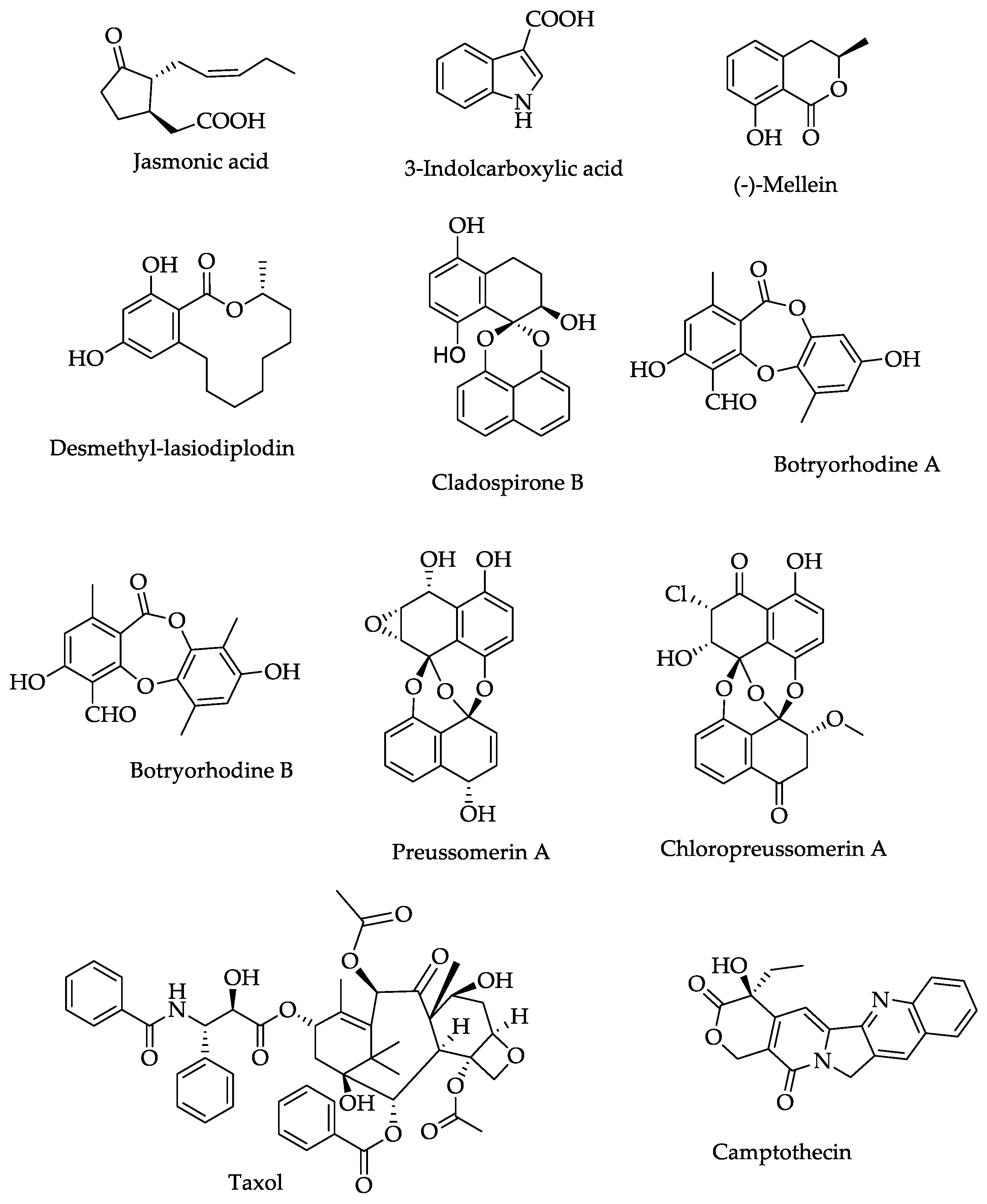
6. Secondary Metabolites and Enzymes of Endophytic Lasiodiplodia theobromae
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Stone, J.K.; Bacon, C.W.; White, J.F. An overview of endophytic microbes: Endophytism defined. Microb. Endophytes 2000, 3, 29–33. [Google Scholar]
- Nicoletti, R. Endophytic Fungi of Citrus Plants. Agriculture 2019, 9, 247. [Google Scholar] [CrossRef]
- Nicoletti, R.; Di Vaio, C.; Cirillo, C. Endophytic fungi of olive tree. Microorganisms 2020, 8, 1321. [Google Scholar] [CrossRef]
- Mehl, J.; Wingfield, M.J.; Roux, J.; Slippers, B. Invasive everywhere? Phylogeographic analysis of the globally distributed tree pathogen Lasiodiplodia theobromae. Forests 2017, 8, 145. [Google Scholar] [CrossRef]
- Punithalingam, E. Plant Diseases Attributed to Botryodiplodia theobromae Pat; J. Cramer: Vaduz, Lichtenstein, 1980; ISBN 9783768212564. [Google Scholar]
- Cilliers, A.J.; Swart, W.J.; Wingfield, M.J. A review of Lasiodiplodia theobromae with particular reference to its occurrence on coniferous seeds. S. Afr. For. J. 1993, 166, 47–52. [Google Scholar]
- Hawksworth, D.L.; Crous, P.W.; Redhead, S.A.; Reynolds, D.R.; Samson, R.A.; Seifert, K.A.; Taylor, J.W.; Wingfield, M.J.; Abaci, Ö.; Aime, C.; et al. The Amsterdam declaration on fungal nomenclature. IMA Fungus 2011, 2, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Mohali, S.; Burgess, T.I.; Wingfield, M.J. Diversity and host association of the tropical tree endophyte Lasiodiplodia theobromae revealed using simple sequence repeat markers. For. Pathol. 2005, 35, 385–396. [Google Scholar] [CrossRef]
- Larignon, P.; Fulchic, R.; Cere, L.; Dubos, B. Observation on black dead arm in French vineyards. Phytopathol. Mediterr. 2001, 40, 336–342. [Google Scholar]
- Slippers, B.; Boissin, E.; Phillips, A.J.L.; Groenewald, J.Z.; Lombard, L.; Wingfield, M.J.; Postma, A.; Burgess, T.; Crous, P.W. Phylogenetic lineages in the botryosphaeriales: A systematic and evolutionary framework. Stud. Mycol. 2013, 76, 32–49. [Google Scholar] [CrossRef]
- Pavlic, D.; Slippers, B.; Coutinho, T.A.; Gryzenhout, M.; Wingfield, M.J. Lasiodiplodia gonubiensis sp. nov., a new Botryosphaeria anamorph from native Syzygium cordatum in South Africa. Stud. Mycol. 2004, 50, 313–322. [Google Scholar]
- Burgess, T.I.; Barber, P.A.; Mohali, S.; Pegg, G.; De Beer, W.; Wingfield, M.J. Three new Lasiodiplodia spp. from the tropics, recognized based on DNA sequence comparisons and morphology. Mycologia 2006, 98, 423–435. [Google Scholar] [CrossRef] [PubMed]
- Damm, U.; Crous, P.W.; Fourie, P.H. Botryosphaeriaceae as potential pathogens of Prunus species in South Africa, with descriptions of Diplodia africana and Lasiodiplodia plurivora sp. nov. Mycologia 2007, 99, 664–680. [Google Scholar] [CrossRef] [PubMed]
- Alves, A.; Crous, P.W.; Correia, A.; Phillips, A.J.L. Morphological and molecular data reveal cryptic speciation in Lasiodiplodia theobromae. Fungal Divers. 2008, 28, 1–13. [Google Scholar]
- Cruywagen, E.M.; Slippers, B.; Roux, J.; Wingfield, M.J. Phylogenetic species recognition and hybridisation in Lasiodiplodia: A case study on species from baobabs. Fungal Biol. 2017, 121, 420–436. [Google Scholar] [CrossRef]
- Coutinho, I.B.L.; Freire, F.C.O.; Lima, C.S.; Lima, J.S.; Gonçalves, F.J.T.; Machado, A.R.; Silva, A.M.S.; Cardoso, J.E. Diversity of genus Lasiodiplodia associated with perennial tropical fruit plants in northeastern Brazil. Plant Pathol. 2017, 66, 90–104. [Google Scholar] [CrossRef]
- Netto, M.S.B.; Lima, W.G.; Correia, K.C.; da Silva, C.F.B.; Thon, M.; Martins, R.B.; Miller, R.N.G.; Michereff, S.J.; Câmara, M.P.S. Analysis of phylogeny, distribution, and pathogenicity of Botryosphaeriaceae species associated with gummosis of Anacardium in Brazil, with a new species of Lasiodiplodia. Fungal Biol. 2017, 121, 437–451. [Google Scholar] [CrossRef]
- Jiang, N.; Wang, X.W.; Liang, Y.M.; Tian, C.M. Lasiodiplodia cinnamomi sp. nov. from Cinnamomum camphora in China. Mycotaxon 2018, 133, 249–259. [Google Scholar] [CrossRef]
- Budiono, B.; Elfita, E.; Muharni, M.; Yohandini, H.; Widjajanti, H. Antioxidant activity of Syzygium samarangense L. and their endophytic fungi. Molekul 2019, 14, 48–55. [Google Scholar] [CrossRef]
- de Silva, N.I.; Phillips, A.J.L.; Liu, J.K.; Lumyong, S.; Hyde, K.D. Phylogeny and morphology of Lasiodiplodia species associated with Magnolia forest plants. Sci. Rep. 2019, 9, 14355. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, S.; Zhao, L.; Sun, X.; He, W.; Zhang, Y.; Dai, Y.C. Lasiodiplodia spp. associated with Aquilaria crassna in Laos. Mycol. Prog. 2019, 18, 683–701. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, Y.; He, W.; Zhang, Y. Stem blight of blueberry caused by Lasiodiplodia vaccinii sp. nov. in China. Plant Dis. 2019, 103, 2041–2050. [Google Scholar] [CrossRef] [PubMed]
- Berraf-Tebbal, A.; Mahamedi, A.E.; Aigoun-Mouhous, W.; Špetík, M.; Čechová, J.; Pokluda, R.; Baránek, M.; Eichmeier, A.; Alves, A. Lasiodiplodia mitidjana sp. nov. and other Botryosphaeriaceae species causing branch canker and dieback of Citrus sinensis in Algeria. PLoS ONE 2020, 15, e0232448. [Google Scholar] [CrossRef] [PubMed]
- Laurent, B.; Marchand, M.; Chancerel, E.; Saint-Jean, G.; Capdevielle, X.; Poeydebat, C.; Bellée, A.; Comont, G.; Villate, L.; Desprez-Loustau, M.-L. A richer community of Botryosphaeriaceae within a less diverse community of fungal endophytes in grapevines than in adjacent forest trees revealed by a mixed metabarcoding strategy. Phytobiomes J. 2020, 4, 252–267. [Google Scholar] [CrossRef]
- Rodríguez-Gálvez, E.; Guerrero, P.; Barradas, C.; Crous, P.W.; Alves, A. Phylogeny and pathogenicity of Lasiodiplodia species associated with dieback of mango in Peru. Fungal Biol. 2017, 121, 452–465. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R. The status of Botryosphaeriaceae species infecting grapevines. Phytopathol. Mediterr. 2011, 50, S5–S45. [Google Scholar]
- Taylor, J.W.; Jacobson, D.J.; Kroken, S.; Kasuga, T.; Geiser, D.M.; Hibbett, D.S.; Fisher, M.C. Phylogenetic species recognition and species concepts in fungi. Fungal Genet. Biol. 2000, 31, 21–32. [Google Scholar] [CrossRef]
- Santos, P.H.D.; Carvalho, B.M.; Aguiar, K.P.; Aredes, F.A.S.; Poltronieri, T.P.S.; Vivas, J.M.S.; Mussi Dias, V.; Bezerra, G.A.; Pinho, D.B.; Pereira, M.G.; et al. Phylogeography and population structure analysis reveals diversity by mutations in Lasiodiplodia theobromae with distinct sources of selection. Genet. Mol. Res. 2017, 16. [Google Scholar] [CrossRef]
- Boyogueno, A.D.B.; Slippers, B.; Perez, G.; Wingfield, M.J.; Roux, J. High gene flow and outcrossing within populations of two cryptic fungal pathogens on a native and non-native host in Cameroon. Fungal Biol. 2012, 116, 343–353. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, L.D.; Hyde, K.D. Taxonomic placement of sterile morphotypes of endophytic fungi from Pinus tabulaeformis (Pinaceae) in northeast China based on rDNA sequences. Fungal Divers. 2005, 20, 235–260. [Google Scholar]
- Ma, Y.M.; Ma, C.C.; Li, T.; Wang, J. A new furan derivative from an endophytic Aspergillus flavus of Cephalotaxus fortunei. Nat. Prod. Res. 2016, 30, 79–84. [Google Scholar] [CrossRef]
- Venkatachalam, R.; Subban, K.; Paul, M.J. Taxol from Botryodiplodia theobromae (BT 115)—An endophytic fungus of Taxus baccata. In Proceedings of the 13th International Biotechnology Symposium and Exhibition, Dalian, China, 12–17 October 2008; pp. 189–190. [Google Scholar]
- Orlandelli, R.C.; Alberto, R.N.; Almeida, T.T.; Azevedo, J.L.; Pamphile, J.A. In vitro antibacterial activity of crude extracts produced by endophytic fungi isolated from Piper hispidum sw. J. Appl. Pharm. Sci. 2012, 2, 137–141. [Google Scholar] [CrossRef]
- Orlandelli, R.C.; Alberto, R.N.; Rubin Filho, C.J.; Pamphile, J.A. Diversity of endophytic fungal community associated with Piper hispidum (Piperaceae) leaves. Genet. Mol. Res. 2012, 11, 1575–1585. [Google Scholar] [CrossRef] [PubMed]
- Sah, B.; Subban, K.; Chelliah, J. Cloning and sequence analysis of 10-deacetylbaccatin III-10-O-acetyl transferase gene and WRKY1 transcription factor from taxol-producing endophytic fungus Lasiodiplodia theobromea. FEMS Microbiol. Lett. 2017, 364, fnx253. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zaher, A.M.; Moharram, A.M.; Davis, R.; Panizzi, P.; Makboul, M.A.; Calderón, A.I. Characterisation of the metabolites of an antibacterial endophyte Botryodiplodia theobromae Pat. of Dracaena draco L. by LC-MS/MS. Nat. Prod. Res. 2015, 29, 2275–2281. [Google Scholar] [CrossRef]
- Richardson, K.A.; Currah, R.S. The fungal community associated with the roots of some rainforest epiphytes of Costa Rica. Selbyana 1995, 16, 49–73. [Google Scholar]
- Duarte dos Santos, C.; Rocha Sousa, E.M.; Leal Candeias, E.; Santos Vitória, N.; Bezerra, J.L.; Newman, M.; Luz, E.D. Lasiodiplodia theobromae as an endophyte on Orchidaceae in Bahia. Agrotrópica 2012, 24, 179–182. [Google Scholar] [CrossRef]
- Govinda Rajulu, M. Endophytic fungi of orchids of Arunachal Pradesh, North Eastern India. Curr. Res. Environ. Appl. Mycol. 2016, 6, 293–299. [Google Scholar] [CrossRef]
- Sawmya, K.; Vasudevan, T.G.; Murali, T.S. Fungal endophytes from two orchid species—Pointer towards organ specificity. Czech Mycol. 2013, 65, 89–101. [Google Scholar] [CrossRef]
- Tibpromma, S.; Hyde, K.D.; Bhat, J.D.; Mortimer, P.E.; Xu, J.; Promputtha, I.; Doilom, M.; Yang, J.-B.; Tang, A.M.C.; Karunarathna, S.C. Identification of endophytic fungi from leaves of Pandanaceae based on their morphotypes and DNA sequence data from southern Thailand. MycoKeys 2018, 33, 25–67. [Google Scholar] [CrossRef]
- Dissanayake, R.K.; Ratnaweera, P.B.; Williams, D.E.; Wijayarathne, C.D.; Wijesundera, R.L.C.; Andersen, R.J.; de Silva, E.D. Antimicrobial activities of mycoleptodiscin B isolated from endophytic fungus Mycoleptodiscus sp. of Calamus thwaitesii Becc. J. Appl. Pharm. Sci. 2016, 6, 1–6. [Google Scholar] [CrossRef]
- Venkatesagowda, B.; Ponugupaty, E. Diversity of plant oil seed-associated fungi isolated from seven oil-bearing seeds and their potential for the production of lipolytic enzymes. World J. Microbiol. Biotechnol. 2012, 28, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Nago, H. (R)-2-octeno-δ-lactone and other volatiles produced by Lasiodiplodia theobromae. Biosci. Biotechnol. Biochem. 1994, 58, 1262–1266. [Google Scholar] [CrossRef]
- Rodrigues, K.F. The foliar fungal endophytes of the Amazonian palm Euterpe oleracea. Mycologia 1994, 86, 376–385. [Google Scholar] [CrossRef]
- Aun, E.S.S.; Hui, J.Y.S.; Ming, J.W.W.; Yi, J.C.C.; Wong, C.; Mujahid, A.; Müller, M. Screening of endophytic fungi for biofuel feedstock production using palm oil mill effluent as a carbon source. Malays. J. Microbiol. 2017, 13, 203–209. [Google Scholar]
- Sultan, S.; Sun, L.; Blunt, J.W.; Cole, A.L.J.; Munro, M.H.G.; Ramasamy, K.; Weber, J.-F.F. Evolving trends in the dereplication of natural product extracts. 3: Further lasiodiplodins from Lasiodiplodia theobromae, an endophyte from Mapania kurzii. Tetrahedron Lett. 2014, 55, 453–455. [Google Scholar] [CrossRef]
- Amirita, A.; Sindhu, P.; Swetha, J.; Vasanthi, N.S.; Kannan, K.P. Enumeration of endophytic fungi from medicinal plants and screeening of extracellular enzymes. World J. Sci. Technol. 2012, 2, 13–19. [Google Scholar]
- Zakaria, L.; Nuraini, W.; Aziz, W.; Pisang, D. Molecular identification of endophytic fungi from banana leaves (Musa spp.). Trop. Life Sci. Res. 2018, 29, 201–211. [Google Scholar] [CrossRef]
- Sakalidis, M.L.; Hardy, G.E.S.J.; Burgess, T.I. Endophytes as potential pathogens of the baobab species Adansonia gregorii: A focus on the Botryosphaeriaceae. Fungal Ecol. 2011, 4, 1–14. [Google Scholar] [CrossRef]
- Mishra, A.; Gond, S.K.; Kumar, A.; Sharma, V.K.; Verma, S.K.; Kharwar, R.N.; Sieber, T.N. Season and tissue type affect fungal endophyte communities of the indian medicinal plant Tinospora cordifolia more strongly than geographic location. Microb. Ecol. 2012, 64, 388–398. [Google Scholar] [CrossRef]
- Jin, B.; Jiang, F.; Xu, F.; Ding, Z. An antitumor activity endophytic fungus A33 isolated from Viscum Coloratum of Chinese. In Proceedings of the 2011 International Conference on Remote Sensing, Environment and Transportation Engineering, RSETE 2011, Nanjing, China, 24–26 June 2011; pp. 7368–7371. [Google Scholar]
- Duan, X.; Xu, F.; Qin, D.; Gao, T.; Shen, W.; Zuo, S.; Yu, B.; Xu, J.; Peng, Y.; Dong, J. Diversity and bioactivities of fungal endophytes from Distylium chinense, a rare waterlogging tolerant plant endemic to the Three Gorges Reservoir. BMC Microbiol. 2019, 19, 278. [Google Scholar] [CrossRef]
- Dissanayake, A.J.; Purahong, W.; Wubet, T.; Hyde, K.D.; Zhang, W.; Xu, H.; Zhang, G.; Fu, C.; Liu, M.; Xing, Q.; et al. Direct comparison of culture-dependent and culture-independent molecular approaches reveal the diversity of fungal endophytic communities in stems of grapevine (Vitis vinifera). Fungal Divers. 2018, 90, 85–107. [Google Scholar] [CrossRef]
- Burruano, S.; Alfonzo, A.; Conigliaro, G.; Mondello, V.; Torta, L. First observations on the interaction between Lasiodiplodia theobromae and Epicoccum purpurascens, both endophytes in grapevine buds. In Proceedings of the XV Convegno Nazionale della Società Italiana di Patologia Vegetale (SIPaV), Locorotondo, Italy, 28 September–1 October 2009. [Google Scholar]
- Suryanarayanan, T.S.; Murali, T.S.; Venkatesan, G. Occurrence and distribution of fungal endophytes in tropical forests across a rainfall gradient. Can. J. Bot. 2002, 80, 818–826. [Google Scholar] [CrossRef]
- Roopa, G.; Madhusudhan, M.C.; Sunil, K.C.R.; Lisa, N.; Calvin, R.; Poornima, R.; Zeinab, N.; Kini, K.R.; Prakash, H.S.; Geetha, N. Identification of taxol-producing endophytic fungi isolated from Salacia oblonga through genomic mining approach. J. Genet. Eng. Biotechnol. 2015, 13, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Jami, F.; Slippers, B.; Wingfield, M.J.; Loots, M.T.; Gryzenhout, M. Temporal and spatial variation of Botryosphaeriaceae associated with Acacia karroo in South Africa. Fungal Ecol. 2015, 15, 51–62. [Google Scholar] [CrossRef]
- Tejesvi, M.V.; Mahesh, B.; Nalini, M.S.; Prakash, H.S.; Kini, K.R.; Subbiah, V.; Shetty, H.S. Fungal endophyte assemblages from ethnopharmaceutically important medicinal trees. Can. J. Microbiol. 2006, 52, 427–435. [Google Scholar] [CrossRef]
- Murali, T.S.; Suryanarayanan, T.S.; Venkatesan, G. Fungal endophyte communities in two tropical forests of southern India: Diversity and host affiliation. Mycol. Prog. 2007, 6, 191–199. [Google Scholar] [CrossRef]
- Arora, P.; Wani, Z.A.; Ahmad, T.; Sultan, P.; Gupta, S.; Riyaz-ul-hassan, S. Community structure, spatial distribution, diversity and functional characterization of culturable endophytic fungi associated with Glycyrrhiza glabra L. Fungal Biol. 2019, 123, 373–383. [Google Scholar] [CrossRef]
- Sheik, S.; Chandrashekar, K.R. Fungal endophytes of an endemic plant Humboldtia brunonis wall of western Ghats (India) and their antimicrobial and DPPH radical scavenging potentiality. Orient. Pharm. Exp. Med. 2018, 18, 115–125. [Google Scholar] [CrossRef]
- Prazeres, I.; Diogo, J.; Bezerra, P.; De Souza, C.M.; Cavalcanti, S.; Lucia, V.; Lima, D.M. Endophytic mycobiota from leaves of Indigofera suffruticosa Miller (Fabaceae): The relationship between seasonal change in Atlantic Coastal Forest and tropical dry forest (Caatinga), Brazil. Afr. J. Microbiol. Res. 2015, 9, 1227–1235. [Google Scholar]
- Gonçalves, F.J.T.; Freire, F.D.C.O.; Lima, J.S.; Melo, J.G.M.; Câmara, M.P.S. Patogenicidade de espécies de Botryosphaeriaceae endofíticas de plantas da Caatinga do estado do Ceará em manga e umbu-cajá. Summa Phytopath. 2016, 42, 43–52. [Google Scholar] [CrossRef]
- López-González, R.C.; Gómez-Cornelio, S.; Susana, C.; Garrido, E.; Oropeza-Mariano, O.; Heil, M.; Partida-Martínez, L.P. The age of lima bean leaves influences the richness and diversity of the endophytic fungal community, but not the antagonistic effect of endophytes against Colletotrichum lindemuthianum. Fungal Ecol. 2017, 26, 1–10. [Google Scholar] [CrossRef]
- Jinu, M.V.; Gini, C.K.; Jayabaskaran, C. In vitro antioxidant activity of cholestanol glucoside from an endophytic fungus Lasiodiplodia theobromae isolated from Saraca asoca. J. Chem. Pharm. Res. 2015, 7, 952–962. [Google Scholar]
- Jinu, M.V.; Jayabaskaran, C. Diversity and anticancer activity of endophytic fungi associated with the medicinal plant Saraca asoca. Curr. Res. Environ. Appl. Mycol. 2015, 5, 169–179. [Google Scholar] [CrossRef]
- Yao, Y.Q.; Lan, F.; Ming, Y.; Ji, Q.; Wei, G.; Shao, R.; Liang, H.; Li, B. Endophytic fungi harbored in the root of Sophora tonkinensis Gapnep: Diversity and biocontrol potential against phytopathogens. Microbiol. Open 2017, 6, e00437. [Google Scholar] [CrossRef] [PubMed]
- Rukachaisirikul, V.; Arunpanichlert, J.; Sukpondma, Y. Metabolites from the endophytic fungi Botryosphaeria rhodina PSU-M35 and PSU-M114. Tetrahedron 2009, 65, 10590–10595. [Google Scholar] [CrossRef]
- Gazis, R.; Chaverri, P. Diversity of fungal endophytes in leaves and stems of wild rubber trees (Hevea brasiliensis) in Peru. Fungal Ecol. 2010, 3, 240–254. [Google Scholar] [CrossRef]
- Samaga, P.V.; Vittal, R.R. Diversity and bioactive potential of endophytic fungi from Nothapodytes foetida, Hypericum mysorense and Hypericum japonicum collected from Western Ghats of India. Ann. Microbiol. 2015, 66, 229–244. [Google Scholar] [CrossRef]
- Guerrero, J.J.G.; General, M.A.; Serrano, J.E. Culturable foliar fungal endophytes of mangrove epecies in Bicol region, Philippines. Philipp. J. Sci. 2018, 147, 563–574. [Google Scholar]
- Zhou, J.; Li, G.; Deng, Q.; Zheng, D.; Yang, X.; Xu, J. Cytotoxic constituents from the mangrove endophytic Pestalotiopsis sp. induce G 0 /G 1 cell cycle arrest and apoptosis in human cancer cells. Nat. Prod. Res. 2018, 32, 2968–2972. [Google Scholar] [CrossRef]
- Li, R.; Yan, D.; Feng, X.; Sun, X. Diversity of Botryosphaeria spp., as endophytes in poplars in Beijing, based on molecular operational taxonomic units. Sci. Silvae Sin. 2014, 50, 109–115. [Google Scholar]
- Huang, J.H.; Xiang, M.M.; Jiang, Z.D. Endophytic fungi of bitter melon (Momordica Charantia) in Guangdong province, China. Gt. Lakes Entomol. 2012, 45, 2. [Google Scholar]
- Suryanarayanan, T.S.; Venkatachalam, A.; Rajulu, M.G. A comparison of endophyte assemblages in transgenic and non-transgenic cotton plant tissues. Curr. Sci. 2011, 101, 1472–1474. [Google Scholar]
- Rubini, M.R.; Silva-ribeiro, R.T.; Pomella, A.W.V.; Maki, C.S. Diversity of endophytic fungal community of cacao (Theobroma cacao L.) and biological control of Crinipellis perniciosa, causal agent of Witches’ Broom Disease. Int. J. Mol. Sci. 2005, 1, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Chaithra, M.; Vanitha, S.; Ramanathan, A.; Jegadeeshwari, V.; Rajesh, V. Morphological and molecular characterization of endophytic fungi associated with Cocoa (Theobroma cacao L.) in India. Curr. Appl. Sci. Technol. 2020, 38, 1–8. [Google Scholar] [CrossRef]
- Evans, H.C.; Holmes, K.A.; Thomas, S.E. Endophytes and mycoparasites associated with an indigenous forest tree. Mycol. Prog. 2003, 2, 149–160. [Google Scholar] [CrossRef]
- Chhipa, H.; Kaushik, N. Fungal and bacterial diversity isolated from Aquilaria malaccensis tree and soil, induces agarospirol formation within 3 months after artificial infection. Front. Microbiol. 2017, 8, 1286. [Google Scholar] [CrossRef]
- Cui, J.; Guo, S.; Xiao, P. Antitumor and antimicrobial activities of endophytic fungi from medicinal parts of Aquilaria sinensis. J. Zhejiang Univ. Sci. B 2011, 12, 385–392. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, W.M.; Pan, Q.L.; Li, H.H.; Tao, M.H.; Gao, X.X. Isolation and molecular identifcation of endophytic fungi from Aquilaria sinensis. J. Fungal Res. 2009, 7, 37–42. [Google Scholar]
- Tejesvi, M.V.; Mahesh, B.; Nalini, M.S.; Prakash, H.S.; Kini, K.R.; Subbiah, V.; Shetty, H.S. Endophytic fungal assemblages from inner bark and twig of Terminalia arjuna W. & A. (Combretaceae). World J. Microbiol. Biotechnol. 2005, 21, 1535–1540. [Google Scholar]
- Patil, M.P.; Patil, R.H.; Patil, S.G.; Maheshwari, V.L. Endophytic mycoflora of indian medicinal plant, Terminalia arjuna and their biological activities. Int. J. Biotechnol. Wellness Ind. 2014, 3, 53–61. [Google Scholar]
- Begoude, B.A.D.; Slippers, B. Botryosphaeriaceae associated with Terminalia catappa in Cameroon, South Africa and Madagascar. Mycol. Prog. 2010, 9, 101–123. [Google Scholar] [CrossRef]
- Toghueo, R.M.K.; Zabalgogeazcoa, I.; De Aldana, B.R.V.; Boyom, F.F. Enzymatic activity of endophytic fungi from the medicinal plants Terminalia catappa, Terminalia mantaly and Cananga odorata. S. Afr. J. Bot. 2017, 109, 146–153. [Google Scholar] [CrossRef]
- Begoude, B.A.D.; Slippers, B.; Wingfield, M.J.; Roux, J. The pathogenic potential of endophytic Botryosphaeriaceous fungi on Terminalia species in Cameroon. For. Pathol. 2011, 41, 281–292. [Google Scholar] [CrossRef][Green Version]
- Suryavamshi, G.; Shivanna, M.B. Diversity and antibacterial activity of endophytic fungi in Memecylon umbellatum Burm. F.—A medicinal plant in the Western Ghats of Karnataka, India. Indian J. Ecol. 2020, 47, 171–180. [Google Scholar]
- Urdaneta, L.; Araujo, D.; Quirós, M.; Rodríguez, D.; Poleo, N.; Petit, Y. Endophytic mycobiota associated to guava floral buds (Psidium guajava L.) and to flat mite (Brevipalpus phoenicis)(Geijskes)(Acari: Tenuipalpidae). UDO Agríc. 2009, 9, 166–174. [Google Scholar]
- Janakiraman, V.; Govindarajan, K.; Magesh, C.R. Biosynthesis of silver nanoparticles from endophytic fungi, and its cytotoxic activity. Bionanoscience 2019, 9, 573–579. [Google Scholar] [CrossRef]
- Freire, F.C.; Bezerra, J.L. Foliar endophytic fungi of Ceará State (Brazil): A preliminary study. Summa Phytopath. 2001, 27, 304–308. [Google Scholar]
- Cardoso, J.E.; Bezerra, M.A.; Viana, F.M.P.; de Sousa, T.R.M.; Cysne, A.Q.; Farias, F. Endophyte occurrence of Lasiodiplodia theobromae in cashew tissues and its transmission by vegetative propagules. Summa Phytopath. 2009, 35, 262–266. [Google Scholar] [CrossRef]
- Johnson, G.I.; Mead, A.J.; Cooke, A.W.; Dean, J.R. Mango stem end rot pathogens—Fruit infection by endophytic colonisation of the inflorescence and pedicel. Ann. Appl. Biol. 1992, 120, 225–234. [Google Scholar] [CrossRef]
- Morales, R.V.; Rodríguez, G.M. Micobiota endofítica asociada al cultivo Del mango ’Haden’(Mangifera indica L.) en el oriente de Venezuela. Rev. Cientifìca UDO Agríc. 2009, 9, 393–402. [Google Scholar]
- González, E.; Umana, G.; Arauz, L.F. Population fluctuation of Botryodiplodia theobromae Pat. in mango. Agron. Costarric. 1999, 23, 21–29. [Google Scholar]
- Aishwarya, S.; Nagam, N.; Vijaya, T.; Netala, R.V. Screening and identification of heavy metal-tolerant endophytic fungi Lasiodiplodia theobromae from Boswellia ovalifoliolata an endemic plant of tirumala hills. Asian J. Pharm. Clin. Res. 2017, 10, 488–491. [Google Scholar]
- El-Nagerabi, S.A.F.; Elshafie, A.E.; Alkhanjari, S.S. Endophytic fungi associated with endogenous Boswellia sacra. Biodiversitas 2014, 15, 24–30. [Google Scholar] [CrossRef]
- Linnakoski, R.; Puhakka-tarvainen, H.; Pappinen, A. Endophytic fungi isolated from Khaya anthotheca in Ghana. Fungal Ecol. 2012, 5, 298–308. [Google Scholar] [CrossRef]
- Zhao, W.; Bai, J.; McCollum, G.; Baldwin, E. High incidence of preharvest colonization of huanglongbing-symptomatic Citrus sinensis fruit by Lasiodiplodia theobromae (Diplodia natalensis) and exacerbation of postharvest fruit decay by that fungus. Appl. Environ. Microbiol. 2015, 81, 364–372. [Google Scholar] [CrossRef]
- Senthilkumar, N.; Mohan, V.; Murugesan, S. Studies on endophytic fungi of commercially important tropical tree species in India. Kavaka 2014, 31, 22–31. [Google Scholar]
- Osorio, J.A.; Crous, C.J.; de Beer, Z.W.; Wingfield, M.J.; Roux, J. Endophytic Botryosphaeriaceae, including five new species, associated with mangrove trees in South Africa. Fungal Biol. 2017, 121, 361–393. [Google Scholar] [CrossRef]
- Verma, S.K.; Gond, S.K.; Mishra, A.; Sharma, V.K.; Kumar, J.; Singh, D.K.; Kumar, A.; Goutam, J.; Kharwar, R.N. Impact of environmental variables on the isolation, diversity and antibacterial activity of endophytic fungal communities from Madhuca indica Gmel. at different locations in India. Ann. Microbiol. 2014, 64, 721–734. [Google Scholar] [CrossRef]
- Shweta, S.; Gurumurthy, B.R.; Vasanthakumari, M.M.; Ravikanth, G.; Dayanandan, S.; Storms, R.; Shivanna, M.B.; Uma Shaanker, R. Endophyte fungal diversity in Nothapodytes nimmoniana along its distributional gradient in the Western Ghats, India: Are camptothecine (anticancer alkaloid) producing endophytes restricted to specific clades? Curr. Sci. 2015, 109, 127–138. [Google Scholar]
- Dhayanithy, G.; Subban, K.; Chelliah, J. Diversity and biological activities of endophytic fungi associated with Catharanthus roseus. BMC Microbiol. 2019, 19, 22. [Google Scholar] [CrossRef]
- Akther, T.; Mathipi, V.; Kumar, N.S.; Davoodbasha, M.; Srinivasan, H. Fungal-mediated synthesis of pharmaceutically active silver nanoparticles and anticancer property against A549 cells through apoptosis. Environ. Sci. Pollut. Res. 2019, 26, 13649–13657. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Chagas, M.B.; dos Santos, I.P.; da Silva, L.C.N.; dos Santos Correia, M.T.; de Araújo, J.M.; da Silva Cavalcanti, M.; de Menezes Lima, V.L. Antimicrobial activity of cultivable endophytic fungi associated with Hancornia speciosa gomes bark. Open Microbiol. J. 2017, 11, 179–188. [Google Scholar] [CrossRef]
- Suryanarayanan, T.S.; Thennarasan, S. Temporal variation in endophyte assemblages of Plumeria rubra leaves. Fungal Divers. 2004, 15, 197–204. [Google Scholar]
- Qadri, M.; Johri, S.; Shah, B.A.; Khajuria, A.; Sidiq, T.; Lattoo, S.K.; Abdin, M.Z.; Riyaz-Ul-Hassan, S. Identification and bioactive potential of endophytic fungi isolated from selected plants of the Western Himalayas. SpringerPlus 2013, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, J.; Bayman, P. Fungal epiphytes and endophytes of coffee leaves (Coffea arabica). Microb. Ecol. 2005, 50, 1–8. [Google Scholar] [CrossRef]
- Pandi, M.; Kumaran, R.S.; Choi, Y.K.; Kim, H.J.; Muthumary, J. Isolation and detection of taxol, an anticancer drug produced from Lasiodiplodia theobromae, an endophytic fungus of the medicinal plant Morinda citrifolia. Afr. J. Biotechnol. 2011, 10, 1428–1435. [Google Scholar]
- Sheik, S.; Chandrashekar, K.R.; Swaroop, K.; Somashekarappa, H.M. Lasiodiplactone A, a novel lactone from the mangrove endophytic fungus Lasiodiplodia theobromae ZJ-HQ1. Int. Biodeterior. Biodegrad. 2015, 105, 21–29. [Google Scholar] [CrossRef]
- Chen, S.; Chen, D.; Cai, R.; Cui, H.; Long, Y.; Lu, Y.; Li, C.; She, Z. Cytotoxic and antibacterial preussomerins from the mangrove endophytic fungus Lasiodiplodia theobromae ZJ-HQ1. J. Nat. Prod. 2016, 79, 2397–2402. [Google Scholar] [CrossRef]
- Chen, S.; Liu, Z.; Liu, H.; Long, Y.; Chen, D.; Lu, Y.; She, Z. Lasiodiplactone A, a novel lactone from the mangrove endophytic fungus Lasiodiplodia. Org. Biomol. Chem. 2017, 73, 6338–6341. [Google Scholar] [CrossRef]
- Moron, L.S.; Lim, Y. Antimicrobial activities of crude culture extracts from mangrove fungal endophytes collected in Luzon Island, Philippines. Philipp. Sci. Lett. 2018, 11, 28–36. [Google Scholar]
- Mazlan, N.; Tate, R.; Clements, C.; Edrada-Ebel, R. Metabolomics studies of endophytic metabolites from Malaysian mangrove plant in the search for new potential antibiotics. Planta Med. 2013, 79, PA22. [Google Scholar] [CrossRef]
- Cannon, P.F.; Simmons, C.M. Diversity and host preference of leaf endophytic fungi in the Iwokrama forest reserve, Guyana diversity and host preference of leaf endophytic fungi in the. Mycologia 2017, 5514, 210–220. [Google Scholar]
- Maheswari, S.; Rajagopal, K. Biodiversity of endophytic fungi in Kigelia pinnata during two different seasons. Curr. Sci. 2013, 515–518. [Google Scholar]
- Rajendran, L.; Rajagopal, K.; Subbarayan, K. Efficiency of fungal taxol on human liver carcinoma cell lines. Am. J. Res. Commun. 2013, 1, 112–121. [Google Scholar]
- Singh, D.K.; Sharma, V.K.; Kumar, J.; Mishra, A.; Verma, S.K.; Sieber, T.N.; Kharwar, R.N. Diversity of endophytic mycobiota of tropical tree Tectona grandis Linn.f.: Spatiotemporal and tissue type effects. Sci. Rep. 2017, 7, 3745. [Google Scholar] [CrossRef] [PubMed]
- Balbool, B.A.; Ahmed, A.A.; Moubasher, M.H.; Helmy, E.A. Production of L-asparaginase (L-ASN) from endophytic Lasiodiplodia theobromae hosted Teucrium polium in Egypt. Microb. Biosyst. 2018, 3, 46–55. [Google Scholar]
- Sunayana, N.; Nalini, M.S.; Kumara Sampath, K.K.; Prakash, H.S. Diversity studies on the endophytic fungi of Vitex negundo L. Miycosphere 2014, 5, 578–590. [Google Scholar] [CrossRef]
- Kamal, N.; Viegelmann, C.V.; Clements, C.J.; Edrada-Ebel, R.A. Metabolomics-guided isolation of anti-trypanosomal metabolites from the endophytic fungus Lasiodiplodia theobromae. Planta Med. 2017, 83, 565–573. [Google Scholar] [CrossRef]
- De Errasti, A.; Carmarán, C.C.; Novas, M.V. Diversity and significance of fungal endophytes from living stems of naturalized trees from Argentina. Fungal Divers. 2010, 41, 29–40. [Google Scholar] [CrossRef]
- El-hawary, S.S.; Sayed, A.M.; Rateb, M.E.; Bakeer, W.; Abouzid, S.F.; Mohammed, R.; Sayed, A.M.; Rateb, M.E. Secondary metabolites from fungal endophytes of Solanum nigrum. Nat. Prod. Res. 2017, 31, 2568–2571. [Google Scholar] [CrossRef]
- Kannan, K.P.; Muthumary, J. Comparative analysis of endophytic mycobiota in different tissues of medicinal plants. Afr. J. Microbiol. Res. 2012, 6, 4219–4225. [Google Scholar]
- Abdou, R.; Scherlach, K.; Dahse, H.; Sattler, I.; Hertweck, C. Phytochemistry Botryorhodines A–D, antifungal and cytotoxic depsidones from Botryosphaeria rhodina, an endophyte of the medicinal plant Bidens pilosa. Phytochemistry 2010, 71, 110–116. [Google Scholar] [CrossRef] [PubMed]
- George, T.S.; Samy, K.; Guru, S.; Sankaranarayanan, N. Extraction, purification and characterization of chitosan from endophytic fungi isolated from medicinal plants. World J. Sci. Technol. 2015, 1, 43–48. [Google Scholar]
- Vardhana, J.; Kathiravan, G.; Dhivya, R. Biodiversity of endophytic fungi and its seasonal recurrence from some plants. Res. J. Pharm. Technol. 2017, 10, 490–496. [Google Scholar] [CrossRef]
- Mullen, J.M.; Gilliam, C.H.; Hagan, A.K.; Morgan-Jones, G. Canker of dogwood caused by Lasiodiplodia theobromae, a disease influenced by drought stress or cultivar selection. Plant Dis. 1991, 75, 886–889. [Google Scholar] [CrossRef]
- Delaye, L.; García-guzmán, G.; Heil, M. Endophytes versus biotrophic and necrotrophic pathogens—Are fungal lifestyles evolutionarily stable traits? Fungal Divers. 2013, 1, 125–135. [Google Scholar] [CrossRef]
- Slippers, B.; Wingfield, M.J. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and impact. Fungal Biol. Rev. 2007, 21, 90–106. [Google Scholar] [CrossRef]
- Crous, P.W.; Groenewald, J.Z.; Slippers, B.; Wingfield, M.J.; Crous, P.W. Global food and fibre security threatened by current inefficiencies in fungal identification. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20160024. [Google Scholar] [CrossRef]
- Martin, R.R.; Constable, F.; Tzanetakis, I.E. Quarantine regulations and the impact of modern detection methods. Annu. Rev. Phytopathol. 2016, 54, 189–208. [Google Scholar] [CrossRef]
- Baker, R.; Bragard, C.; Candresse, T.; Gilioli, G.; Grégoire, J.; Holb, I.; Rafoss, T.; Rossi, V.; Schans, J.; Schrader, G.; et al. Scientific opinion on the pest categorisation of Cryphonectria parasitica (Murrill) Barr. 1. EFSA J. 2014, 12, 3859. [Google Scholar]
- Jeger, M.; Bragard, C.; Caf, D.; Candresse, T.; Chatzivassiliou, E.; Dehnen-schmutz, K.; Gilioli, G.; Gregoire, J.; Anton, J.; Miret, J.; et al. Pest categorisation of Gremmeniella abietina. EFSA J. 2017, 15, 5030. [Google Scholar]
- EFSA Call for Proposals GP/EFSA/ALPHA/2020/01—Entrusting Support Tasks in the Area of Plant Health Commodity Risk Assessment for High Risk Plants (Plants for Planting for Ornamental Purpose). Available online: http://www.efsa.europa.eu/en/art36grants/article36/gpefsaalpha202001-entrusting-support-tasks-area-plant-health (accessed on 12 September 2020).
- Aschehoug, E.T.; Metlen, K.L.; Callaway, R.M.; Newcombe, G. Fungal endophytes directly increase the competitive effects of an invasive forb. Ecology 2012, 93, 3–8. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, Y.; Yang, H.; Wang, P. Detection of quiescent infection of mango stem end rot pathogen Lasiodiplodia theobromae in shoot and pre-plucked mango fruit by seminested PCR. Plant Pathol. Bull. 2009, 18, 225–235. [Google Scholar]
- Chhipa, H.; Chowdhary, K.; Kaushik, N. Artificial production of agarwood oil in Aquilaria sp. by fungi: A review. Phytochem. Rev. 2017, 16, 835–860. [Google Scholar] [CrossRef]
- Sajitha, K.L.; Florence, E.J.M.; Arun, S. Screening of bacterial biocontrols against sapstain fungus (Lasiodiplodia theobromae Pat.) of rubberwood (Hevea brasiliensis Muell. Arg.). Res. Microbiol. 2014, 165, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Mohali, S.; Encinas, O.; Mora, N. Manchado azul en madera de Pinus oocarpa y Azadirachta indica en Venezuela. Fitopatol. Venez. 2002, 15, 30–32. [Google Scholar]
- Mohali, S.R.; Castro-Medina, F.; Úrbez-Torres, J.R.; Gubler, W.D. First report of Lasiodiplodia theobromae and L. venezuelensis associated with blue stain on Ficus insipida wood from the Natural Forest of Venezuela. For. Pathol. 2017, 47, e12355. [Google Scholar] [CrossRef]
- Ludwig-Müller, J. Plants and endophytes: Equal partners in secondary metabolite production? Biotechnol. Lett. 2015, 37, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, R.; Fiorentino, A. Plant bioactive metabolites and drugs produced by endophytic fungi of Spermatophyta. Agriculture 2015, 5, 918–970. [Google Scholar] [CrossRef]
- Caruso, G.; Abdelhamid, M.; Kalisz, A.; Sekara, A. Linking endophytic fungi to medicinal plants therapeutic activity. A case study on Asteraceae. Agriculture 2020, 10, 286. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Alves, A.; Andolfi, A. Secondary metabolites of Lasiodiplodia theobromae: Distribution, chemical diversity, bioactivity, and implications of their occurrence. Toxins 2020, 12, 457. [Google Scholar] [CrossRef] [PubMed]
- Félix, C.; Salvatore, M.M.; Dellagreca, M.; Meneses, R.; Duarte, A.S.; Salvatore, F.; Naviglio, D.; Gallo, M.; Jorrín-Novo, J.V.; Alves, A.; et al. Production of toxic metabolites by two strains of Lasiodiplodia theobromae, isolated from a coconut tree and a human patient. Mycologia 2018, 110, 642–653. [Google Scholar] [CrossRef] [PubMed]
- Félix, C.; Salvatore, M.M.; DellaGreca, M.; Ferreira, V.; Duarte, A.S.; Salvatore, F.; Naviglio, D.; Gallo, M.; Alves, A.; Esteves, A.C.; et al. Secondary metabolites produced by grapevine strains of Lasiodiplodia theobromae grown at two different temperatures. Mycologia 2019, 111, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Keller, N.P.; Turner, G.; Bennett, J.W. Fungal secondary metabolism-from biochemistry to genomics. Nat. Rev. Microbiol. 2005, 3, 937–947. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Nicoletti, R.; DellaGreca, M.; Andolfi, A. Occurrence and properties of thiosilvatins. Mar. Drugs 2019, 17, 664. [Google Scholar] [CrossRef]
- Uranga, C.C.; Beld, J.; Mrse, A.; Córdova-Guerrero, I.; Burkart, M.D.; Hernández-Martínez, R. Fatty acid esters produced by Lasiodiplodia theobromae function as growth regulators in tobacco seedlings. Biochem. Biophys. Res. Commun. 2016, 472, 339–345. [Google Scholar] [CrossRef]
- Jernerén, F.; Eng, F.; Hamberg, M.; Oliw, E.H. Linolenate 9R-dioxygenase and allene oxide synthase activities of Lasiodiplodia theobromae. Lipids 2012, 47, 65–73. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Félix, C.; Lima, F.; Ferreira, V.; Duarte, A.S.; Salvatore, F.; Alves, A.; Esteves, A.C.; Andolfi, A. Effect of γ-aminobutyric Acid (GABA) on the metabolome of two strains of Lasiodiplodia theobromae isolated from grapevine. Molecules 2020, 25, 3833. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, H.; Li, W.; Tao, M.; Pan, Q.; Sun, Z.; Ye, W.; Li, H.; Zhang, W. 2-(2-Phenylethyl)chromones from endophytic fungal strain Botryosphaeria rhodina A13 from Aquilaria sinensis. Chin. Herb. Med. 2017, 9, 58–62. [Google Scholar] [CrossRef]
- Valayil, M.J.; Kuriakose, G.C.; Jayabaskaran, C. Isolation, purification and characterization of a novel steroidal saponin cholestanol glucoside from Lasiodiplodia theobromae that induces apoptosis in A549 cells. Anticancer Agents Med. Chem. 2016, 16, 865–874. [Google Scholar] [CrossRef]
- Vasanthakumari, M.M.; Jadhav, S.S.; Sachin, N.; Vinod, G.; Shweta, S.; Manjunatha, B.L.; Kumara, P.M.; Ravikanth, G.; Nataraja, K.N.; Uma Shaanker, R. Restoration of camptothecine production in attenuated endophytic fungus on re-inoculation into host plant and treatment with DNA methyltransferase inhibitor. World J. Microbiol. Biotechnol. 2015, 31, 1629–1639. [Google Scholar] [CrossRef]
- Nicoletti, R. Antitumor and immunomodulatory compounds from fungi. In Reference Module in Life Sciences (Planned for Publication in Encyclopaedia of Mycology); Zaragoza, O., Ed.; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Pandi, M.; Rajapriya, P.; Suresh, G.; Ravichandran, N.; Manikandan, R.; Thiagarajan, R.; Muthumary, J. A fungal taxol from Botryodiplodia theobromae Pat., attenuates 7, 12 dimethyl benz(a)anthracene (DMBA)-induced biochemical changes during mammary gland carcinogenesis. Biomed. Prev. Nutr. 2011, 1, 95–102. [Google Scholar] [CrossRef]
- Magnus, V.; Šimaga, Š.; Iskrić, S.; Kveder, S. Metabolism of Tryptophan, Indole-3-acetic acid, and related compounds in parasitic plants from the genus Orobanche. Plant. Physiol. 1982, 69, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Stahl, E.; Bellwon, P.; Huber, S.; Schlaeppi, K.; Bernsdorff, F.; Vallat-Michel, A.; Mauch, F.; Zeier, J. Regulatory and functional aspects of indolic metabolism in plant systemic acquired resistance. Mol. Plant. 2016, 9, 662–681. [Google Scholar] [CrossRef]
- Bartel, B. Auxin biosynthesis. Annu. Rev. Plant. Biol. 1997, 48, 51–66. [Google Scholar] [CrossRef]
- Félix, C.; Libório, S.; Nunes, M.; Félix, R.; Duarte, A.S.; Alves, A.; Esteves, A.C. Lasiodiplodia theobromae as a producer of biotechnologically relevant enzymes. Int. J. Mol. Sci. 2018, 19, 29. [Google Scholar] [CrossRef] [PubMed]
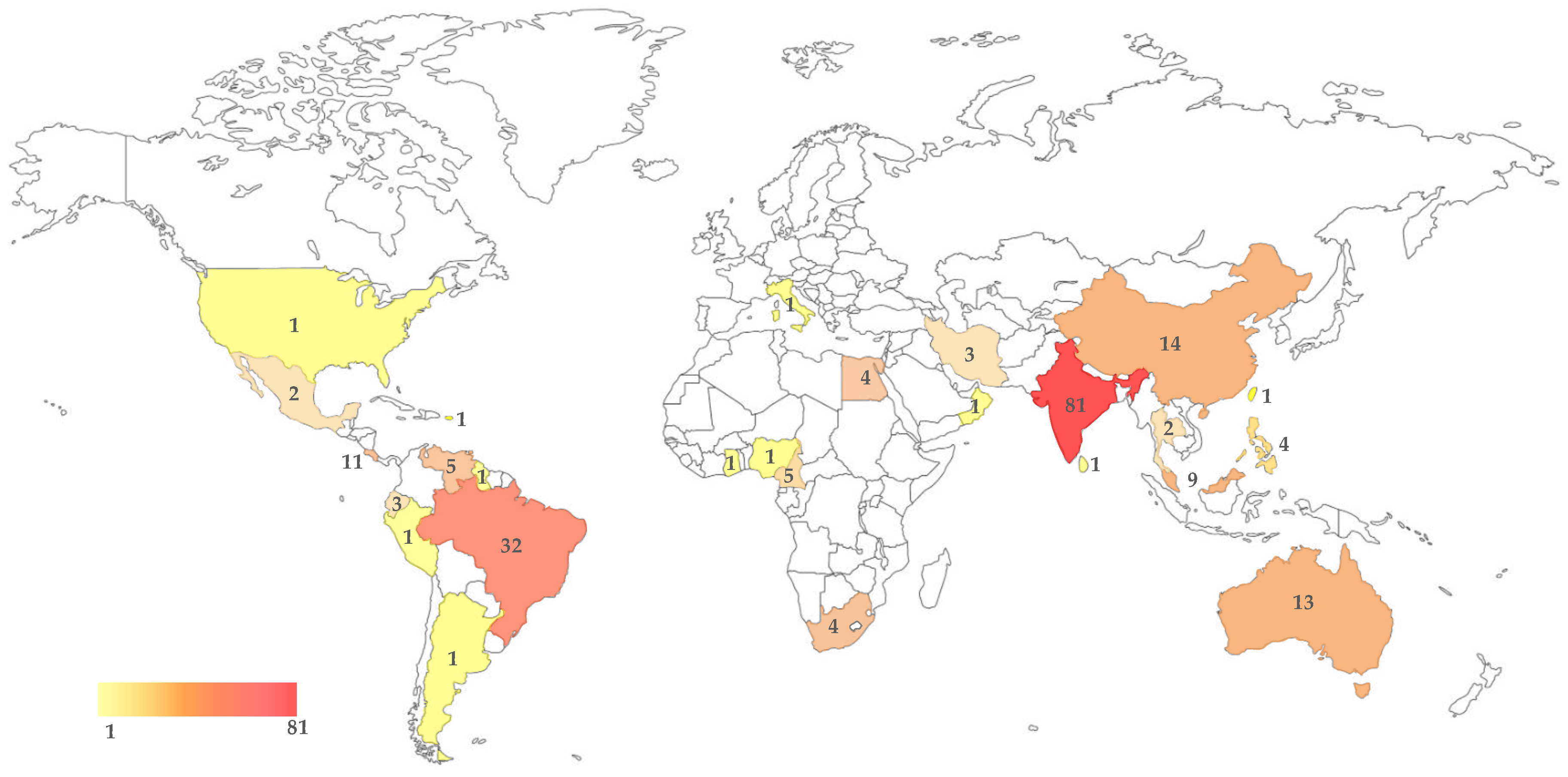
| Source | Origin | Ref. | |
|---|---|---|---|
| Pinophyta | |||
| Pinales, Pinaceae | Pinus elliottii | South Africa | [8] |
| Pinus caribaea var. hondurensis | Venezuela | [8] | |
| Pinus pseudostrobus | Mexico | [8] | |
| Pinus tabulaeformis | China | [30] | |
| Pinales, Taxaceae | Cephalotaxus hainanensis | China | [31] |
| Taxus baccata | India | [32] | |
| Taxus chinensis | China | GenBank | |
| Magnoliids | |||
| Magnoliales, Annonaceae | Annona muricata | Malaysia | GenBank |
| Piperales, Piperaceae | Piper hispidum | Brazil | [33,34] |
| Piper nigrum | India | [35] | |
| Monocots | |||
| Asparagales, Asparagaceae | Dracaena draco | Egypt | [36] |
| Asparagales, Orchidaceae | Campylocentrum micranthum | Costa Rica | [37] |
| Cattleya sp. | Brazil | [38] | |
| Cymbidium aloifolium | India | [39] | |
| Dendrobium moschatum | India | [39] | |
| Encyclia fragrans | Costa Rica | [37] | |
| Epidendrum difforme | Costa Rica | [37] | |
| Epidendrum octomerioides | Costa Rica | [37] | |
| Epidendrum radicans | India | GenBank | |
| Eria flava | India | [39] | |
| Nidema boothii | Costa Rica | [37] | |
| Oncidium sp. | Brazil | [38] | |
| Paphiopedilum fairrieanum | India | [39] | |
| Phalaenopsis sp. | Brazil | [38] | |
| Pholidota imbricata | India | [39] | |
| Pholidota pallida | India | [40] | |
| Pleurothallis guanacastensis | Costa Rica | [37] | |
| Pleurothallis phyllocardioides | Costa Rica | [37] | |
| Sobralia mucronata | Costa Rica | [37] | |
| Sobralia sp. | Costa Rica | [37] | |
| Trichosalpinx blasdellii | Costa Rica | [37] | |
| Vanilla planifolia | India | [39] | |
| Pandanales, Pandanaceae | Pandanus sp. | Thailand | [41] |
| Arecales, Arecaceae | Calamus thwaitesii | Sri Lanka | [42] |
| Cocos nucifera | Brazil | [28] | |
| India | [43] | ||
| Philippines | [44] | ||
| Euterpe oleracea | Brazil | [45] | |
| Nypa fruticans | Malaysia | [46] | |
| Poales, Cyperaceae | Mapania kurzii | Malaysia | [47] |
| Poales, Poaceae | Cynodon dactylon | India | GenBank |
| Zingiberales, Costaceae | Costus igneus | India | [48] |
| Zingiberales, Musaceae | Musa spp. | Malaysia | [49] |
| Eudicots | |||
| Proteales, Proteaceae | Grevillea agrifolia | Australia | [50] |
| Ranunculales, Menispermaceae | Tinospora cordifolia | India | [51] |
| Santalales, Santalaceae | Viscum coloratum | China | [52] |
| Saxifragales, Hamamelidaceae | Distilium chinense | China | [53] |
| Vitales, Vitaceae | Vitis vinifera | China | [54] |
| Italy | [55] | ||
| Celastrales, Celastraceae | Elaeodendrum glaucum | India | [56] |
| Salacia oblonga | India | [57] | |
| Fabales, Fabaceae | Acacia karroo | South Africa | [58] |
| Acacia mangium | Venezuela | [8] | |
| Acacia synchronicia | Australia | [50] | |
| Albizzia lebbeck | India | Genbank | |
| Arachis hypogaea | India | [56] | |
| Bauhinia racemosa | India | [56] | |
| Butea monosperma | India | [59,60] | |
| Cassia fistula | India | [56] | |
| Crotalaria medicaginea | Australia | [50] | |
| Dalbergia lanceolaria | India | [60] | |
| Dalbergia latifolia | India | [56] | |
| Glycyrrhiza glabra | India | [61] | |
| Humboldtia brunonis | India | [62] | |
| Indigofera suffruticosa | Brazil | [63] | |
| Libidibia (Caesalpinia) ferrea | Brazil | [64] | |
| Lysiphyllum cunninghamii | Australia | [50] | |
| Mimosa caesalpinifolia | Brazil | [64] | |
| Ougeinia oojeinensis | India | [60] | |
| Phaseolus lunatus | Mexico | [65] | |
| Pongamia pinnata | India | [43] | |
| Saraca asoca | India | [66,67] | |
| Sophora tonkinensis | China | [68] | |
| Malpighiales, Chrysobalanaceae | Licania rigida | Brazil | [64] |
| Malpighiales, Clusiaceae | Garcinia mangostana | Thailand | [69] |
| Malpighiales, Euphorbiaceae | Croton campestris | Brazil | [64] |
| Croton sonderianus | Brazil | [64] | |
| Givotia rottleriformis | India | [60] | |
| Hevea brasiliensis | Malaysia | GenBank | |
| Peru | [70] | ||
| Malpighiales, Hypericaceae | Hypericum mysorense | India | [71] |
| Malpighiales, Rhizophoraceae | Bruguiera cylindrica | Philippines | [72] |
| Ceriops tagal | China | GenBank | |
| Rhizophora mucronata | China | [73] | |
| Malpighiales, Salicaceae | Populus sp. | China | [74] |
| Oxalidales, Elaeocarpaceae | Elaeocarpus ganitrus | India | GenBank |
| Elaeocarpus tuberculatus | India | [56] | |
| Rosales, Moraceae | Artocarpus altilis | Ecuador | Genbank |
| Ficus opposita | Australia | [50] | |
| Ficus racemosa | India | GenBank | |
| Ficus trigona | Ecuador | GenBank | |
| Rosales, Rhamnaceae | Ziziphus xylopyrus | India | [60] |
| Rosales, Ulmaceae | Zelkova carpinifolia | Iran | GenBank |
| Cucurbitales, Cucurbitaceae | Momordica charantia | China | [75] |
| Fagales, Fagaceae | Quercus castaneifolia | Iran | GenBank |
| Fagales, Juglandaceae | Pterocarya fraxinifolia | Iran | GenBank |
| Brassicales, Moringaceae | Moringa oleifera | Brazil | [64] |
| Malvales, Malvaceae | Adansonia digitata | Australia | [50] |
| Cameroon | [15] | ||
| Adansonia gregorii | Australia | [50] | |
| Adansonia za | Australia | [50] | |
| Gossypium hirsutum | India | [76] | |
| Grewia tiliaefolia | India | [56] | |
| Helicteres isora | India | [60] | |
| Kydia calycina | India | [60] | |
| Theobroma cacao | Brazil | [77] | |
| India | [78] | ||
| Theobroma gileri | Ecuador | [79] | |
| Malvales, Thymelaeaceae | Aquilaria malaccensis | India | [80] |
| Aquilaria sinensis | China | [81,82] | |
| Taiwan | GenBank | ||
| Myrtales, Combretaceae | Anogeissus latifolia | India | [60] |
| Combretum leprosum | Brazil | [64] | |
| Lumnitzera littorea | Philippines | [72] | |
| Terminalia arjuna | India | [83,84] | |
| Terminalia bellerica | India | [56] | |
| Terminalia catappa | Cameroon | [85,86] | |
| Terminalia crenulata | India | [60] | |
| Terminalia ivorensis | Cameroon | [87] | |
| Terminalia mantaly | Cameroon | [86,87] | |
| Terminalia pterocarya | Australia | [50] | |
| Terminalia superba | Cameroon | [87] | |
| Terminalia tomentosa | India | [56] | |
| Myrtales, Lythraceae | Lagerstroemia microcarpa | India | [60] |
| Lagerstroemia parviflora | India | [60] | |
| Myrtales, Melastomataceae | Memecylon umbellatum | India | [88] |
| Myrtales, Myrtaceae | Calytrix sp. | Australia | [50] |
| Corymbia sp. | Australia | [50] | |
| Eucalyptus sp. | Australia | [50] | |
| Eucalyptus urophylla | Venezuela | [8] | |
| Eugenia uniflora | Brazil | [64] | |
| Psidium guajava | Venezuela | [89] | |
| Brazil | [64] | ||
| India | [90] | ||
| Nigeria | GenBank | ||
| Psidium rufum | Brazil | [64] | |
| Syzygium cordatum | South Africa | [11] | |
| Syzygium cumini | India | [60] | |
| Sapindales, Anacardiaceae | Anacardium occidentale | Brazil | [91,92] |
| Astronium fraxinifolium | Brazil | [64] | |
| Mangifera indica | Australia | [93] | |
| Brazil | [91] | ||
| Venezuela | [94] | ||
| Costa Rica | [95] | ||
| Myracrodruon urundeuva | Brazil | [64] | |
| Spondias mombin | Brazil | [64] | |
| Spondias sp. | Brazil | [64] | |
| Sapindales, Burseraceae | Boswellia ovalifoliata | India | [96] |
| Boswellia sacra | Oman | [97] | |
| Protium heptaphyllum | Brazil | [64] | |
| Sapindales, Meliaceae | Azadirachta indica | India | [43] |
| Khaya anthotheca | Ghana | [98] | |
| Sapindales, Rutaceae | Citrus sinensis | USA | [99] |
| Sapindales, Sapindaceae | Nephelium lappaceum | Malaysia | GenBank |
| Paullinia cupana | Brazil | GenBank | |
| Sapindales, Simaroubaceae | Ailanthus excelsa | India | [100] |
| Simarouba amara | Brazil | [64] | |
| Ericales, Ebenaceae | Diospyros montana | India | [60] |
| Ericales, Lecythidaceae | Barringtonia racemosa | South Africa | [101] |
| Careya arborea | India | [60] | |
| Ericales, Sapotaceae | Madhuca indica | India | [102] |
| Icacinales, Icacinaceae | Nothapodytes nimmoniana | India | [103] |
| Pyrenacantha sp. | India | GenBank | |
| Boraginales, Boraginaceae | Auxemma oncocalyx | Brazil | [64] |
| Cordia obliqua | India | [60] | |
| Cordia trichotoma | Brazil | [64] | |
| Cordia wallichi | India | [60] | |
| Gentianales, Apocynaceae | Alstonia scholaris | India | [56] |
| Catharanthus roseus | India | [90,104,105] | |
| Hancornia speciosa | Brazil | [106] | |
| Holarrhena antidysenterica | India | [59] | |
| Plumeria rubra | India | [107] | |
| Rauwolfia serpentina | India | [108] | |
| Gentianales, Loganiaceae | Strychnos potatorum | India | [60] |
| Gentianales, Rubiaceae | Coffea arabica | Puerto Rico | [109] |
| Ixora nigricans | India | [60] | |
| Morinda citrifolia | India | [110] | |
| Psychotria flavida | India | [62,111] | |
| Psychotria sp. | Brazil | [64] | |
| Lamiales, Acanthaceae | Acanthus ilicifolius | China | [112,113] |
| Avicennia lanata | Philippines | [114] | |
| Malaysia | [115] | ||
| Lamiales, Bignoniaceae | Jacaranda sp. | Guyana | [116] |
| Kigelia pinnata | India | [117] | |
| Radermachera xylocarpa | India | [56] | |
| Stereospermum angustifolium | India | [60] | |
| Lamiales, Lamiaceae | Gmelina arborea | India | [60] |
| Plectranthus amboinicus | India | [118] | |
| Pogostemon cablin | China | GenBank | |
| Premna tomentosa | India | [60] | |
| Tectona grandis | India | [60,119] | |
| Teucrium polium | Egypt | [120] | |
| Vitex negundo | India | [121] | |
| Vitex pinnata | Malaysia | [122] | |
| Lamiales, Oleaceae | Ligustrum lucidum | Argentina | [123] |
| Olea dioica | India | [56] | |
| Solanales, Solanaceae | Solanum melongena | Brazil | GenBank |
| Solanum nigrum | Egypt | [124] | |
| Solanum surratense | India | [125] | |
| Solanum torvum | India | [125] | |
| Withania somnifera | India | [125] | |
| Apiales, Araliaceae | Dendropanax laurifolius | Malaysia | GenBank |
| Asterales, Asteraceae | Bidens pilosa | Egypt | [126] |
| Bioactivity | Source | Sample tested | Ref. |
|---|---|---|---|
| Antibacterial | Acanthus ilicifolius | Secondary metabolites | [112] |
| Aquilaria sinensis | Culture filtrate extract | [81] | |
| Calamus thwaitesii | Culture filtrate extract | [42] | |
| Dracaena draco | Culture filtrate extract | [36] | |
| Garcinia mangostana | Secondary metabolites | [69] | |
| Hancornia speciosa | Culture filtrate extract | [106] | |
| Humboldtia brunonis | Culture filtrate extract | [62] | |
| Madhuca indica | Culture filtrate extract | [102] | |
| Piper hispidum | Culture filtrate extract | [33] | |
| Terminalia arjuna | Culture filtrate extract | [84] | |
| Antifungal | A. sinensis | Culture filtrate extract | [81] |
| Avicennia lanata | Culture filtrate extract | [114] | |
| Bidens pilosa | Culture filtrate extract and secondary metabolites | [126] | |
| H. speciosa | Culture filtrate extract | [106] | |
| H. brunonis | Culture filtrate extract | [62] | |
| T. arjuna | Culture filtrate extract | [84] | |
| Anti-inflammatory | Acanthus ilicifolius | Secondary metabolites | [113] |
| Antioxidant | Catharanthus roseus | Culture filtrate and mycelial extracts | [104] |
| C. roseus | Silver nanoparticles | [105] | |
| T. arjuna | Culture filtrate extract | [84] | |
| Antiprotozoal | A. lanata | Culture filtrate extract and chromatographic fraction | [115] |
| Vitex pinnata | Secondary metabolites | [122] | |
| Cytotoxic | Acanthus ilicifolius | Secondary metabolites | [112] |
| A. sinensis | Culture filtrate extract | [81] | |
| B. pilosa | Culture filtrate extract and secondary metabolites | [126] | |
| C. roseus | Silver nanoparticles | [90] | |
| C. roseus | Culture filtrate and mycelial extracts | [104] | |
| Morinda citrifolia | Secondary metabolite | [110] | |
| Plectranthus amboinicus | Secondary metabolite | [118] | |
| Enzymatic | Azadirachta indica | Isolate | [43] |
| Cocos nucifera | Isolate | [43] | |
| Pongamia pinnata | Isolate | [43] | |
| Psychotria flavida | Isolate | [111] | |
| Terminalia catappa | Isolate | [86] | |
| Terminalia mantaly | Isolate | [86] | |
| Heavy metal tolerance | Boswellia ovalifoliata | Isolate | [96] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvatore, M.M.; Andolfi, A.; Nicoletti, R. The Thin Line between Pathogenicity and Endophytism: The Case of Lasiodiplodia theobromae. Agriculture 2020, 10, 488. https://doi.org/10.3390/agriculture10100488
Salvatore MM, Andolfi A, Nicoletti R. The Thin Line between Pathogenicity and Endophytism: The Case of Lasiodiplodia theobromae. Agriculture. 2020; 10(10):488. https://doi.org/10.3390/agriculture10100488
Chicago/Turabian StyleSalvatore, Maria Michela, Anna Andolfi, and Rosario Nicoletti. 2020. "The Thin Line between Pathogenicity and Endophytism: The Case of Lasiodiplodia theobromae" Agriculture 10, no. 10: 488. https://doi.org/10.3390/agriculture10100488
APA StyleSalvatore, M. M., Andolfi, A., & Nicoletti, R. (2020). The Thin Line between Pathogenicity and Endophytism: The Case of Lasiodiplodia theobromae. Agriculture, 10(10), 488. https://doi.org/10.3390/agriculture10100488







