Walnut Shell Biochar Increases Seed Germination and Early Growth of Seedlings of Fodder Crops
Abstract
:1. Introduction
2. Materials and Methods
2.1. Biochar Production and Characterization
2.2. Biochar Bioassay for Fodder Seeds
2.3. Statistical Analysis
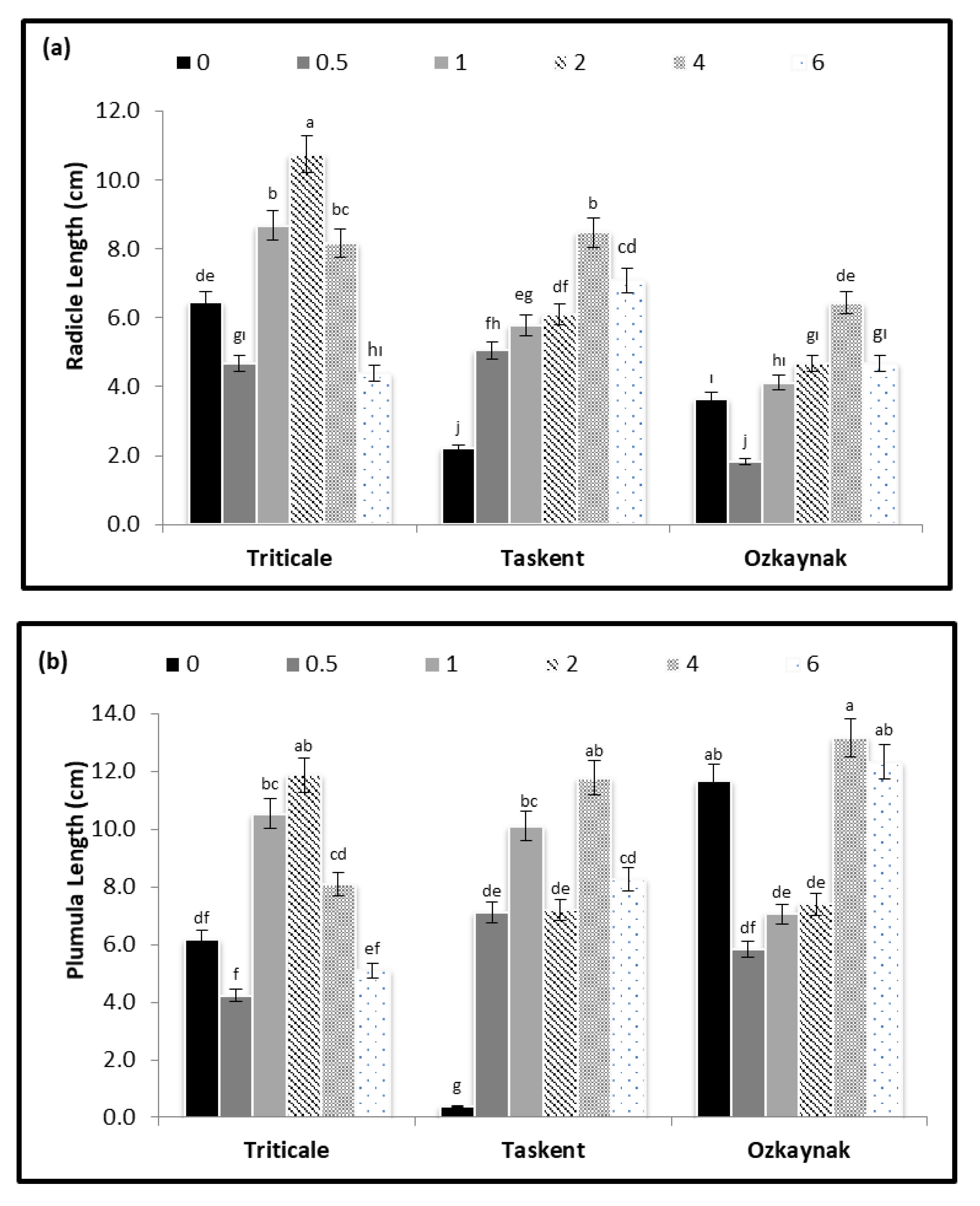
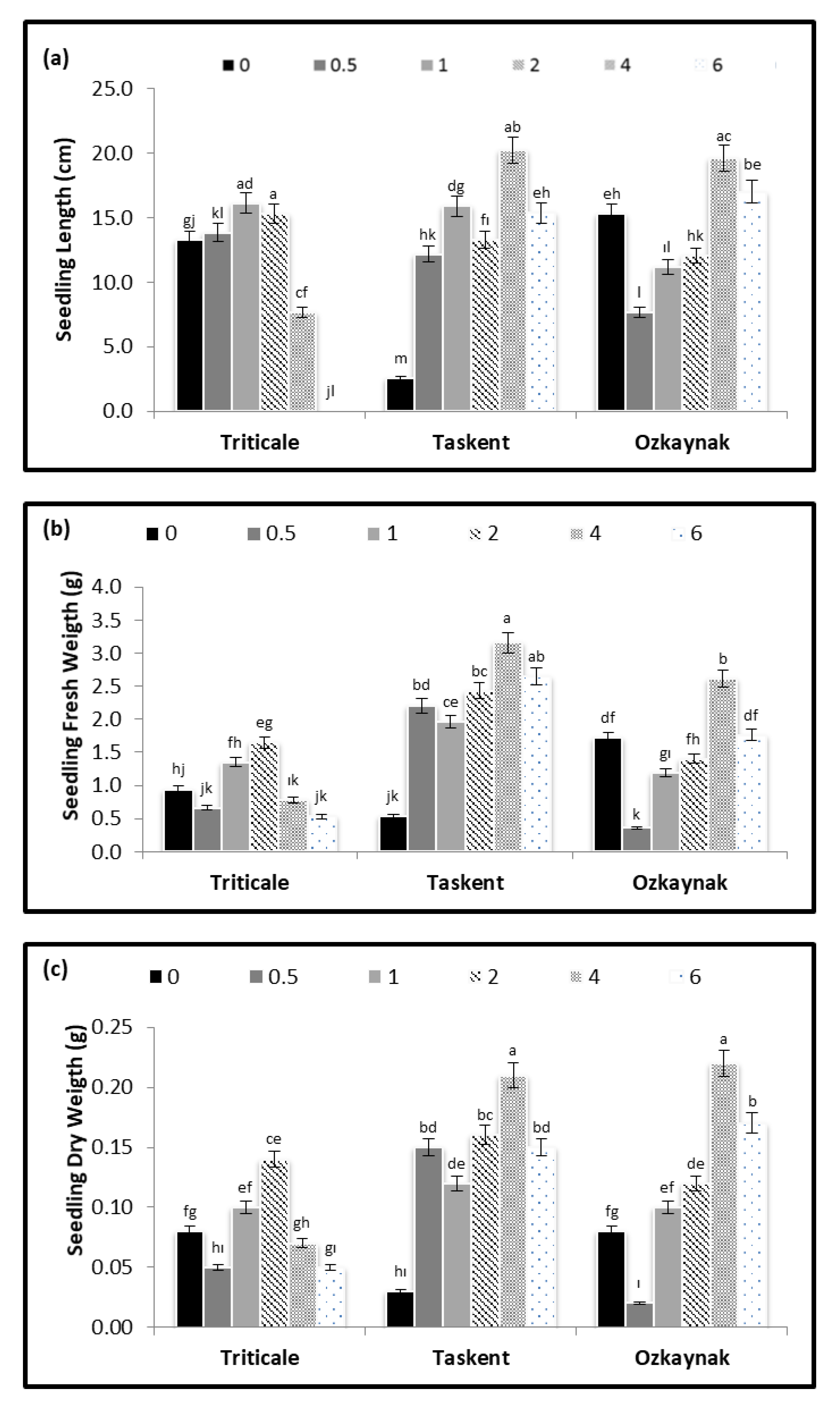
3. Results
3.1. Biochar Properties
3.2. Germination and Plant Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brevedan, R.E.; Egli, D.B. Short Period Water Stress during Seed Filling, Leaf Senescence and Yield of Soybean. Crop Sci. 2003, 43, 2083–2088. [Google Scholar]
- Hamayun, M.; Khan, S.A.; Shinwari, Z.K.; Khan, A.L.; Ahmad, N.; Lee, I. Effect of Polyethylene Glycol Induced Drought Stress on Physio-Hormonal Attributes of Soybean. Pak. J. Bot. 2010, 42, 977–986. [Google Scholar]
- Angevine, M.W.; Chabot, B.F. Seed Germination Syndromes in Higher Plants. In Topics in Plant Population Biology; Solbrig, O.T., Jain, S., Johnson, G.B., Raven, P.H., Eds.; Palgrave: London, UK, 1979. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Germination Ecophysiology of Herbaceous Plant Species in A Temperate Region. Amer. J. Bot. 1988, 75, 286–305. [Google Scholar] [CrossRef]
- Battaglia, M.L.; Lee, C.; Thomason, W. Corn Yield Components and Yield Responses to Defoliation at Different Row Widths. Agron. J. 2018, 110, 1–16. [Google Scholar] [CrossRef]
- Battaglia, M.L.; Lee, C.; Thomason, W.; Van Mullekom, J. Effects of Corn Row Width and Defoliation Timing and Intensity on Canopy Light Interception. Crop Sci. 2019, 59, 1718–1731. [Google Scholar] [CrossRef]
- Rogovska, N.; Laird, D.; Cruse, R.M.; Trabue, S.; Heaton, E. Germination Tests for Assessing Biochar Quality. J. Environ. Qual. 2011, 41, 1014–1022. [Google Scholar]
- Ketterings, Q.; Czymmek, K. Removal of Phosphorus by Field Crops; Agronomy Fact Sheet Series; Cornell University Cooperative Extension, 2007; Available online: http://nmsp.cals.cornell.edu/publications/factsheets/factsheet28.pdf (accessed on 23 September 2020).
- Kumar, P.; Lai, L.; Battaglia, M.L.; Kumar, S.; Owens, V.; Fike, J.; Galbraith, J.; Hong, C.O.; Faris, R.; Crawford, R.; et al. Impacts of Nitrogen Fertilization Rate and Landscape Position on Select Soil Properties in Switchgrass Field at Four Sites in the USA. CATENA 2019, 180, 183–193. [Google Scholar]
- Kumar, S.; Lai, L.; Kumar, P.; Feliciano, Y.M.V.; Battaglia, M.L.; Hong, C.O.; Owens, V.N.; Fike, J.; Farris, R.; Galbraith, J. Impacts of Nitrogen Rate and Landscape Position on Soils and Switchgrass Root Growth Parameters. Agron. J. 2019, 111, 1046–1059. [Google Scholar]
- Adeyemi, O.; Keshavarz-Afshar, R.; Jahanzad, E.; Battaglia, M.L.; Luo, Y.; Sadeghpour, A. Effect of Wheat Cover Crop and Split Nitrogen Application on Corn Yield and Nitrogen Use Efficiency. Agronomy 2020, 10, 1081. [Google Scholar] [CrossRef]
- Khan, A.A.; Jilani, G.; Akhtar, M.S.; Naqvi, S.M.S.; Rasheed, M. Phosphorus Solubilizing Bacteria, Occurrence, Mechanisms and Their Role in Crop Production. J. Agric. Biol. Sci. 2009, 1, 48–58. [Google Scholar]
- Battaglia, M.L.; Groover, G.; Thomason, W.E. Harvesting and Nutrient Replacement Costs Associated with Corn Stover Removal in Virginia. Va. Coop. Ext. Publ. 2018, CSES-229NP. Available online: https://www.pubs.ext.vt.edu/content/dam/pubs_ext_vt_edu/CSES/cses-229/CSES-229.pdf (accessed on 23 September 2020).
- Czymmek, K.; Ketterings, Q.; Ros, M.; Battaglia, M.L.; Cela, S.; Crittenden, S.; Gates, D.; Walter, T.; Latessa, S.; Klaiber, L.; et al. The New York Phosphorus Index 2.0; Agronomy Fact Sheet Series; Cornell University Cooperative Extension, 2020; Available online: http://nmsp.cals.cornell.edu/publications/factsheets/factsheet110.pdf (accessed on 23 September 2020).
- Solaiman, Z.M.; Murphy, D.V.; Abbott, L.K. Biochars Influence Seed Germination and Early Growth of Seedlings. Plant Soil. 2012, 353, 273–287. [Google Scholar] [CrossRef]
- Naeem, M.A.; Khalid, M.; Aon, M.; Abbas, G.; Tahir, M.; Amjad, M.; Murtaza, B.; Yang, A.; Akhtar, S.S. Effect of Wheat and Rice Straw Biochar Produced at Different Temperatures on Maize Growth and Nutrient Dynamics of A Calcareous Soil. Arch. Agron. Soil Sci. 2017, 63, 2048–2061. [Google Scholar] [CrossRef]
- Olszyk, D.M.; Shiroyama, T.; Novak, J.M.; Johnson, M.G. A Rapid-Test for Screening Biochar Effects on Seed Germination. Commun. Soil Sci. Plan. 2018, 49, 2025–2041. [Google Scholar] [CrossRef]
- Adnan, M.; Fahad, S.; Zamin, M.; Shah, S.; Mian, I.A.; Danish, S.; Zafar-ul-Hye, M.; Battaglia, M.L.; Naz, R.M.M.; Saeed, B.; et al. Coupling Phosphate-Solubilizing Bacteria with Phosphorus Supplements Improve Maize Phosphorus Acquisition and Growth under Lime Induced Salinity Stress. Plants 2020, 9, 900. [Google Scholar] [CrossRef] [PubMed]
- Diatta, A.A.; Thomason, W.E.; Abaye, O.; Thompson, T.L.; Battaglia, M.L.; Vaughan, L.J.; Lo, M.; Leme, J.F.D.C. Assessment of Nitrogen Fixation by Mungbean Genotypes in Different Soil Textures Using 15N Natural Abundance Method. J. Soil Sci. Plant Nut. 2020. [CrossRef]
- Diatta, A.A.; Fike, J.H.; Battaglia, M.L.; Galbraith, J.; Baig, M.B. Effects of Biochar on Soil Fertility and Crop Productivity in Arid Regions: A review. Arab. J. Geosci. 2020, 13, 595. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management: Science and Technology, 1st ed.; Sterling, V.A., Ed.; Earthscan: London, UK, 2009. [Google Scholar]
- Free, H.F.; McGill, C.R.; Rowarth, J.S.; Hedley, M.J. The Effect of Biochars on Maize (Zea Mays) Germination. N. Z. J. Agric. Res. 2010, 53, 1–4. [Google Scholar] [CrossRef]
- Sänger, A.; Reibe, K.; Mumme, J.; Kaupenjohann, M.; Ellmer, F.; Roß, C.L.; Meyer-Aurich, A. Biochar Application to Sandy Soil: Effects of Different Biochars and N Fertilization on Crop Yields in A 3-Year Field Experiment. Arch. Agron. Soil Sci. 2017, 63, 213–229. [Google Scholar] [CrossRef]
- Yamato, M.; Okimori, Y.; Wibowo, I.F.; Anshori, S.; Ogawa, M. Effect of the Application of Charred Bark of Acacia mangium on the Yield of Maize, Cowpea and Peanut, and Soil Chemical Properties in South Sumatra, Indonesia. Soil Sci. Plant Nut. 2006, 52, 489–495. [Google Scholar] [CrossRef]
- Ronsse, F.; Van Hecke, S.; Dickinson, D.; Prins, W. Production and Characterization of Slow Pyrolysis Biochar: Influence of Feedstock Type and Pyrolysis Conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Pluchon, N.; Gundale, M.J.; Nilsson, M.C.; Kardol, P.; Wardle, D.A. Stimulation of Boreal Tree Seedling Growth by Wood-Derived Charcoal: Effects of Charcoal Properties, Seedling Species and Soil Fertility. Funct. Ecol. 2014, 28, 766–775. [Google Scholar] [CrossRef]
- Kammann, C.I.; Linsel, S.; Geoßling, J.W.; Koyro, H.W. Influence of Biochar on Drought Tolerance of Chenopodium quinoa Willd and on Soil Plant Relations. Plant Soil. 2011, 345, 195–210. [Google Scholar] [CrossRef]
- Barrow, C.J. Biochar: Potential for Countering Land Degradation and for Improving Agriculture. Appl Geogr. 2012, 34, 21–28. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and Its Effects on Plant Productivity and Nutrient Cycling: A Meta-Analysis. GCB Bioenerg. 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Joseph, S.D.; Camps-Arbestain, M.; Lin, Y.; Munroe, P. An Investigation into the Reactions of Biochar in Soil. Soil Res. 2010, 48, 501–515. [Google Scholar] [CrossRef]
- Sohi, S.P.; Krull, E.; Lopez-Capel, E.; Bol, R. A Review of Biochar and Its Use and Function in Soil. Adv. Agron. 2010, 105, 47–82. [Google Scholar]
- He, L.; Zhao, X.; Wang, S.Q.; Xing, G.X. The Effects of Rice-Straw Biochar Addition on Nitrification Activity and Nitrous Oxide Emissions in Two Oxisols. Soil Till. Res. 2016, 164, 52–62. [Google Scholar] [CrossRef]
- Sheng, G.; Yang, Y.; Huang, M.; Yang, K. Influence of pH on Pesticide Sorption by Soil Containing Wheat Residue-Derived Char. Environ. Pollut. 2005, 134, 457–463. [Google Scholar] [CrossRef]
- Atkinson, J.A.; Fitzgerald, J.D.; Hipps, N.A. Potential Mechanisms for Achieving Agricultural Benefits from Biochar Application to Temperate Soils: A Review. Plant Soil. 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Wardle, D.A.; Zackrisson, O.; Nilsson, M.C. The Charcoal Effect on Boreal Forests: Mechanisms and Ecological Consequences. Oecologia 1998, 115, 419–426. [Google Scholar] [CrossRef]
- Chan, K.Y.; Van Zwieten, L.; Meszaros, I.; Downie, A.; Joseph, S. Using Poultry Litter Biochars as Soil Amendments. Soil Res. 2008, 46, 437–444. [Google Scholar] [CrossRef]
- Jeffery, S.; Verheijen, F.G.A.; van der Velde, M.; Bastos, A.C. A Quantitative Review of the Effects of Biochar Application to Soils on Crop Productivity Using Meta-Analysis. Agric. Ecosyst. Environ. 2011, 144, 175–187. [Google Scholar] [CrossRef]
- Deenik, J.L.; McClellan, T.; Uehara, G.; Antal, M.J.; Campbell, S. Charcoal Volatile Matter Content Influences Plant Growth and Soil Nitrogen Transformations. Soil Sci. Soc. Am. J. 2010, 74, 1259–1270. [Google Scholar] [CrossRef]
- Busch, D.; Kammann, C.; Grünhage, L.; Müller, C. Simple Biotoxicity Tests for Evaluation of Carbonaceous Soil Additives: Establishment and Reproducibility of Four Test Procedures. J. Environ. Qual. 2011, 40, 1–10. [Google Scholar] [CrossRef]
- Gascó, G.; Cely, P.; Paz-Ferreiro, J.; Plaza, C.; Méndez, A. Relation between Biochar Properties and Effects on Seed Germination and Plant Development. Biol. Agric. Hortic. 2016, 32, 237–247. [Google Scholar] [CrossRef]
- Kookana, R.S.; Sarmah, A.K.; Van Zwieten, L.; Krull, E.; Singh, B. Biochar Application to Soil: Agronomic and Environmental Benefits and Unintended Consequences. Adv. Agron. 2011, 112, 103–143. [Google Scholar]
- Rosende, M.; Beesley, L.; Moreno-Jimenez, E.; Miró, M. Automatic Flow-Through Dynamic Extraction: A Fast Tool to Evaluate Char-Based Remediation of Multi-Element Contaminated Mine Soils. Talanta 2016, 148, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Sütyemez, M. Bitkisel Üretimde Kahramanmaraş. Kahramanmaraş ta Tarım Yaşam 2015, 4, 88. [Google Scholar]
- Weidhuner, A.; Keshavarz Afshar, R.; Luo, Y.; Battaglia, M.; Sadeghpour, A. Sample Grinding Size Affects Nitrogen and Carbon Estimate of A Wheat Cover Crop. Agron. J. 2019, 111, 3398–3402. [Google Scholar] [CrossRef]
- Rhoades, J.D. Electrical Conductivity Methods for Measuring and Mapping Soil Salinity. Adv. Agron. 1993, 49, 201–251. [Google Scholar]
- G&lser, C.; Pek&en, A. Using Tea Waste as A New Casing Material in Mushroom (Agaricus Bisporus (L.) Sing.) Cultivation. Bioresour. Technol. 2003, 88, 153–156. [Google Scholar]
- Walkley, A.; Black, A.I. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-total. In Methods of Soil Analysis; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Jones, J.B., Jr.; Case, V.W. Sampling, handling, and analyzing plant tissue samples, chapter 15. In Soil Testing and Plant Analysis, 3rd ed.; Westerman, R.L., Ed.; SSSA: Madison, WI, USA, 1990; pp. 390–420. [Google Scholar]
- Morrison, D.A.; Morris, E.C. Pseudoreplication in Experimental Designs for the Manipulation of Seed Germination Treatments. Austral. Ecol. 2000, 25, 292–296. [Google Scholar] [CrossRef]
- Liao, S.; Pan, B.; Hao, L.; Zang, D.; Xing, B. Detecting Free Radicals in Biochars and Determining Their Ability to Inhibit the Germination and Growth of Corn, Wheat and Rice Seedlings. Environ. Sci. Technol. 2014, 48, 8581−8587. [Google Scholar] [CrossRef]
- Solaiman, Z.M.; Blackwell, P.; Abbott, L.K.; Storer, P. Direct and Residual Effect of Biochar Application on Mycorrhizal Roocolonizationon, Growth and Nutrition of Wheat. Soil Res. 2010, 48, 546–554. [Google Scholar] [CrossRef]
- Maquire, J.D. Speed of Germination Aid in Selection and Evaluation for Seedling Emergence and Vigor. Crop Sci. 1962, 2, 176–177. [Google Scholar] [CrossRef]
- Anonymous. Association of Official Seed Analysts, Rules for Testing Seeds. J. Seed Tech. 1984, 6, 1–125. [Google Scholar]
- Abdul-Baki, A.A.; Anderson, J.D. Vigor Determination in Soybean Seed by Multiple Criteria. Crop Sci. 1973, 13, 630–633. [Google Scholar] [CrossRef]
- Anonymous. Association of Official Seed Analysts, Seed Vigor Testing Handbook; ADSA: Boise, ID, USA, 1983. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H. Principles and Procedures of Statistics; McGraw-Hill: New York, NY, USA, 1960; p. 481. [Google Scholar]
- USDA. Natural Resources Conservation Service, Soil Quality Indicators: pH, January 1998. Available online: https://www.nrcs.usda.gov/Internet/FSE_DOCUMENTS/nrcs142p2_052208.pdf (accessed on 23 September 2020).
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-Char Sequestration in Terrestrial Ecosystems. Mitig. Adapt. Strateg. Glob. Chang. 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Novak, J.M.; Lima, I.; Xing, B.; Gaskin, J.W. Characterization and Their Effects on A Loamy Sand. Annal. Environ. Sci. 2009, 3, 195–206. [Google Scholar]
- Mendez, A.; Gomez, A.; Paz-Ferreiro, J.; Gasco, G. Effects of Sewage Sludge Biochar on Plant Metal Availability After Application to a Mediterranean Soil. Chemosphere 2012, 89, 1354–1359. [Google Scholar] [PubMed]
- Liang, C.; Zhu, X.; Fu, S.; Mendez, A.; Gasco, G.; Paz-Ferreiro, J. Biochar Alters the Resistance and Resilience to Drought in A Tropical Soil. Environ. Res. Lett. 2014, 9, 064013. [Google Scholar]
- Lu, H.; Li, Z.; Fu, S.; Mendez, A.; Gasco, G.; Paz-Ferreiro, J. Combining phtoextraction and Biochar Addition Improves Soil Biochemical Properties in A Soil Contaminated with Cd. Chemosphere 2015, 119, 209–216. [Google Scholar]
- Paz-Ferreiro, J.; Fu, S.; Mendez, A.; Gasco, G. Interactive Effects of Biochar and the Earthworm Pontoscolex Corethrurus on Plant Productivity and Soil Enzyme Activities. J. Soil Sediment. 2014, 14, 483–494. [Google Scholar]
- Pierce, G.L.; Warren, G.L.; Mikkelsen, R.L.; Linker, H.M. Effects of Soil Calcium and pH on Seed Germination and Subsequent Growth of Large Crabgrass (Digitaria sanguinalis). Weed Technol. 1999, 13, 421–424. [Google Scholar]
- Chidumayo, E.N. Effects of Woocarbonizationon on Soil and Initial Development of Seedlings in Miombo Woodland, Zambia. For. Ecol. Manag. 1994, 70, 353–357. [Google Scholar]
- Cely, P.; Gasco, G.; Paz-Ferreiro, J.; Mendez, A. Agronomic Properties of Biochars from Different Manure Wastes. J. Anal. Appl. Pyrol. 2015, 111, 173–182. [Google Scholar]
- Major, J. A Guide to Conducting Biochar Trials—International Biochar Initiative; 2009; pp. 1–30. Available online: www.biochar-international.org (accessed on 23 September 2020).
- Rondon, M.A.; Lehmann, J.; Ram&rez, J.; Hurtado, M. Biological Nitrogen Fixation by Common Beans (Phaseolus vulgaris L.) Increases with Bio-Char Additions. Biol. Fertil. Soil. 2007, 43, 699–708. [Google Scholar] [CrossRef]
- Sarma, B.; Gogoi, N. Germination and Seedling Growth of Okra (Abelmoschus esculentus L.) as Influenced by Organic Amendments. Cogent Food Agric. 2015, 1, 1030906. [Google Scholar]
- Gaskin, J.W.; Speir, A.; Morris, L.M.; Ogden, L.; Harris, K.; Lee, D.; Das, K.C. Potential for pyrolysis char to affect soil moisture and nutrient status of a loamy sand soil. In Proceedings of the 2007 Georgia Water Resources Conference; Georgia Institute of Technology: Athens, Greece, 2007. [Google Scholar]
- Asai, H.; Samson, B.K.; Stephan, H.M.; Songyikhangsuthor, K.; Homma, K.; Kiyono, Y.; Inoue, Y.; Shiraiwa, T.; Horie, T. Biochar Amendment Techniques for Upland Rice Production in Northern Laos: 1. Soil Physical Properties, Leaf SPAD and Grain Yield. Field Crops Res. 2009, 111, 81–84. [Google Scholar]
- Meng, H.; Hua, S.; Shamsi, I.H.; Jilani, G.; Li, Y.; Jiang, L. Cadmium-Induced Stress on the Seed Germination and Seedling Growth of Brassica napus L., and Its Alleviation through Exogenous Plant Growth Regulators. Plant Growth Regul. 2009, 58, 47–59. [Google Scholar] [CrossRef]
- Solaiman, Z.M. Use of Biochar for Sustainable Agriculture. J. Integr. Field Sci. 2018, 15, 8–17. [Google Scholar]

| Properties | Mean | Properties | Mean |
|---|---|---|---|
| pH (1:2.5) | 8.25 | Cu (ppm) | 9.56 |
| EC (1:2.5, μS cm−1) | 570.00 | Fe (ppm) | 328.44 |
| OC (%) | 79.60 | K (ppm) | 6132.38 |
| TN (%) | 0.12 | Mg (ppm) | 596.25 |
| WHC (%) | 61.29 | Mn (ppm) | 77.88 |
| Al (ppm) | 327.47 | Na (ppm) | 420.18 |
| B (ppm) | 19.78 | Ni (ppm) | 7.52 |
| Ca (ppm) | 6648.85 | P (ppm) | 224.13 |
| Cd (ppm) | 0.02 | Pb (ppm) | 1.46 |
| Co (ppm) | 0.22 | Zn (ppm) | 11.58 |
| Cr (ppm) | 5.20 |
| ANOVA | GR (%) | RL (cm) | PL (cm) | SL (cm) | SFW (g) | SDW (g) | VI |
|---|---|---|---|---|---|---|---|
| Cultivar | ** | * | ns | ns | * | ** | ** |
| Biochar | * | * | ns | * | ns | ** | ** |
| Cultivar × Biochar | * | * | * | * | * | * | * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uslu, O.S.; Babur, E.; Alma, M.H.; Solaiman, Z.M. Walnut Shell Biochar Increases Seed Germination and Early Growth of Seedlings of Fodder Crops. Agriculture 2020, 10, 427. https://doi.org/10.3390/agriculture10100427
Uslu OS, Babur E, Alma MH, Solaiman ZM. Walnut Shell Biochar Increases Seed Germination and Early Growth of Seedlings of Fodder Crops. Agriculture. 2020; 10(10):427. https://doi.org/10.3390/agriculture10100427
Chicago/Turabian StyleUslu, Omer Suha, Emre Babur, Mehmet Hakkı Alma, and Zakaria M. Solaiman. 2020. "Walnut Shell Biochar Increases Seed Germination and Early Growth of Seedlings of Fodder Crops" Agriculture 10, no. 10: 427. https://doi.org/10.3390/agriculture10100427
APA StyleUslu, O. S., Babur, E., Alma, M. H., & Solaiman, Z. M. (2020). Walnut Shell Biochar Increases Seed Germination and Early Growth of Seedlings of Fodder Crops. Agriculture, 10(10), 427. https://doi.org/10.3390/agriculture10100427








