Motor Control Stabilisation Exercise for Patients with Non-Specific Low Back Pain: A Prospective Meta-Analysis with Multilevel Meta-Regressions on Intervention Effects
Abstract
:1. Introduction
2. Methods
2.1. Design
2.1.1. Meta-Level
2.1.2. Design at the Study Level
2.2. Recruitment, Screening and Inclusion and Exclusion Criteria (Study Level)
2.3. Randomization
2.4. Outcomes
2.5. Effect Estimators
2.6. Intervention
2.7. Risk of Bias within Studies
2.8. Measures of Treatment Effects—Main Effects
2.9. Measures of Treatment Effects–Assessment of Heterogeneity
2.10. Measures of an Interaction of the Treatment Effects–Multilevel Sensitivity Meta-Regressions
2.11. Risk of Bias Across Studies
2.12. Effect Estimators’ Level of Evidence
3. Results
3.1. Participants Flow
3.2. Participants and Study Characteristics
3.3. Risk of Bias Within Studies
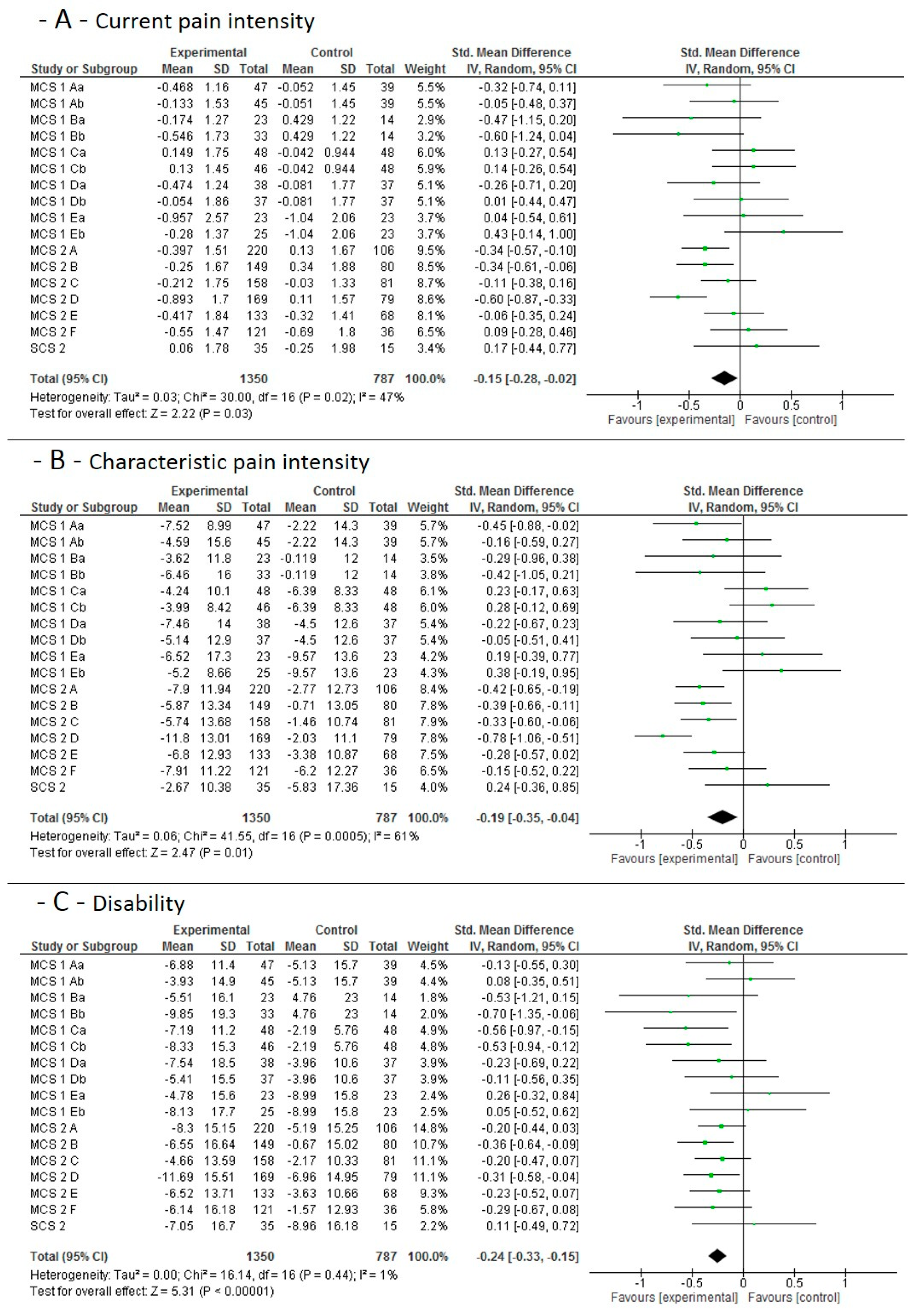
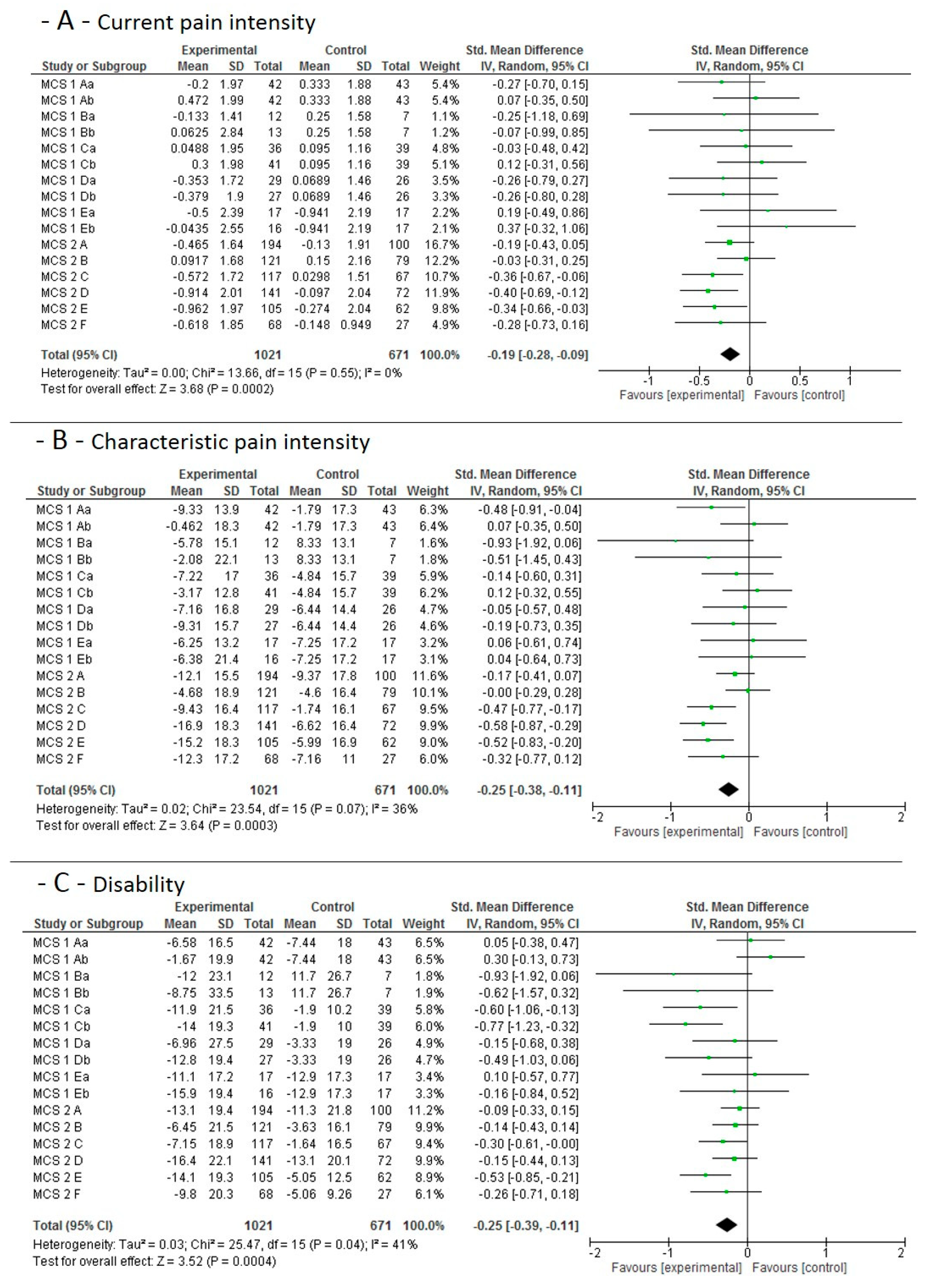
3.4. Main Effect Estimates
3.4.1. Short-Term Effects
3.4.2. Mid-Term Main Study Period Effects
3.4.3. Long-Term and Sustainability Effects
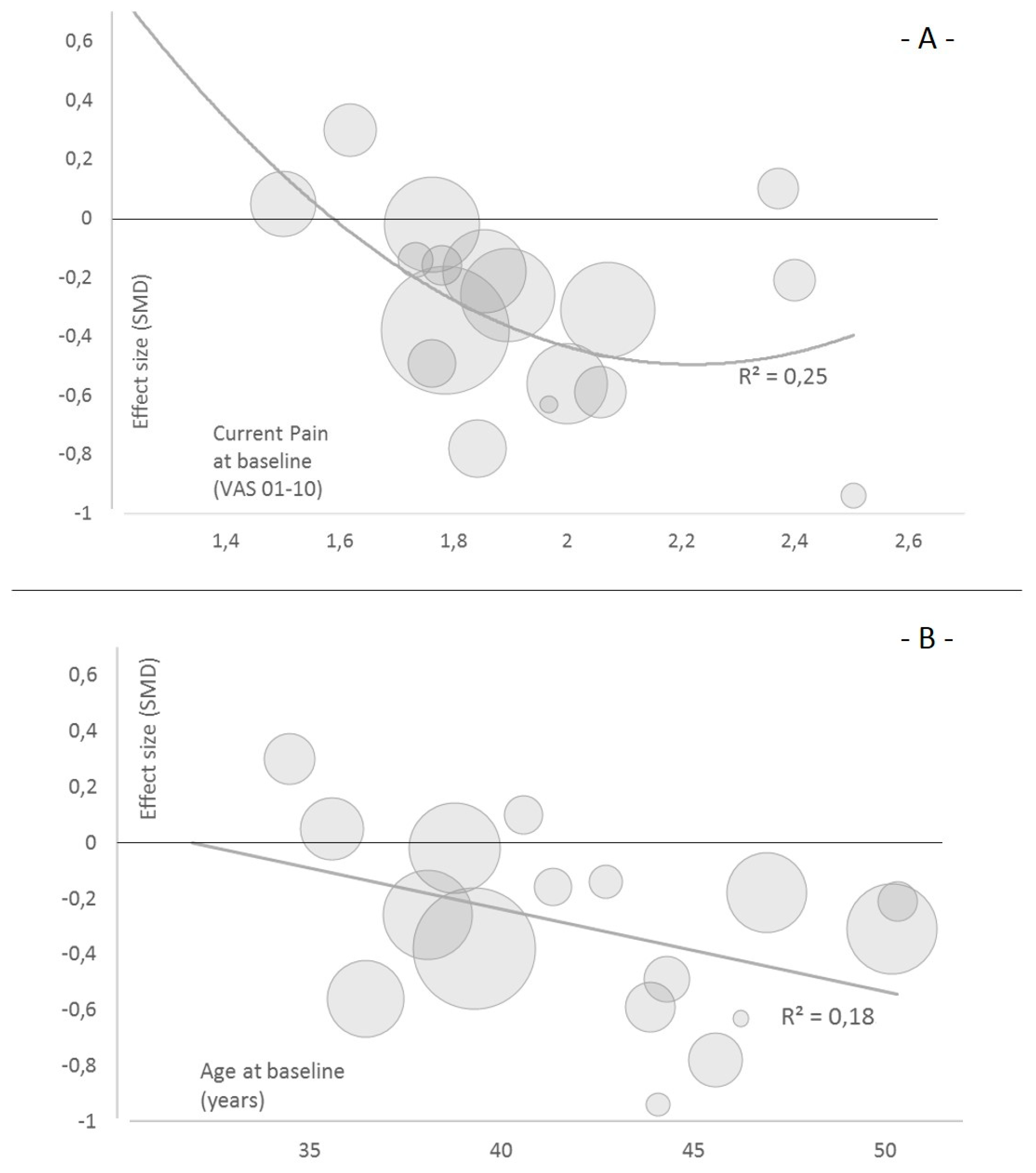
3.5. Sensitivity Analyses
4. Discussion
4.1. Summary of Evidence and Hypothesis Verification
4.2. Comparison with other Evidence
4.3. Practical Relevance
4.4. Definition of the Intervention
4.5. Limitations
4.5.1. Study and Outcome Level
4.5.2. Meta-Level
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Borghuis, J.; Hof, A.L.; Lemmink, K.A.P.M. The importance of sensory-motor control in providing core stability: Implications for measurement and training. Sports Med. 2008, 38, 893–916. [Google Scholar] [CrossRef]
- Brown, S.H.M.; McGill, S.M. The intrinsic stiffness of the in vivo lumbar spine in response to quick releases: Implications for reflexive requirements. J. Electromyogr. Kinesiol. 2009, 19, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Hartvigsen, J.; Lings, S.; Leboeuf-Yde, C.; Bakketeig, L. Psychosocial factors at work in relation to low back pain and consequences of low back pain; a systematic, critical review of prospective cohort studies. Occup. Environ. Med. 2004, 61, e2. [Google Scholar] [PubMed]
- Searle, A.; Spink, M.; Ho, A.; Chuter, V. Exercise interventions for the treatment of chronic low back pain: A systematic review and meta-analysis of randomised controlled trials. Clin. Rehabil. 2015, 29, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Saragiotto, B.T.; Maher, C.G.; Yamato, T.P.; Costa, L.O.P.; Menezes Costa, L.C.; Ostelo, R.W.J.G.; Macedo, L.G. Motor control exercise for chronic non-specific low-back pain. Cochrane Database Syst. Rev. 2016, CD012004. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.C.W.; Poh, R.L.C.; Low, A.Y.; Wong, W.P. Effects of Pilates-based exercises on pain and disability in individuals with persistent nonspecific low back pain: A systematic review with meta-analysis. J. Orthop. Sports Phys. Ther. 2011, 41, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Neto, M.; Lopes, J.M.; Conceição, C.S.; Araujo, A.; Brasileiro, A.; Sousa, C.; Carvalho, V.O.; Arcanjo, F.L. Stabilization exercise compared to general exercises or manual therapy for the management of low back pain: A systematic review and meta-analysis. Phys. Ther. Sport 2017, 23, 136–142. [Google Scholar] [CrossRef]
- Wang, X.-Q.; Zheng, J.-J.; Yu, Z.-W.; Bi, X.; Lou, S.-J.; Liu, J.; Cai, B.; Hua, Y.-H.; Wu, M.; Wei, M.-L.; et al. A meta-analysis of core stability exercise versus general exercise for chronic low back pain. PLoS ONE 2012, 7, e52082. [Google Scholar] [CrossRef] [Green Version]
- Riemann, B.L.; Lephart, S.M. The Sensorimotor System, Part I: The Physiologic Basis of Functional Joint Stability. J. Athl. Train. 2002, 37, 71–79. [Google Scholar]
- Owen, P.J.; Miller, C.T.; Mundell, N.L.; Verswijveren, S.J.; Tagliaferri, S.D.; Brisby, H.; Bowe, S.J.; Belavy, D.L. Which specific modes of exercise training are most effective for treating low back pain? Network meta-analysis. Br. J. Sports Med. 2019. [Google Scholar] [CrossRef]
- Sharan, D.; Rajkumar, J.S.; Mohandoss, M.; Ranganathan, R. Myofascial low back pain treatment. Curr. Pain Headache Rep. 2014, 18, 449. [Google Scholar] [CrossRef]
- Egan, M.; Seeger, D.; Schöps, P. Physiotherapie und physikalische Therapie in der Schmerzmedizin. Schmerz 2015, 29, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Pogue, J.; Yusuf, S. Overcoming the limitations of current meta-analysis of randomised controlled trials. Lancet 1998, 351, 47–52. [Google Scholar] [CrossRef]
- Margitić, S.E.; Morgan, T.M.; Sager, M.A.; Furberg, C.D. Lessons learned from a prospective meta-analysis. J. Am. Geriatr. Soc. 1995, 43, 435–439. [Google Scholar] [CrossRef]
- Ghersi, D.; Berlin, J.; Askie, L.; On Behalf of the Cochrane Prospective Meta-Analysis Methods Group. Prospective meta-analysis. In Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [Updated March 2011]; Higgins, J.P.T., Green, S., Eds.; The Cochrane Collaboration: London, UK, 2011. [Google Scholar]
- Seidler, A.L.; Hunter, K.E.; Cheyne, S.; Ghersi, D.; Berlin, J.A.; Askie, L. A guide to prospective meta-analysis. BMJ 2019, 367, l5342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bundesärztekammer, Kassenärztliche Bundesvereinigung (KBV); Arbeitsgemeinschaft der Wissenschaftli-chen Medizinischen Fachgesellschaften. Nationale VersorgungsLeitlinie Nicht-spezifischer Kreuzschmerz. 2. Auflage. Version 1; 2017. Available online: https://www.leitlinien.de/mdb/downloads/nvl/kreuzschmerz/kreuzschmerz-2aufl-vers1-kurz.pdf (accessed on 17 September 2020).
- Wippert, P.-M.; Wiebking, C. Stress and Alterations in the Pain Matrix: A Biopsychosocial Perspective on Back Pain and Its Prevention and Treatment. Int. J. Environ. Res. Public Health 2018, 15, 785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer, F.; Arampatzis, A.; Banzer, W.; Beck, H.; Brüggemann, G.-P.; Hasenbring, M.; Kellmann, M.; Kleinert, J.; Schiltenwolf, M.; Schmidt, H.; et al. Medicine in Spine Exercise [MiSpEx]—A national research network to evaluate back pain. Dtsch. Z. Sportmed. 2018, 2018, 229–235. [Google Scholar] [CrossRef]
- Hönning, A.; Stengel, D.; Güthoff, C. Statistical strategies to address main research questions of the MiSpEx network and meta-analytical approaches. Dtsch. Z. Sportmed. 2018, 2018, 236–239. [Google Scholar] [CrossRef]
- Niederer, D.; Vogt, L.; Wippert, P.-M.; Puschmann, A.-K.; Pfeifer, A.-C.; Schiltenwolf, M.; Banzer, W.; Mayer, F. Medicine in spine exercise (MiSpEx) for nonspecific low back pain patients: Study protocol for a multicentre, single-blind randomized controlled trial. Trials 2016, 17, 507. [Google Scholar] [CrossRef]
- Thong, I.S.K.; Jensen, M.P.; Miró, J.; Tan, G. The validity of pain intensity measures: What do the NRS, VAS, VRS, and FPS-R measure? Scand. J. Pain 2018, 18, 99–107. [Google Scholar] [CrossRef]
- von Korff, M.; Ormel, J.; Keefe, F.J.; Dworkin, S.F. Grading the severity of chronic pain. Pain 1992, 50, 133–149. [Google Scholar] [CrossRef]
- Wippert, P.-M.; de Witt Huberts, J.; Klipker, K.; Gantz, S.; Schiltenwolf, M.; Mayer, F. Beschreibung und empirische Fundierung des verhaltenstherapeutischen Moduls der MiSpEx-Intervention: Randomisierte, kontrollierte Studie zu chronischem unspezifischem Rückenschmerz. Schmerz 2015, 29, 658–663. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hedges, L.V.; Tipton, E.; Johnson, M.C. Robust variance estimation in meta-regression with dependent effect size estimates. Res. Synth. Methods 2010, 1, 39–65. [Google Scholar] [CrossRef] [PubMed]
- Atkins, D.; Best, D.; Briss, P.A.; Eccles, M.; Falck-Ytter, Y.; Flottorp, S.; Guyatt, G.H.; Harbour, R.T.; Haugh, M.C.; Henry, D.; et al. Grading quality of evidence and strength of recommendations. BMJ 2004, 328, 1490. [Google Scholar] [CrossRef] [Green Version]
- Shekelle, P.G.; Woolf, S.H.; Eccles, M.; Grimshaw, J. Developing clinical guidelines. West. J. Med. 1999, 170, 348–351. [Google Scholar]
- Arampatzis, A.; Schroll, A.; Catalá, M.M.; Laube, G.; Schüler, S.; Dreinhofer, K. A random-perturbation therapy in chronic non-specific low-back pain patients: A randomised controlled trial. Eur. J. Appl. Physiol. 2017, 117, 2547–2560. [Google Scholar] [CrossRef]
- Niederer, D.; Müller, J. Sustainability effects of motor control stabilisation exercises on pain and function in chronic nonspecific low back pain patients: A systematic review with meta-analysis and meta-regression. PLoS ONE 2020, 15, e0227423. [Google Scholar] [CrossRef]
- Brooks, C.; Siegler, J.C.; Cheema, B.S.; Marshall, P.W.M. No relationship between body mass index and changes in pain and disability after exercise rehabilitation for patients with mild to moderate chronic low back pain. Spine (Phila PA 1976) 2013, 38, 2190–2195. [Google Scholar] [CrossRef]
- Farin, E.; Gramm, L.; Schmidt, E. The patient-physician relationship in patients with chronic low back pain as a predictor of outcomes after rehabilitation. J. Behav. Med. 2013, 36, 246–258. [Google Scholar] [CrossRef]
- Garcia, A.N.; Costa, L.D.C.M.; Hancock, M.; Costa, L.O.P. Identifying Patients with Chronic Low Back Pain Who Respond Best to Mechanical Diagnosis and Therapy: Secondary Analysis of a Randomized Controlled Trial. Phys. Ther. 2016, 96, 623–630. [Google Scholar] [CrossRef] [PubMed]
- van der Hulst, M.; Vollenbroek-Hutten, M.M.R.; Ijzerman, M.J. A systematic review of sociodemographic, physical, and psychological predictors of multidisciplinary rehabilitation-or, back school treatment outcome in patients with chronic low back pain. Spine (Phila PA 1976) 2005, 30, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Cecchi, F.; Pasquini, G.; Paperini, A.; Boni, R.; Castagnoli, C.; Pistritto, S.; Macchi, C. Predictors of response to exercise therapy for chronic low back pain: Result of a prospective study with one year follow-up. Eur. J. Phys. Rehabil. Med. 2014, 50, 143–151. [Google Scholar] [PubMed]
- Hill, J.C.; Whitehurst, D.G.T.; Lewis, M.; Bryan, S.; Dunn, K.M.; Foster, N.E.; Konstantinou, K.; Main, C.J.; Mason, E.; Somerville, S.; et al. Comparison of stratified primary care management for low back pain with current best practice (STarT Back): A randomised controlled trial. Lancet 2011, 378, 1560–1571. [Google Scholar] [CrossRef] [Green Version]
- Hides, J.A.; Murphy, M.; Jang, E.; Blackwell, L.; Sexton, M.; Sexton, C.; Mendis, M.D. Predicting a beneficial response to motor control training in patients with low back pain: A longitudinal cohort study. Eur. Spine J. 2019. [Google Scholar] [CrossRef]
- Wippert, P.-M.; Puschmann, A.-K.; Drießlein, D.; Arampatzis, A.; Banzer, W.; Beck, H.; Schiltenwolf, M.; Schmidt, H.; Schneider, C.; Mayer, F. Development of a risk stratification and prevention index for stratified care in chronic low back pain. Focus: Yellow flags (MiSpEx network). Pain Rep. 2017, 2, e623. [Google Scholar] [CrossRef]
- O’Sullivan, P.B.; Phyty, G.D.; Twomey, L.T.; Allison, G.T. Evaluation of specific stabilizing exercise in the treatment of chronic low back pain with radiologic diagnosis of spondylolysis or spondylolisthesis. Spine (Phila Pa. 1976) 1997, 22, 2959–2967. [Google Scholar] [CrossRef]


| Study ID | Females (%) | Age (Years) | Body Mass Index (kg/m2) | Baseline Pain Intensity (VAS/NRS 0–10 cm)/Points) | Baseline Pain Intensity (Points 0–100) | Habitual Training/Exercise Volume (Minutes Per Week) | Mean MCE Training Volume (Treatment) during Intervention (1–12 Weeks) | Status in the Publication Process | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| MCS 1 A | 63 | 35.3 | 12.1 | 23.8 | 3.79 | 1.57 | 1.5 | 33.2 | 17.1 | 242 | 165 | 2.0 | In preparation |
| MCS 1 B | 65 | 45.5 | 8.04 | 24.6 | 2.98 | 2.09 | 2.5 | 34.6 | 23 | 208 | 113 | 1.9 | |
| MCS 1 C | 65 | 37.5 | 13.3 | 24.2 | 4.94 | 2.06 | 2.06 | 39.7 | 17.6 | 232 | 181 | 2.7 | |
| MCS 1 D | 65 | 41.6 | 13 | 24.9 | 3.56 | 2.16 | 1.73 | 35 | 20 | 182 | 111 | 2.5 | |
| MCS 1 E | 61 | 38.3 | 11.6 | 23.5 | 2.62 | 2.43 | 2.37 | 35.7 | 21.6 | 229 | 125 | 2.7 | |
| MCS 2 A | 49 | 38.9 | 12.4 | 25.4 | 3.95 | 1.65 | 1.76 | 25 | 20.7 | 2292 | 125 | 2.1 | In preparation |
| MCS 2 B | 55 | 46.9 | 11.9 | 24.6 | 3.78 | 1.64 | 1.85 | 29 | 19.1 | 276 | 312 | 2.0 | |
| MCS 2 C | 59 | 38.6 | 13.9 | 24.1 | 3.5 | 1.5 | 1.79 | 32.4 | 17.8 | 198 | 175 | 2.7 | |
| MCS 2 D | 49 | 48.1 | 13,0 | 26.2 | 4.91 | 2.66 | 2.1 | 36.2 | 15 | 336 | 321 | 2.7 | |
| MCS 2 E | 65 | 37.2 | 11.9 | 23.6 | 3.65 | 1.86 | 1.9 | 33.8 | 20.1 | 227 | 207 | 1.7 | |
| MCS 2 F | 56 | 35.4 | 13.4 | 23.5 | 3.71 | 1.82 | 2 | 35.8 | 16.1 | 217 | 211 | 2.2 | |
| SCS 1 | 48 | 31.8 | 5.88 | 23.3 | 3.9 | 4.05 | 1.2 | N/A | N/A | N/A | N/A | 2.3 | Published [29] |
| SCS 2 | 56 | 49.2 | 13.2 | N/A | N/A | 4.1 | 2.4 | 50.4 | 16.5 | N/A | N/A | N/A | In preparation |
| Study | Selection Bias: Random Sequence Generation | Selection Bias: Allocation Concealment | Performance Bias: Blinding of Participants and Personnel | Detection Bias: Blinding of Outcome Assessment | Attrition Bias: Incomplete Outcome Data | Reporting Bias: Selective Reporting | Other Bias |
|---|---|---|---|---|---|---|---|
| MCS 1 A | low | high | high | high | low | low | low |
| MCS 1 B | low | high | high | high | high | low | low |
| MCS 1 C | high | high | high | high | low | low | low |
| MCS 1 D | low | high | high | high | high | low | low |
| MCS 1 E | low | high | high | high | high | low | low |
| MCS 2 A | low | unknown | high | low | low | low | low |
| MCS 2 B | low | unknown | high | low | low | low | low |
| MCS 2 C | low | unknown | high | low | low | low | low |
| MCS 2 D | low | unknown | high | low | low | low | low |
| MCS 2 E | low | unknown | high | low | low | low | low |
| MCS 2 F | low | unknown | high | low | high | low | low |
| SCS 1 | unknown | unknown | high | high | low | low | low |
| SCS 2 | low | unknown | high | high | high | low | low |
| Model 1—Multilevel Meta-Regression | |||||
| N effect sizes included | Tau-square | ||||
| 51 | 0.036 | ||||
| Predictor/Moderator | Estimate | Standard Error | 95% Confidence interval | ||
| Lower level | Upper level | significance | |||
| Duration of the intervention (weeks) | −0.01 | 0.02 | −0.04 | 0.02 | n.s. |
| Intervention frequency (units/week) | −0.78 | 1.71 | −4.14 | 2.59 | n.s. |
| Habitual training volume (minutes/week) | 0.03 | 0.07 | −0.11 | 0.16 | n.s. |
| Intervention session duration (minutes/training) | −0.08 | 0.18 | −0.44 | 0.29 | n.s. |
| Female proportion | −1.27 | 2.04 | −5.27 | 2.72 | n.s. |
| Risk of BIAS sum score | 0.06 | 0.37 | −0.66 | 0.77 | n.s. |
| Mean age at baseline (years) | −0.01 | 0.04 | −0.08 | 0.06 | n.s. |
| Current pain (VAS or NRS) | −0.95 | 0.81 | −2.54 | 0.63 | n.s. |
| Body mass index (kg/m2) | 0.05 | 0.23 | −0.4 | 0.5 | n.s. |
| Characteristic pain intensity (0–100) | 0 | 0.03 | −0.06 | 0.07 | n.s. |
| Model 2—Single Level Meta-Regression | |||||
| Mean effect size | N effect sizes included | R-square | Homogeneity Q | Homogeneity p-value | |
| Descriptives | −0.2772 | 17 | 0.0252 | 0.0077 | >0.05 |
| Independent variable | B | Standard error | 95% CI | Z-value | p-value |
| MCE versus other | 0.211 | 3.16 | −6.3; 6.1 | −0.035 | >0.05 |
| MCE + behavioral versus other | −0.109 | 3.15 | −4.03; 3.55 | 0.0067 | >0.05 |
| MCE + perturbation versus other | −0.0782 | 2.97 | −5.9; 5.74 | −0.026 | >0.05 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niederer, D.; Engel, T.; Vogt, L.; Arampatzis, A.; Banzer, W.; Beck, H.; Moreno Catalá, M.; Brenner-Fliesser, M.; Güthoff, C.; Haag, T.; et al. Motor Control Stabilisation Exercise for Patients with Non-Specific Low Back Pain: A Prospective Meta-Analysis with Multilevel Meta-Regressions on Intervention Effects. J. Clin. Med. 2020, 9, 3058. https://doi.org/10.3390/jcm9093058
Niederer D, Engel T, Vogt L, Arampatzis A, Banzer W, Beck H, Moreno Catalá M, Brenner-Fliesser M, Güthoff C, Haag T, et al. Motor Control Stabilisation Exercise for Patients with Non-Specific Low Back Pain: A Prospective Meta-Analysis with Multilevel Meta-Regressions on Intervention Effects. Journal of Clinical Medicine. 2020; 9(9):3058. https://doi.org/10.3390/jcm9093058
Chicago/Turabian StyleNiederer, Daniel, Tilman Engel, Lutz Vogt, Adamantios Arampatzis, Winfried Banzer, Heidrun Beck, María Moreno Catalá, Michael Brenner-Fliesser, Claas Güthoff, Thore Haag, and et al. 2020. "Motor Control Stabilisation Exercise for Patients with Non-Specific Low Back Pain: A Prospective Meta-Analysis with Multilevel Meta-Regressions on Intervention Effects" Journal of Clinical Medicine 9, no. 9: 3058. https://doi.org/10.3390/jcm9093058







