Assessing the Relationship between Sense of Agency, the Bodily-Self and Stress: Four Virtual-Reality Experiments in Healthy Individuals
Abstract
1. Introduction
1.1. Sense of Agency and the Bodily-Self
1.2. Embodied SoA and Its Metacognition: Exteroceptive and Interoceptive Contributions
1.3. Open Questions: The Effect of Stress
1.4. The Present Study: Goals and Predictions
2. Participants
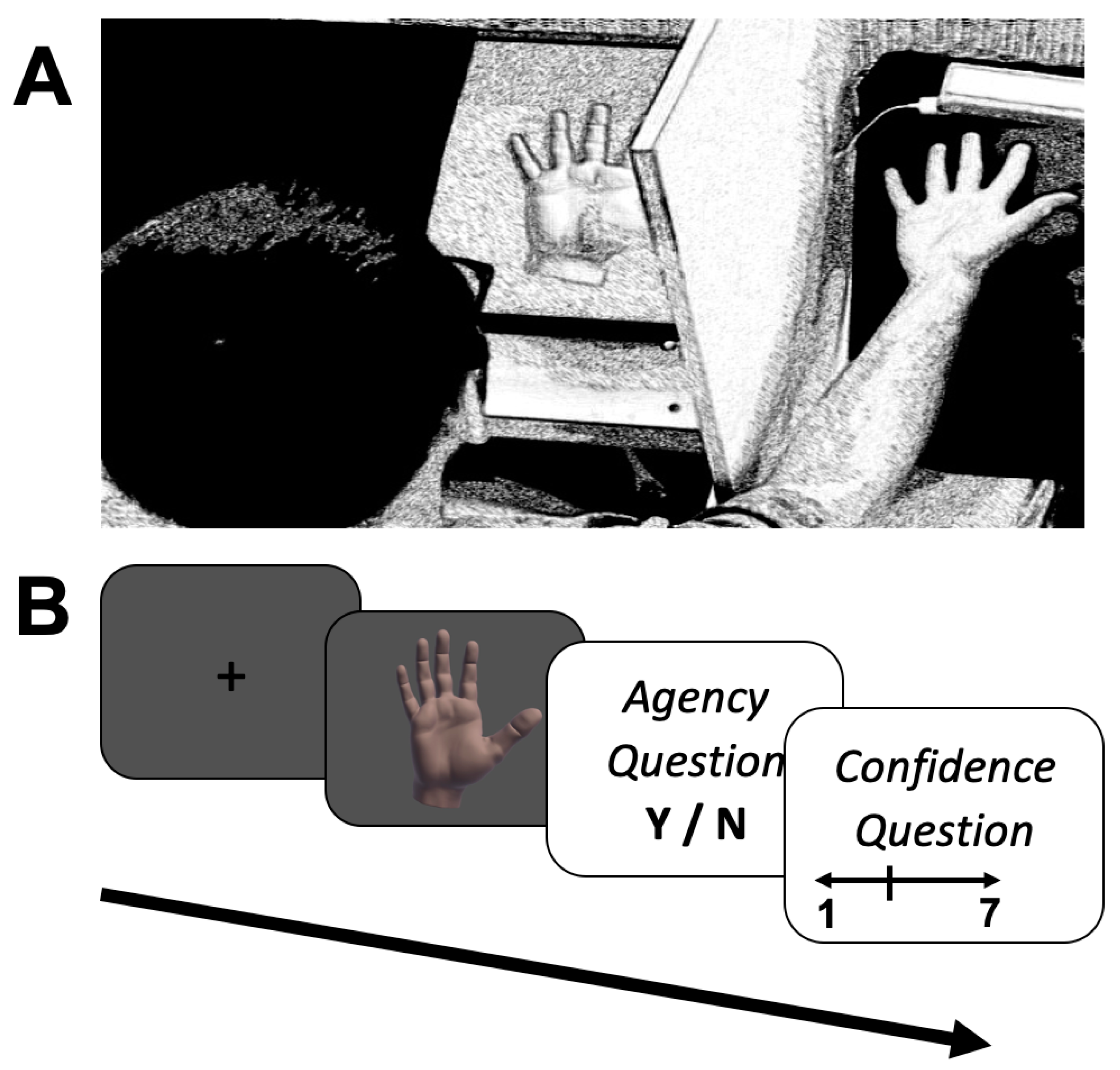
3. Procedure
3.1. Experiment 1
3.2. Experiment 2
3.3. Experiment 3
3.4. Experiment 4
4. Data Analysis
5. Results
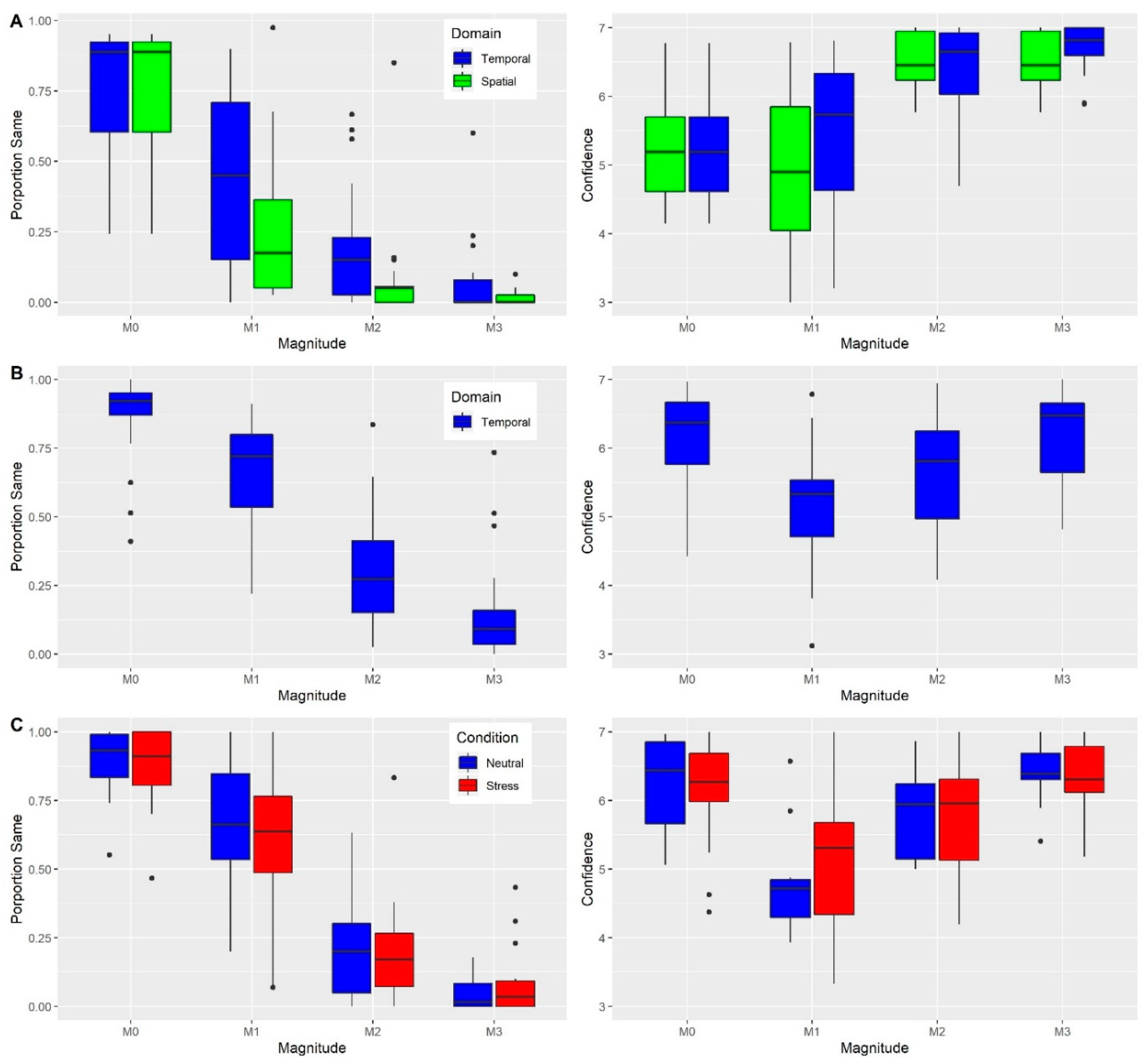
5.1. Experiment 1
5.2. Experiment 2
5.3. Experiment 3
5.4. Experiment 4
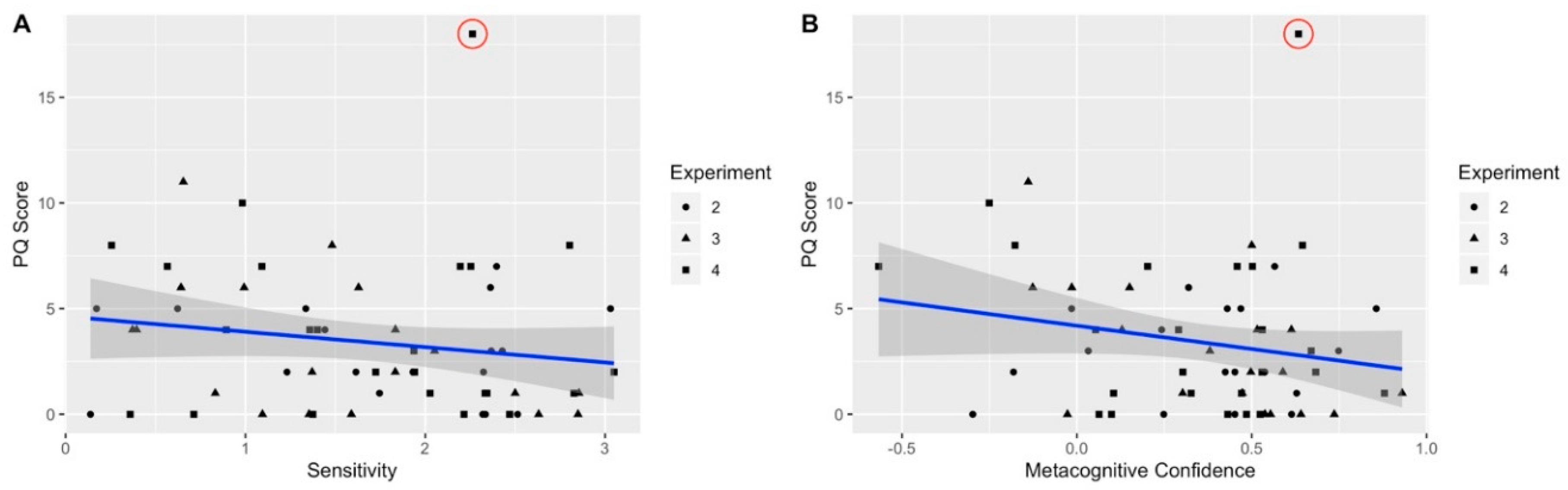
5.5. The Relation between APS and SoA across Experiments
6. Discussion
7. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Blanke, O.; Metzinger, T. Full-body illusions and minimal phenomenal selfhood. Trends Cogn. Sci. 2009, 13, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Blanke, O.; Slater, M.; Serino, A. Behavioral, Neural, and Computational Principles of Bodily Self-Consciousness. Neuron 2015, 88, 145–166. [Google Scholar] [CrossRef] [PubMed]
- Sass, L.A.; Parnas, J. Schizophrenia, consciousness, and the self. Schizophr. Bull. 2003, 29, 427–444. [Google Scholar] [CrossRef] [PubMed]
- Salomon, R. The Assembly of the Self from Sensory and Motor Foundations. Soc. Cogn. 2017, 35, 87–106. [Google Scholar] [CrossRef]
- Gallagher, S. The Natural Philosophy of Agency. Philos. Compass 2007, 2, 347–357. [Google Scholar] [CrossRef]
- Metzinger, T. Self models. Scholarpedia 2007, 2, 4174. [Google Scholar] [CrossRef]
- Botvinick, M.; Cohen, J. Rubber hands ‘feel’ touch that eyes see. Nature 1998, 391, 756. [Google Scholar] [CrossRef]
- Ehrsson, H.H.; Wiech, K.; Weiskopf, N.; Dolan, R.J.; Passingham, R.E. Threatening a rubber hand that you feel is yours elicits a cortical anxiety response. Proc. Natl. Acad. Sci. USA 2007, 104, 9828–9833. [Google Scholar] [CrossRef]
- Lenggenhager, B.; Tadi, T.; Metzinger, T.; Blanke, O. Video ergo sum: Manipulating bodily self-consciousness. Science 2007, 317, 1096–1099. [Google Scholar] [CrossRef]
- De Vignemont, F.; Fourneret, P. The sense of agency: A philosophical and empirical review of the “Who” system. Conscious. Cogn. 2004, 13, 1–19. [Google Scholar] [CrossRef]
- Haggard, P. Sense of agency in the human brain. Nat. Rev. Neurosci. 2017, 18, 196. [Google Scholar] [CrossRef] [PubMed]
- Kalckert, A.; Ehrsson, H.H. The moving rubber hand illusion revisited: Comparing movements and visuotactile stimulation to induce illusory ownership. Conscious. Cogn. 2014, 26, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Aarts, H.; Custers, R.; Wegner, D.M. On the inference of personal authorship: Enhancing experienced agency by priming effect information. Conscious. Cogn. 2005, 14, 439–458. [Google Scholar] [CrossRef]
- Moore, J.W.; Fletcher, P.C. Sense of agency in health and disease: A review of cue integration approaches. Conscious. Cogn. 2012, 21, 59–68. [Google Scholar] [CrossRef]
- Synofzik, M.; Vosgerau, G.; Lindner, A. Me or not me–An optimal integration of agency cues? Conscious. Cogn. 2009, 18, 1065–1068. [Google Scholar] [CrossRef] [PubMed]
- Wegner, D.M.; Sparrow, B.; Winerman, L. Vicarious agency: Experiencing control over the movements of others. J. Personal. Soc. Psychol. 2004, 86, 838. [Google Scholar] [CrossRef] [PubMed]
- Wegner, D.M. The mind’s best trick: How we experience conscious will. Trends Cogn. Sci. 2003, 7, 65–69. [Google Scholar] [CrossRef]
- Frith, C.; Blakemore, S.-J.; Wolpert, D. Abnormalities in the awareness and control of action. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2000, 355, 1771–1788. [Google Scholar] [CrossRef]
- Wolpert, D.M. Computational approaches to motor control. Trends Cogn. Sci. 1997, 1, 209–216. [Google Scholar] [CrossRef]
- Jeannerod, M. The sense of agency and its disturbances in schizophrenia: A reappraisal. Exp. Brain Res. 2009, 192, 527. [Google Scholar] [CrossRef]
- Christensen, M.S.; Grünbaum, T. Sense of agency for movements. Conscious. Cogn. 2018, 65, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.S.; Grünbaum, T. Sense of moving: Moving closer to the movement. Sensat. Mov. 2017, 64–84. [Google Scholar] [CrossRef]
- Anscombe, G.E.M. Intention; Harvard University Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Farrer, C.; Bouchereau, M.; Jeannerod, M.; Franck, N. Effect of distorted visual feedback on the sense of agency. Behav. Neurol. 2008, 19, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Gentsch, A.; Kathmann, N.; Schütz-Bosbach, S. Reliability of sensory predictions determines the experience of self-agency. Behav. Brain Res. 2012, 228, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Kalckert, A.; Ehrsson, H.H. Moving a rubber hand that feels like your own: A dissociation of ownership and agency. Front. Hum. Neurosci. 2012, 6, 40. [Google Scholar] [CrossRef]
- Ma, K.; Hommel, B. The role of agency for perceived ownership in the virtual hand illusion. Conscious. Cogn. 2015, 36, 277–288. [Google Scholar] [CrossRef]
- Salomon, R.; Fernandez, N.B.; Van Elk, M.; Vachicouras, N.; Sabatier, F.; Tychinskaya, A.; Llobera, J.; Blanke, O. Changing motor perception by sensorimotor conflicts and body ownership. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef]
- Salomon, R.; Lim, M.; Kannape, O.; Llobera, J.; Blanke, O. “Self pop-out”: Agency enhances self-recognition in visual search. Exp. Brain Res. 2013, 228, 173–181. [Google Scholar] [CrossRef]
- Jeannerod, M. Visual and action cues contribute to the self-other distinction. Nat. Neurosci. 2004, 7, 422–423. [Google Scholar] [CrossRef]
- Sirigu, A.; Daprati, E.; Pradat-Diehl, P.; Franck, N.; Jeannerod, M. Perception of self-generated movement following left parietal lesion. Brain 1999, 122, 1867–1874. [Google Scholar] [CrossRef]
- Caspar, E.A.; Cleeremans, A.; Haggard, P. The relationship between human agency and embodiment. Conscious. Cogn. 2015, 33, 226–236. [Google Scholar] [CrossRef]
- Engbert, K.; Wohlschläger, A.; Haggard, P. Who is causing what? The sense of agency is relational and efferent-triggered. Cognition 2008, 107, 693–704. [Google Scholar] [CrossRef] [PubMed]
- Salomon, R.; Malach, R.; Lamy, D. Involvement of the intrinsic/default system in movement-related self recognition. PLoS ONE 2009, 4, e7527. [Google Scholar] [CrossRef] [PubMed]
- Frith, C.; Done, D. Experiences of alien control in schizophrenia reflect a disorder in the central monitoring of action. Psychol. Med. 2009, 19, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, D.M.; Kawato, M. Multiple paired forward and inverse models for motor control. Neural Netw. 1998, 11, 1317–1329. [Google Scholar] [CrossRef]
- Wolpert, D.M.; Ghahramani, Z.; Jordan, M.I. An internal model for sensorimotor integration. Science 1995, 269, 1880. [Google Scholar] [CrossRef]
- Blakemore, S.-J.; Wolpert, D.M.; Frith, C.D. Central cancellation of self-produced tickle sensation. Nat. Neurosci. 1998, 1, 635–640. [Google Scholar] [CrossRef]
- Nielsen, T. Volition: A new experimental approach. Scand. J. Psychol. 1963, 4, 225–230. [Google Scholar] [CrossRef]
- Sato, A.; Yasuda, A. Illusion of sense of self-agency: Discrepancy between the predicted and actual sensory consequences of actions modulates the sense of self-agency, but not the sense of self-ownership. Cognition 2005, 94, 241–255. [Google Scholar] [CrossRef]
- Synofzik, M.; Thier, P.; Lindner, A. Internalizing agency of self-action: Perception of one’s own hand movements depends on an adaptable prediction about the sensory action outcome. J. Neurophysiol. 2006, 96, 1592. [Google Scholar] [CrossRef]
- Bergouignan, L.; Nyberg, L.; Ehrsson, H.H. Out-of-body-induced hippocampal amnesia. Proc. Natl. Acad. Sci. USA 2014, 111, 4421–4426. [Google Scholar] [CrossRef] [PubMed]
- Bohil, C.J.; Alicea, B.; Biocca, F.A. Virtual reality in neuroscience research and therapy. Nat. Rev. Neurosci. 2011, 12, 752. [Google Scholar] [CrossRef]
- Debarba, H.G.; Bovet, S.; Salomon, R.; Blanke, O.; Herbelin, B.; Boulic, R. Characterizing first and third person viewpoints and their alternation for embodied interaction in virtual reality. PLoS ONE 2017, 12, e0190109. [Google Scholar] [CrossRef] [PubMed]
- Kannape, O.A.; Schwabe, L.; Tadi, T.; Blanke, O. The limits of agency in walking humans. Neuropsychologia 2010, 48, 1628–1636. [Google Scholar] [CrossRef]
- Krugwasser, A.R.; Harel, E.V.; Salomon, R. The boundaries of the self: The sense of agency across different sensorimotor aspects. J. Vis. 2019, 19, 14. [Google Scholar] [CrossRef] [PubMed]
- Synofzik, M.; Thier, P.; Leube, D.T.; Schlotterbeck, P.; Lindner, A. Misattributions of agency in schizophrenia are based on imprecise predictions about the sensory consequences of one’s actions. Brain 2010, 133, 262–271. [Google Scholar] [CrossRef]
- Limanowski, J.; Kirilina, E.; Blankenburg, F. Neuronal correlates of continuous manual tracking under varying visual movement feedback in a virtual reality environment. NeuroImage 2017, 146, 81–89. [Google Scholar] [CrossRef]
- Herbelin, B.; Salomon, R.; Serino, A.; Blanke, O. 5. Neural Mechanisms of Bodily Self-Consciousness and the Experience of Presence in Virtual Reality. Hum. Comput. Conflu. Transform. Hum. Exp. Symbiotic Technol. 2015. [Google Scholar] [CrossRef]
- Sanchez-Vives, M.V.; Slater, M. From presence to consciousness through virtual reality. Nat. Rev. Neurosci. 2005, 6, 332–339. [Google Scholar] [CrossRef]
- Park, H.-D.; Bernasconi, F.; Bello-Ruiz, J.; Pfeiffer, C.; Salomon, R.; Blanke, O. Transient Modulations of Neural Responses to Heartbeats Covary with Bodily Self-Consciousness. J. Neurosci. 2016, 36, 8453–8460. [Google Scholar] [CrossRef]
- Park, H.-D.; Blanke, O. Coupling Inner and Outer Body for Self-Consciousness. Trends Cogn. Sci. 2019, 23, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Garfinkel, S.N.; Critchley, H.D.; Seth, A.K. Multisensory integration across exteroceptive and interoceptive domains modulates self-experience in the rubber-hand illusion. Neuropsychologia 2013, 51, 2909–2917. [Google Scholar] [CrossRef] [PubMed]
- Aspell, J.E.; Heydrich, L.; Marillier, G.; Lavanchy, T.; Herbelin, B.; Blanke, O. Turning Body and Self Inside Out: Visualized Heartbeats Alter Bodily Self-Consciousness and Tactile Perception. Psychol. Sci. 2013, 24, 2445–2453. [Google Scholar]
- Bury, G.; García-Huéscar, M.; Bhattacharya, J.; Ruiz, M.H. Cardiac afferent activity modulates early neural signature of error detection during skilled performance. NeuroImage 2019, 199, 704–717. [Google Scholar] [CrossRef]
- Wessel, J.R.; Danielmeier, C.; Ullsperger, M. Error awareness revisited: Accumulation of multimodal evidence from central and autonomic nervous systems. J. Cogn. Neurosci. 2011, 23, 3021–3036. [Google Scholar] [CrossRef]
- Chambon, V.; Filevich, E.; Haggard, P. What is the human sense of agency, and is it Metacognitive? In The Cognitive Neuroscience of Metacognition; Springer: Berlin/Heidelberg, Germany, 2014; pp. 321–342. [Google Scholar]
- Metcalfe, J.; Van Snellenberg, J.X.; DeRosse, P.; Balsam, P.; Malhotra, A.K. Judgements of agency in schizophrenia: An impairment in autonoetic metacognition. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 1391–1400. [Google Scholar] [CrossRef] [PubMed]
- Carlson, E.B.; Dalenberg, C.; McDade-Montez, E. Dissociation in posttraumatic stress disorder part I: Definitions and review of research. Psychol. Trauma Theory Res. Pract. Policy 2012, 4, 479–489. [Google Scholar] [CrossRef]
- Sass, L.; Pienkos, E.; Nelson, B.; Medford, N. Anomalous self-experience in depersonalization and schizophrenia: A comparative investigation. Conscious. Cogn. 2013, 22, 430–441. [Google Scholar] [CrossRef]
- Schauer, M.; Elbert, T. Dissociation following traumatic stress etiology and treatment. J. Psychol. 2010, 218, 109–127. [Google Scholar] [CrossRef]
- Stein, D.J.; Koenen, K.C.; Friedman, M.J.; Hill, E.; McLaughlin, K.A.; Petukhova, M.; Ruscio, A.M.; Shahly, V.; Spiegel, D.; Borges, G.; et al. Dissociation in posttraumatic stress disorder: Evidence from the world mental health surveys. Biol. Psychiatry 2013, 73, 302–312. [Google Scholar] [CrossRef]
- Fowles, D.C. Schizophrenia: Diathesis-stress revisited. Annu. Rev. Psychol. 1992, 43, 303–336. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.F.; Diforio, D. Schizophrenia: A neural diathesis-stress model. Psychol. Rev. 1997, 104, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Pruessner, M.; Cullen, A.E.; Aas, M.; Walker, E.F. The neural diathesis-stress model of schizophrenia revisited: An update on recent findings considering illness stage and neurobiological and methodological complexities. Neurosci. Biobehav. Rev. 2017, 73, 191–218. [Google Scholar] [CrossRef]
- Croft, J.; Heron, J.; Teufel, C.; Cannon, M.; Wolke, D.; Thompson, A.; Houtepen, L.; Zammit, S. Association of Trauma Type, Age of Exposure, and Frequency in Childhood and Adolescence with Psychotic Experiences in Early Adulthood. JAMA Psychiatry 2019, 76, 79–86. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.J.; McLaughlin, K.A.; Saha, S.; Aguilar-Gaxiola, S.; Al-Hamzawi, A.; Alonso, J.; Bruffaerts, R.; De Girolamo, G.; De Jonge, P.; Esan, O.; et al. The association between childhood adversities and subsequent first onset of psychotic experiences: A cross-national analysis of 23 998 respondents from 17 countries. Psychol. Med. 2017, 47, 1230–1245. [Google Scholar] [CrossRef]
- Frith, C. The Cognitive Neuropsychology of Schizophrenia; Psychology Press: London, UK, 2014. [Google Scholar]
- Nelson, B.; Whitford, T.J.; Lavoie, S.; Sass, L.A. What are the neurocognitive correlates of basic self-disturbance in schizophrenia?: Integrating phenomenology and neurocognition. Part 2 (Aberrant salience). Schizophr. Res. 2014, 152, 20–27. [Google Scholar] [CrossRef]
- Asai, T.; Tanno, Y. Highly schizotypal students have a weaker sense of self-agency. Psychiatry Clin. Neurosci. 2008, 62, 115–119. [Google Scholar] [CrossRef]
- Nelson, B.; Lavoie, S.; Li, E.; Sass, L.A.; Koren, D.; McGorry, P.D.; Jack, B.N.; Parnas, J.; Polari, A.; Allott, K.; et al. The neurophenomenology of early psychosis: An integrative empirical study. Conscious. Cogn. 2020, 77, 102845. [Google Scholar] [CrossRef]
- Eysenck, M.W.; Derakshan, N.; Santos, R.; Calvo, M.G. Anxiety and cognitive performance: Attentional control theory. Emotion 2007, 7, 336–353. [Google Scholar] [CrossRef]
- Kalckert, A.; Ehrsson, H.H. The spatial distance rule in the moving and classical rubber hand illusions. Conscious. Cogn. 2014, 30, 118–132. [Google Scholar] [CrossRef]
- Salomon, R.; Lim, M.; Pfeiffer, C.; Gassert, R.; Blanke, O. Full body illusion is associated with widespread skin temperature reduction. Front. Behav. Neurosci. 2013, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Hara, M.; Pozeg, P.; Rognini, G.; Higuchi, T.; Fukuhara, K.; Yamamoto, A.; Higuchi, T.; Blanke, O.; Salomon, R. Voluntary self-touch increases body ownership. Front. Psychol. 2015, 6, 1509. [Google Scholar] [CrossRef] [PubMed]
- Leube, D.T.; Knoblich, G.; Erb, M.; Grodd, W.; Bartels, M.; Kircher, T.T. The neural correlates of perceiving one’s own movements. Neuroimage 2003, 20, 2084–2090. [Google Scholar] [CrossRef] [PubMed]
- Sato, A. Both motor prediction and conceptual congruency between preview and action-effect contribute to explicit judgment of agency. Cognition 2009, 110, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Stanislaw, H.; Todorov, N. Calculation of signal detection theory measures. Behav. Res. Methods Instrum. Comput. 1999, 31, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Goodman, L.A.; Kruskal, W.H. Measures of Association for Cross Classifications. J. Am. Stat. Assoc. 1954, 49, 732–764. [Google Scholar]
- Nelson, T.O. A comparison of current measures of the accuracy of feeling-of-knowing predictions. Psychol. Bull. 1984, 95, 109. [Google Scholar] [CrossRef]
- Loewy, R.L.; Pearson, R.; Vinogradov, S.; Bearden, C.E.; Cannon, T.D. Psychosis risk screening with the Prodromal Questionnaire—Brief Version (PQ-B). Schizophr. Res. 2011, 129, 42–46. [Google Scholar] [CrossRef]
- Schandry, R. Heart beat perception and emotional experience. Psychophysiology 1981, 18, 483–488. [Google Scholar] [CrossRef]
- Garfinkel, S.N.; Seth, A.K.; Barrett, A.B.; Suzuki, K.; Critchley, H.D. Knowing your own heart: Distinguishing interoceptive accuracy from interoceptive awareness. Biol. Psychol. 2015, 104, 65–74. [Google Scholar] [CrossRef]
- Tsakiris, M.; Tajadura-Jiménez, A.; Costantini, M. Just a heartbeat away from one’s body:Interoceptive sensitivity predicts malleability of body-representations. Proc. R. Soc. B Biol. Sci. 2011, 278, 2470–2476. [Google Scholar] [CrossRef]
- Ardizzi, M.; Ferri, F. Interoceptive influences on peripersonal space boundary. Cognition 2018, 177, 79–86. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gorsuch, R.L.; Lushene, R.E. Manual for the State-Trait Anxiety Inventory (Self Evaluation Questionnaire). Palo Alto Calif. Consult. Psychol. 1970, 22, 1–24. [Google Scholar]
- Kirschbaum, C.; Pirke, K.-M.; Hellhammer, D.H. The ‘Trier Social Stress Test—A tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology 1993, 28, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Dedovic, K.; Renwick, R.; Mahani, N.K.; Engert, V. The Montreal Imaging Stress Task: Using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. J. Psychiatry Neurosci. 2005, 30, 319–325. [Google Scholar]
- G.Nautilus Wireless 64-Channel EEG Acquisition System; g.tec Medical Engineering GmbH: Schiedlberg, Austria; Available online: https://www.gtec.at/product/gnautilus-pro/ (accessed on 1 August 2019).
- Thayer, J.F.; Lane, R.D. A model of neurovisceral integration in emotion regulation and dysregulation. J. Affect. Disord. 2000, 61, 201–216. [Google Scholar] [CrossRef]
- Kim, H.G.; Cheon, E.J.; Bai, D.S.; Lee, Y.H.; Koo, B.H. Stress and heart rate variability: A meta-analysis and review of the literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Quintana, D.S.; Alvares, G.A.; Heathers, J.A.J. Guidelines for Reporting Articles on Psychiatry and Heart rate variability (GRAPH): Recommendations to advance research communication. Transl. Psychiatry 2016, 6, e803. [Google Scholar] [CrossRef]
- Castro, M.N.; Villarreal, M.F.; Bolotinsky, N.; Papávero, E.; Goldschmidt, M.G.; Costanzo, E.Y.; Drucaroff, L.; Wainsztein, A.; de Achával, D.; Pahissa, J.; et al. Brain activation induced by psychological stress in patients with schizophrenia. Schizophr. Res. 2015, 168, 313–321. [Google Scholar] [CrossRef] [PubMed]
- MathWorks, T. Matlab Version 9.6.0 (R2019a); The MathWorks: Natick, MA, USA, 2019. [Google Scholar]
- JASP, Version 0.9; Computer software; JASP Team: Amsterdam, The Netherlands, 2018.
- Wagenmakers, E.J.; Marsman, M.; Jamil, T.; Ly, A.; Verhagen, J.; Love, J.; Selker, R.; Gronau, Q.F.; Šmíra, M.; Epskamp, S.; et al. Bayesian inference for psychology. Part I: Theoretical advantages and practical ramifications. Psychon. Bull. Rev. 2018, 25, 35–57. [Google Scholar] [CrossRef]
- Kruschke, J.K.; Liddell, T.M. Bayesian data analysis for newcomers. Psychon. Bull. Rev. 2018, 25, 155–177. [Google Scholar] [CrossRef]
- Keysers, C.; Gazzola, V.; Wagenmakers, E.-J. Using Bayes factor hypothesis testing in neuroscience to establish evidence of absence. Nat. Neurosci. 2020, 23, 788–799. [Google Scholar] [CrossRef] [PubMed]
- Savill, M.; Ambrosio, J.D.; Cannon, T.D.; Loewy, R.L. Psychosis risk screening in different populations using the Prodromal Questionnaire: A systematic review. Early Interv. Psychiatry 2017, 12, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, S. Philosophical conceptions of the self: Implications for cognitive science. Trends Cogn. Sci. 2000, 4, 14–21. [Google Scholar] [CrossRef]
- Marshall, A.C.; Gentsch, A.; Schütz-Bosbach, S. The interaction between interoceptive and action states within a framework of predictive coding. Front. Psychol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Salomon, R.; Ronchi, R.; Dönz, J.; Bello-Ruiz, J.; Herbelin, B.; Martet, R.; Faivre, N.; Schaller, K.; Blanke, O. The insula mediates access to awareness of visual stimuli presented synchronously to the heartbeat. J. Neurosci. 2016, 36, 5115–5127. [Google Scholar] [CrossRef]
- Seth, A.K. Interoceptive inference, emotion, and the embodied self. Trends Cogn. Sci. 2013, 17, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Haggard, P.; Clark, S.; Kalogeras, J. Voluntary action and conscious awareness. Nat. Neurosci. 2002, 5, 382–385. [Google Scholar] [CrossRef]
- Tsakiris, M.; Prabhu, G.; Haggard, P. Having a body versus moving your body: How agency structures body-ownership. Conscious. Cogn. 2006, 15, 423–432. [Google Scholar] [CrossRef]
- Van Os, J.; Linscott, R.J.; Myin-Germeys, I.; Delespaul, P.; Krabbendam, L. A systematic review and meta-analysis of the psychosis continuum: Evidence for a psychosis proneness--persistence--impairment model of psychotic disorder. Psychol. Med. 2009, 39, 179–195. [Google Scholar] [CrossRef]
- Drori, G.; Bar-Tal, P.; Stern, Y.; Zvilichovsky, Y.; Salomon, R. UnReal? Investigating the Sense of Reality and Psychotic Symptoms with Virtual Reality. J. Clin. Med. 2020, 9, 1627. [Google Scholar] [CrossRef] [PubMed]
- Koren, D.; Scheyer, R.; Reznik, N.; Adres, M.; Apter, A.; Parnas, J.; Seidman, L.J. Basic self-disturbance, neurocognition and metacognition: A pilot study among help-seeking adolescents with and without attenuated psychosis syndrome. Early Interv. Psychiatry 2017. [Google Scholar] [CrossRef]
- Koren, D.; Scheyer, R.; Stern, Y.; Adres, M.; Reznik, N.; Apter, A.; Seidman, L.J. Metacognition strengthens the association between neurocognition and attenuated psychosis syndrome: Preliminary evidence from a pilot study among treatment-seeking versus healthy adolescents. Schizophr. Res. 2019, 210, 207–214. [Google Scholar] [CrossRef]
- Koren, D.; Seidman, L.J.; Goldsmith, M.; Harvey, P.D. Real-world cognitive—And metacognitive—Dysfunction in schizophrenia: A new approach for measuring (and remediating) more “right stuff”. Schizophr. Bull. 2006, 32, 310–326. [Google Scholar] [CrossRef]
- McDonald, M.; Christoforidou, E.; Van Rijsbergen, N.; Gajwani, R.; Gross, J.; Gumley, A.I.; Lawrie, S.M.; Schwannauer, M.; Schultze-Lutter, F.; Uhlhaas, P.J. Using online screening in the general population to detect participants at clinical high-risk for psychosis. Schizophr. Bull. 2018, 45, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Myin-Germeys, I.; van Os, J. Stress-reactivity in psychosis: Evidence for an affective pathway to psychosis. Clin. Psychol. Rev. 2007, 27, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Klippel, A.; Viechtbauer, W.; Reininghaus, U.; Wigman, J.; van Borkulo, C.; Myin-Germeys, I.; Wichers, M. The Cascade of Stress: A Network Approach to Explore Differential Dynamics in Populations Varying in Risk for Psychosis. Schizophr. Bull. 2017, 44, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Reininghaus, U.; Kempton, M.J.; Valmaggia, L.; Craig, T.K.J.; Garety, P.; Onyejiaka, A.; Gayer-Anderson, C.; So, S.H.; Hubbard, K.; Beards, S.; et al. Stress sensitivity, aberrant salience, and threat anticipation in early psychosis: An experience sampling study. Schizophr. Bull. 2016, 42, 712–722. [Google Scholar] [CrossRef] [PubMed]
- Yerkes, R.M.; Dodson, J.D. The relation of strength of stimulus to rapidity of habit-formation. J. Comp. Neurol. Psychol. 1908, 18, 459–482. [Google Scholar] [CrossRef]
- Fusar-Poli, P.; Borgwardt, S.; Bechdolf, A.; Addington, J.; Riecher-Rössler, A.; Schultze-Lutter, F.; Keshavan, M.; Wood, S.; Ruhrmann, S.; Seidman, L.J.; et al. The psychosis high-risk state: A comprehensive state-of-the-art review. Arch. Gen. Psychiatry 2013, 70, 107–120. [Google Scholar] [CrossRef] [PubMed]
- van der Gaag, M.; Smit, F.; Bechdolf, A.; French, P.; Linszen, D.H.; Yung, A.R.; McGorry, P.; Cuijpers, P. Preventing a first episode of psychosis: Meta-analysis of randomized controlled prevention trials of 12 month and longer-term follow-ups. Schizophr. Res. 2013, 149, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Tsuang, M.T.; Van Os, J.; Tandon, R.; Barch, D.M.; Bustillo, J.; Gaebel, W.; Gur, R.E.; Heckers, S.; Malaspina, D.; Owen, M.J.; et al. Attenuated psychosis syndrome in DSM-5. Schizophr. Res. 2013, 150, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Light, G.A.; Swerdlow, N.R. Bending the curve on psychosis outcomes. Lancet Psychiatry 2015, 2, 365–367. [Google Scholar] [CrossRef]
- Tsuang, M.T.; Shapiro, D.I.; Ronzio, A.; Bearden, C.E.; Cadenhead, K.S.; Addington, J.; McGlashan, T.H.; Walker, E.F.; Stone, W.S.; Perkins, D.O.; et al. Association of Neurocognition with Transition to Psychosis. JAMA Psychiatry 2016, 73, 1239. [Google Scholar] [CrossRef]
- Gupta, S.; Ranganathan, M.; Dsouza, D.C. The early identification of psychosis: Can lessons be learnt from cardiac stress testing? Psychopharmacology 2016, 233, 19–37. [Google Scholar] [CrossRef][Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stern, Y.; Koren, D.; Moebus, R.; Panishev, G.; Salomon, R. Assessing the Relationship between Sense of Agency, the Bodily-Self and Stress: Four Virtual-Reality Experiments in Healthy Individuals. J. Clin. Med. 2020, 9, 2931. https://doi.org/10.3390/jcm9092931
Stern Y, Koren D, Moebus R, Panishev G, Salomon R. Assessing the Relationship between Sense of Agency, the Bodily-Self and Stress: Four Virtual-Reality Experiments in Healthy Individuals. Journal of Clinical Medicine. 2020; 9(9):2931. https://doi.org/10.3390/jcm9092931
Chicago/Turabian StyleStern, Yonatan, Danny Koren, Renana Moebus, Gabriella Panishev, and Roy Salomon. 2020. "Assessing the Relationship between Sense of Agency, the Bodily-Self and Stress: Four Virtual-Reality Experiments in Healthy Individuals" Journal of Clinical Medicine 9, no. 9: 2931. https://doi.org/10.3390/jcm9092931
APA StyleStern, Y., Koren, D., Moebus, R., Panishev, G., & Salomon, R. (2020). Assessing the Relationship between Sense of Agency, the Bodily-Self and Stress: Four Virtual-Reality Experiments in Healthy Individuals. Journal of Clinical Medicine, 9(9), 2931. https://doi.org/10.3390/jcm9092931




