Six-Month Predictive Value of Diuretic Resistance Formulas in Discharged Heart Failure Patients after an Acute Decompensation
Abstract
:1. Introduction
2. Methods
2.1. Echocardiography Assessment
2.2. Blood Collection
2.3. Cardiovascular Events
2.4. Statistical Analysis
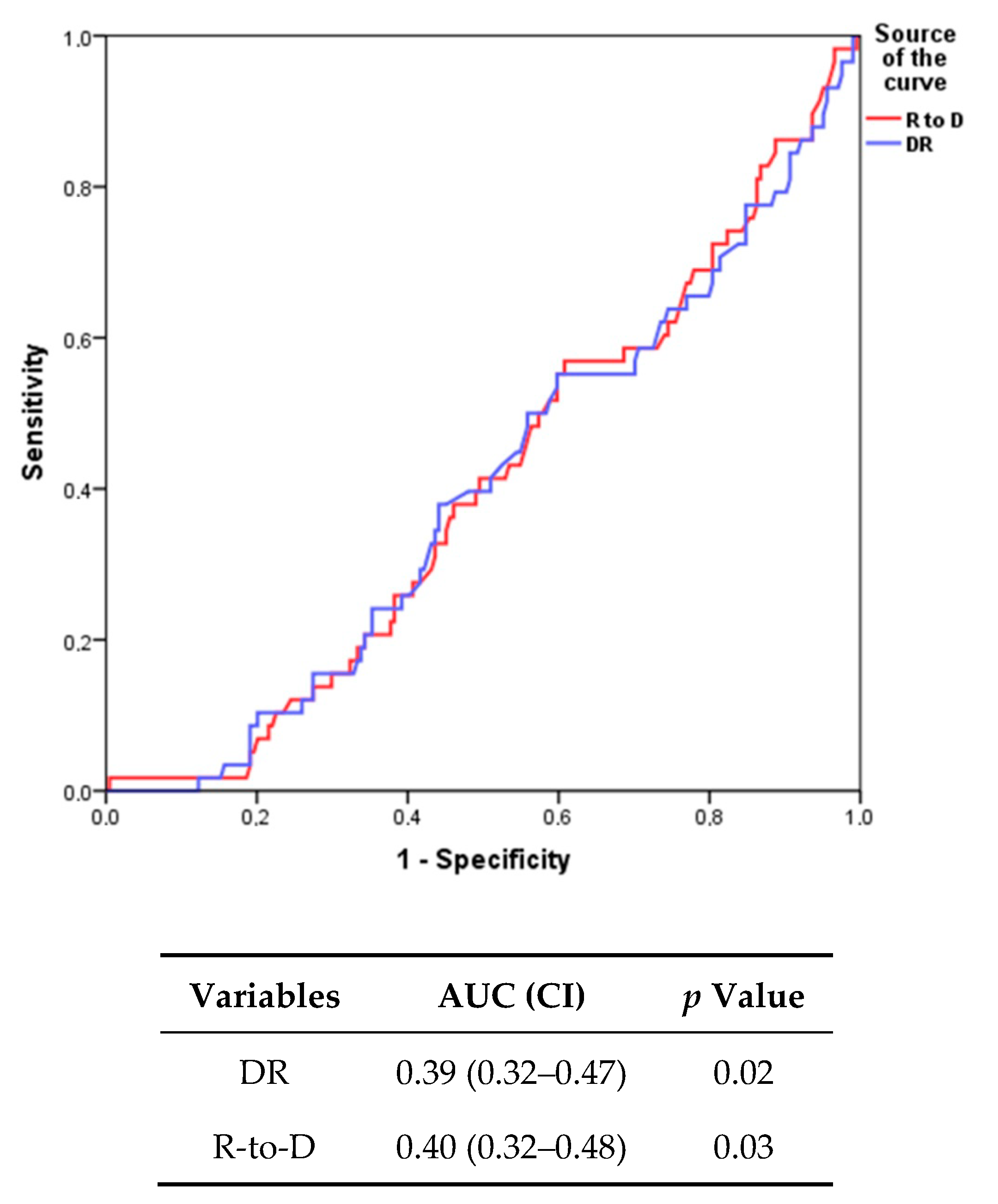
3. Results
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the Diagnosis and Treatment of acute and of chronic Heart Failure of the European Society of Cardiology with the special contribution of the Heart Failure Association. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [PubMed]
- Fonarow, G.C.; Adams, K.F.; Abraham, W.T.; Yancy, C.W.; Boscardin, W.J. Risk Stratification for In-Hospital Mortality in Acutely Decompensated Heart Failure. JAMA 2005, 293, 572–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boland, D.G.; Abraham, W.T. Natriuretic peptides in heart failure. Congest. Heart Fail 1998, 4, 23–33. [Google Scholar]
- McDonagh, T.A.; Robb, S.D.; Murdoch, D.R.; Morton, J.J.; Ford, I.; Morrison, C.E.; Tunsell-Podoe, H.; McMurray, J.J.; Dargie, H.J. Biochemical detection of left-ventricular systolic dysfunction. Lancet 1998, 351, 9–13. [Google Scholar] [CrossRef]
- Maisel, A.S.; Krishnaswamy, P.; Nowak, R.M.; McCord, J.; Hollander, J.; Duc, P.; Omland, T.; Storrow, A.B.; Abraham, W.T.; Wu, A.H.B.; et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N. Engl. J. Med. 2002, 347, 161–167. [Google Scholar] [CrossRef]
- Berger, R.; Huelsman, M.; Strecker, K.; Bojic, A.; Moser, P.; Stanek, B.; Pacher, R. B-type Natriuretic Peptide predicts sudden death in patients with chronic heart failure. Circulation 2002, 105, 2392–2397. [Google Scholar] [CrossRef] [Green Version]
- Feola, M.; Aspromonte, N.; Canali, C.; Ceci, V.; Giovinazzo, P.; Milani, L.; Quarta, G.; Ricci, R.; Scardovi, A.B.; Uslenghi, E.; et al. Prognostic value of plasma brain natriuretic peptide, urea nitrogen and creatinine in outpatients >70 years of age with heart failure. Am. J. Cardiol. 2005, 96, 705–709. [Google Scholar] [CrossRef]
- Van Kimmenade, R.R.; Jannuzzi, J.L.; Ellinor, P.T.; Sharma, U.C.; Bakker, J.A.; Low, A.F.; Martinez, A.; Crijns, H.J.; MacRae, C.A.; Menhereere, P.; et al. Utility of amino-terminal Pro-Brain Natriuretic Peptide, Galectin-3 and Apelin for the evaluation of patients with acute heart failure. J. Am. Coll. Cardiol. 2006, 48, 1217–1224. [Google Scholar] [CrossRef] [Green Version]
- Neuberg, G.W.; Miller, A.B.; O’Connor, C.M.; Belkin, R.N.; Carson, P.E.; Cropp, A.B.; Frid, D.J.; Nye, R.G.; Pressler, M.L.; Wertheimer, J.H.; et al. Diuretic resistance predicts mortality in patients with advanced heart failure. Am. Heart J. 2002, 144, 31–38. [Google Scholar] [CrossRef]
- Ellison, D.H. Diuretic therapy and resistance in congestive heart failure. Cardiology 2001, 96, 132–143. [Google Scholar] [CrossRef]
- Sambandam, K.K. Effective use of loop diuretics in heart failure exacerbation: A Nephrologist’s view. Am. J. Med. Sci. 2014, 347, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.R.; Komajda, M.; Murray-Thomas, T.; Underwood, J.; Ticho, B. Prevalence and impact of worsening renal function in patients hospitalized with decompensated heart failure: Results of prospective outcomes study in heart failure (POSH). Eur. Heart J. 2006, 27, 1216–1222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forman, D.E.; Butler, J.; Wang, Y.; Abraham, W.T.; O’Connor, C.M.; Gottlieb, S.S. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J. Am. Coll. Cardiol. 2004, 43, 61–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aronsonk, D.; Burger, A.J. The relationship between transient and persistent worsening renal function and mortality in patients with acute decompensated heart failure. J. Card. Fail 2010, 16, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Givertz, M.M.; Postmus, D.; Hillege, H.L.; Mansoon, G.A.; Massie, B.M.; Davison, B.A.; Ponikowski, P.; Metra, M.; Teerlink, J.R.; Cleland, J.G.; et al. Renal function trajectories and clinical outcomes in acute heart failure. Circ. Heart Fail 2014, 7, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Valente, M.A.E.; Voors, A.A.; Damman, K.; Van Veldhuisen, D.J.; Massie, B.M.; O’Connor, C.M.; Metra, M.; Ponikowski, P.; Teerlink, J.R.; Cotter, G.; et al. Diuretic response in acute heart failure: Clinical characteristics and prognostic significance. Eur. Heart J. 2014, 35, 1284–1293. [Google Scholar] [CrossRef] [Green Version]
- Feola, M.; Testa, M.; Ferreri, C.; Cardone, M.; Sola, M.; Ariotti, S.; Rosso, G.L. Role of Response-to-Diuretic in predicting prognosis in discharged heart failure patients after an acute decompensation. Arch. Med. Res. 2018, 49, 198–204. [Google Scholar] [CrossRef]
- Filippatos, G.; Parissis, J.T. Heart failure diagnosis and prognosis in the elderly: The proof of the pudding is in the eating. Eur. J. Heart Fail 2011, 13, 467–471. [Google Scholar] [CrossRef] [Green Version]
- Peacock, W.F. Rapid optimization: Strategies for optimal care of decompensated congestive heart failure patients in the emergency department. Rev. Cardiovasc. Med. 2003, 3, 41–48. [Google Scholar]
- Sahn, D.J.; Demaria, A.; Kisslo, J.; Weyman, A. The committee on M mode standardisation of the American Society of Echocardiography: Recommendations regarding quantification in M-mode echocardiography: Results of a survey of echocardiographic measurements. Circulation 1978, 58, 1072–1083. [Google Scholar] [CrossRef] [Green Version]
- Testani, J.M.; Brisco, M.A.; Kociol, R.D.; Jacoby, D.; Bellmkonda, L.; Parikh, C.R.; Coca, S.G.; Tang, W.H. Substantially discrepancy between fluid and weight loss during acute decompensated heart failure treatment. Am. J. Med. 2015, 128, 776–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mullens, W.; Dammman, K.; Harjola, V.P.; Mebazaa, A.; La Rocca, H.P.B.; Martens, P.; Testani, J.M.; Tang, W.H.W.; Orso, F.; Rossignol, P.; et al. The use of diuretics in heart failure wit congestion-a position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail 2019, 21, 137–155. [Google Scholar] [CrossRef]
- Testani, J.M.; Brisco, M.A.; Turner, J.M.; Stpatz, E.S.; Bellumkonda, L.; Parikh, C.R.; Tang, W.W. Loop diuretic efficiency: A metric of diuretic responsiveness with prognostic importance in acute decompensated heart failure. Circ. Heart Fail 2014, 7, 261–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, D.; Shrestha, K.; Testani, J.M.; Verbrugge, F.H.; Dupont, M.; Mullens, W.; Tang, W.H. Insufficient natriuretic response to continous intravenous furosemide is associated with poor long-term outcomes in acute decompensated heart failure. J. Card. Fail 2014, 20, 392–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Testani, J.M.; Hanberg, J.S.; Cheng, S.; Rao, V.; Onyebeke, C.; Laur, O.; Kula, A.; Chen, M.; Wilson, F.P.; Darlington, A.; et al. Rapid and highly accurate prediction of poor diuretic natriuretic response in patients with heart failure. Circ. Heart Fail 2016, 9, e002370. [Google Scholar] [CrossRef] [Green Version]
- Di Lenarda, A.; Scherillo, M.; Maggioni, A.P.; Acquarone, N.; Ambrosio, G.B.; Anniccharico, M.; Bellis, P.; Bellotti, P.; DeMaria, R.; Lavecchia, R.; et al. Current presentation and management of heart failure in cardiology and internal medicine hospital units: A tale of words. The TEMISTOCLE study. Am. Heart J. 2003, 146, 735. [Google Scholar] [CrossRef]
- Hernandez, H.F.; Greiner, M.A.; Fonarow, G.C.; Hammill, B.G.; Heidenreich, P.A.; Yancy, C.W.; Peterson, E.D.; Curtis, L.H. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA 2010, 303, 1716–1722. [Google Scholar] [CrossRef] [Green Version]
- Di Tano, G.; De Maria, R.; Gonzini, L.; Aspromonte, N.; Di Lenarda, A.; Feola, M.; Marini, M.; Milli, M.; Misuraca, G.; Mortara, A.; et al. The 30-day metric in acute heart failure revisited: Data from IN-HF Outcome, an Italian nationwide cardiology registry. Eur. J. Heart Fail 2015, 17, 1032–1041. [Google Scholar] [CrossRef]
- Ergatoudes, C.; Schaufelberger, M.; Pivodic, A.; Dahlstrom, U.; Fu, M. Non-cardiac comorbidities and mortality in patient with heart failure with reduced vs preserved ejection fraction. A study using the Swedish heart Failure Registry. Clin. Res. Cardiol. 2019, 108, 1025–1033. [Google Scholar] [CrossRef] [Green Version]


| Variables | No-Event Group (205 pts) | Event Group (58 pts) | p Value |
|---|---|---|---|
| Age | 77 (70–82) | 79 (76–85) | 0.01 |
| Gender Male (%) | 63 | 64 | 0.88 |
| Sodium (MEq/L) | 140 (138–143) | 140 (136–142) | 0.04 |
| Creatinine (mg/dL) | 1.06 (0.82–1.45) | 1.53 (1.04–2.05) | <0.001 |
| GFR mL/min/1.73 mq | 52 (40–68) | 39 (28–50) | <0.001 |
| Hb (gr/L) | 12 (10.5–13.7) | 12.6 (11.2–13.5) | 0.22 |
| RDW | 14.8 (13.8–16) | 15.9 (14.7–17.7) | <0.001 |
| Pro-BNP (pg/mL) | 5210 (1851–14,901) | 12,922 (7074–27,769) | <0.001 |
| LVEF (%) | 47 (35–55) | 38 (26–50) | 0.001 |
| LVESD (mm) | 37 (30–46) | 45 (35–57) | 0.008 |
| LVEDD (mm) | 51 (46–60) | 59 (48–66) | 0.04 |
| TAPSE (mm) | 17 (15–20) | 16 (15–20) | 0.32 |
| PASP (mmHg) | 37 (30–45) | 45 (35–58) | 0.003 |
| Length of hospital stay (days) | 10 (7–14) | 10 (7–15) | 0.35 |
| Baseline body weight (kg) | 73.9 (63–89.3) | 70.9 (56.8–89.9) | 0.30 |
| Body weight at Day 4 (kg) | 72.4 (60.4–87.8) | 70.9 (58–87.4) | 0.77 |
| Diuresis on Day 4 (mL/die) | 1800 (1300–2600) | 1900 (1400–2800) | 0.44 |
| Diuretic dosage on Day 4 (mg/die) | 60 (50–100) | 100 (50–237) | 0.005 |
| R-to-D (kg/40 mg furosemide) | 1.74 (1.02–3.48) | 1.53 (0.77–2.49) | 0.03 |
| Diuretic response (kg/40 mg furosemide) | 1.50 (0.75–3.20) | 1.29 (0.53–2.17) | 0.02 |
| Diabetes mellitus N (%) | 30 | 50 | 0.004 |
| Coronary artery disease N (%) | 39 | 33 | 0.38 |
| Hypertension (%) | 65 | 81 | 0.03 |
| CKD (%) | 66 | 83 | 0.01 |
| AF (%) | 34 | 53 | 0.02 |
| (A) 180-Day HF Rehospitalization or Death | ||||
| Univariate Analysis | Multivariate Analysis 1 | |||
| Variable | HR (95% CI) | p Value | HR (95% CI) | p Value |
| Q1 R to D (R to D < 0.97 kg/40 mg) | 2.53 (1.11–5.67) | 0.03 | 2.36 (1.02–5.47) | 0.04 |
| Q2 R to D (0.97 ≤ R to D < 1.69 kg/40 mg) | 2.09 (0.88–4.93) | 0.09 | 2.52 (1.04–6.10) | 0.04 |
| Q3 R to D (1.69 ≤ R to D < 3.28 kg/40 mg) | 2.26 (0.97–5.29) | 0.06 | 2.35 (0.99–5.55) | 0.06 |
| Q4 R to D (R to D ≥ 3.28 kg/40 mg) | Reference | Reference | ||
| (B) 180-Day HF Rehospitalization or Death | ||||
| Q1 DR (DR < 0.67 kg/40 mg) | 2.56 (1.17–5.63) | 0.02 | 2.27 (1.01–5.08) | 0.05 |
| Q2 DR (0.67 ≤ DR < 1.44 kg/40 mg) | 1.54 (0.66–3.61) | 0.32 | 1.81 (0.76–4.31) | 0.18 |
| Q3 DR (1.44 ≤ DR < 2.80 kg/40 mg) | 2.13 (0.94–4.81) | 0.07 | 2.23 (0.97–5.12) | 0.06 |
| Q4 DR (DR ≥ 2.80 kg/40 mg) | Reference | Reference | ||
| Characteristics | All Patients | HFrEF | HFpEF | p Value |
|---|---|---|---|---|
| n = 263 | n = 146 | n = 117 | ||
| Age (years) | 78 (70–83) | 79 (73–83) | 76 (68–82) | 0.03 |
| Gender Male (%) | 62 | 71 | 52 | 0.003 |
| Comorbidities (%): | ||||
| CAD | 38 | 47 | 26 | 0.001 |
| Diabetes | 34 | 40 | 27 | 0.04 |
| Hypertension | 69 | 62 | 78 | 0.007 |
| AF | 38 | 41 | 35 | 0.42 |
| CKD | 69 | 74 | 64 | 0.08 |
| Laboratory variables | ||||
| Creatinine (mg/dL) | 1.13 (0.85–1.61) | 1.28 (0.94–1.89) | 1.02 (0.80–1.25) | <0.001 |
| eGFR (mL/min/m2) | 50 (37–66) | 45 (32–61) | 53 (45–73) | <0.001 |
| Hemoglobin (g/L) | 12.1 (10.7–13.7) | 12.9 (11.5–14.2) | 11 (10.1–11.9) | <0.001 |
| RDW | 14.9 (14–16.4) | 15.3 (14.2–16.5) | 14.6 (13.6–16) | 0.02 |
| NTproBNP (pg/mL) | 7851 (2623–17,989) | 10,285 (4027–26,078) | 3880 (1457–10,686) | <0.001 |
| Echocardiography: | ||||
| EDD (mm) | 53 (46–60) | 60 (51–65) | 48 (41–53) | <0.001 |
| ESD (mm) | 39 (31–47) | 46 (39–55) | 32 (28–39) | <0.001 |
| PASP (mmHg) | 40 (30–50) | 39 (30–50) | 40 (31–50) | 0.49 |
| TAPSE (mm) | 17 (15–20) | 16 (15–20) | 18 (15–20) | 0.31 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feola, M.; Rossi, A.; Testa, M.; Ferreri, C.; Palazzuoli, A.; Pastorini, G.; Ruocco, G. Six-Month Predictive Value of Diuretic Resistance Formulas in Discharged Heart Failure Patients after an Acute Decompensation. J. Clin. Med. 2020, 9, 2932. https://doi.org/10.3390/jcm9092932
Feola M, Rossi A, Testa M, Ferreri C, Palazzuoli A, Pastorini G, Ruocco G. Six-Month Predictive Value of Diuretic Resistance Formulas in Discharged Heart Failure Patients after an Acute Decompensation. Journal of Clinical Medicine. 2020; 9(9):2932. https://doi.org/10.3390/jcm9092932
Chicago/Turabian StyleFeola, Mauro, Arianna Rossi, Marzia Testa, Cinzia Ferreri, Alberto Palazzuoli, Guido Pastorini, and Gaetano Ruocco. 2020. "Six-Month Predictive Value of Diuretic Resistance Formulas in Discharged Heart Failure Patients after an Acute Decompensation" Journal of Clinical Medicine 9, no. 9: 2932. https://doi.org/10.3390/jcm9092932
APA StyleFeola, M., Rossi, A., Testa, M., Ferreri, C., Palazzuoli, A., Pastorini, G., & Ruocco, G. (2020). Six-Month Predictive Value of Diuretic Resistance Formulas in Discharged Heart Failure Patients after an Acute Decompensation. Journal of Clinical Medicine, 9(9), 2932. https://doi.org/10.3390/jcm9092932






