Fructosamine-3-Kinase as a Potential Treatment Option for Age-Related Macular Degeneration
Abstract
1. Introduction
2. Experimental Section
2.1. Recombinant Production of Fructosamine-3-Kinase
2.2. Autofluorescence Measurement of AGEs
2.3. FN3K Treatment of AGE-Modified Neural Porcine Retinas
2.4. FN3K Treatment of Human Neural Retinas
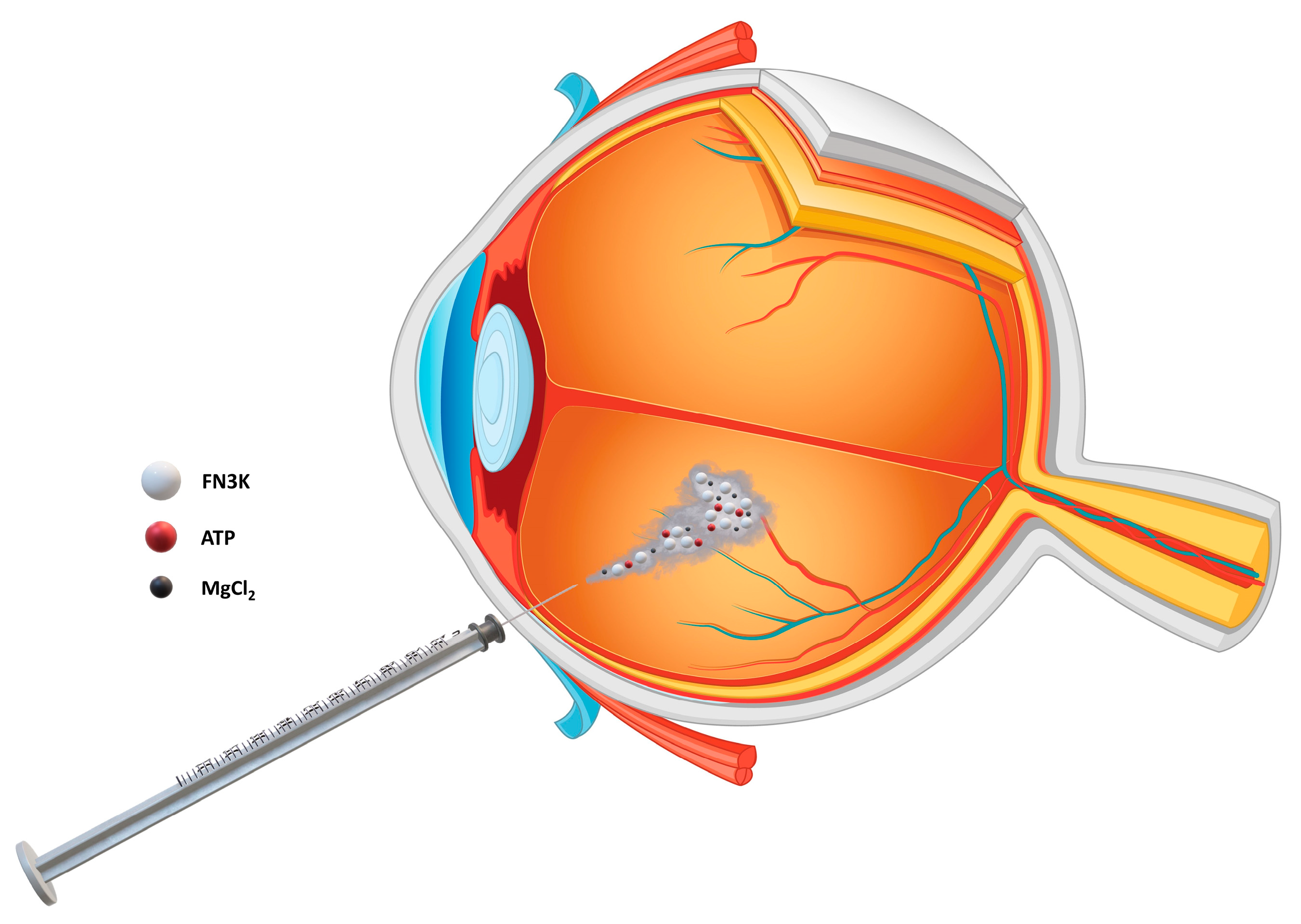
2.5. FN3K Treatment of Murine Eyes
2.6. FN3K Treatment of Human Eye Tissue Sections Containing Drusen
2.7. Near-Infrared Microspectroscopy and Multivariate Data Analysis
2.8. Ex Vivo Intravitreal FN3K Treatment of Post-Mortem Human Eyes
2.9. Statistical Analysis
3. Results
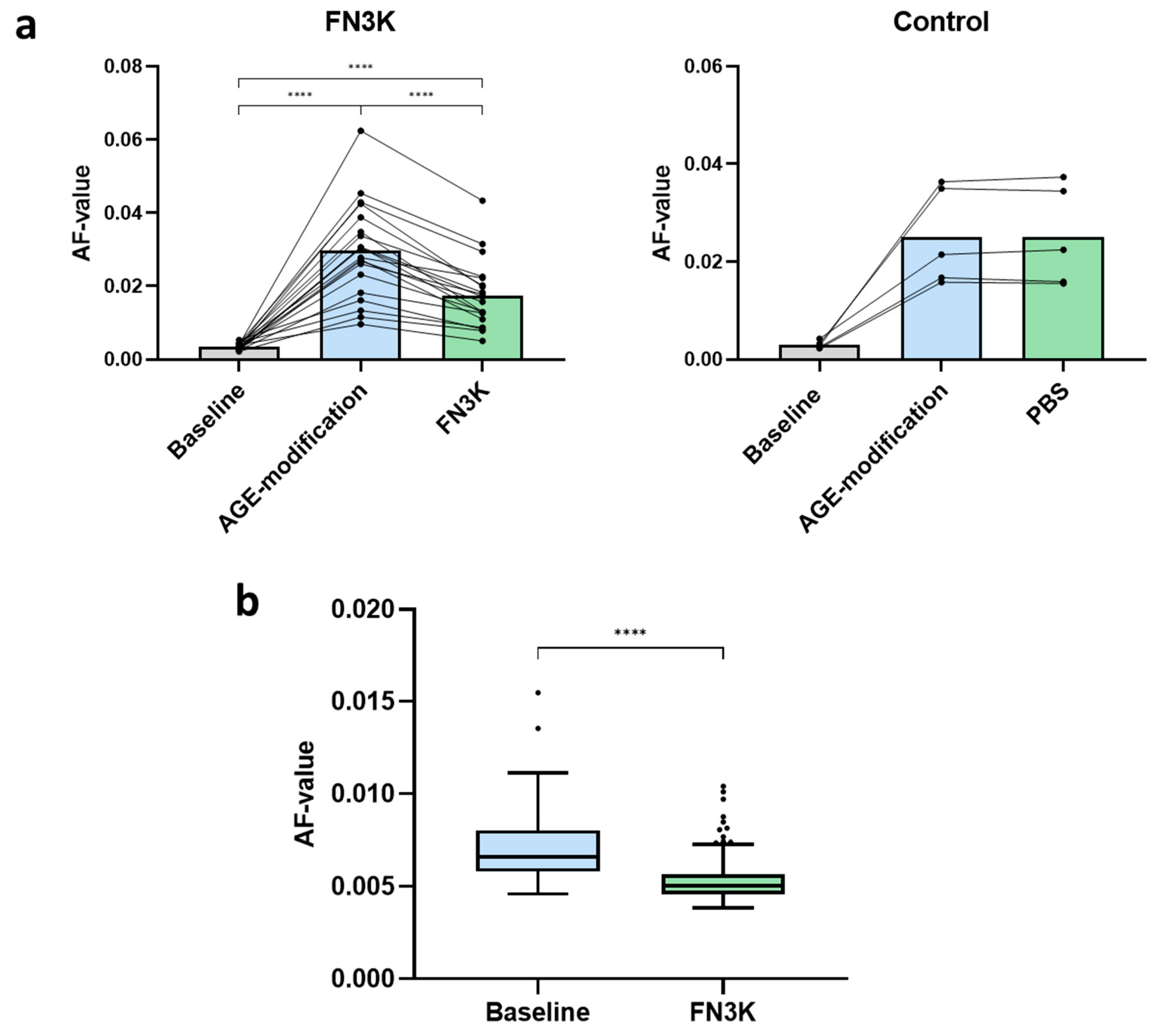
3.1. FN3K Treatment of AGE-Modified Neural Porcine Retinas and Human Neural Retinas Reduces AGE-Related Autofluorescence
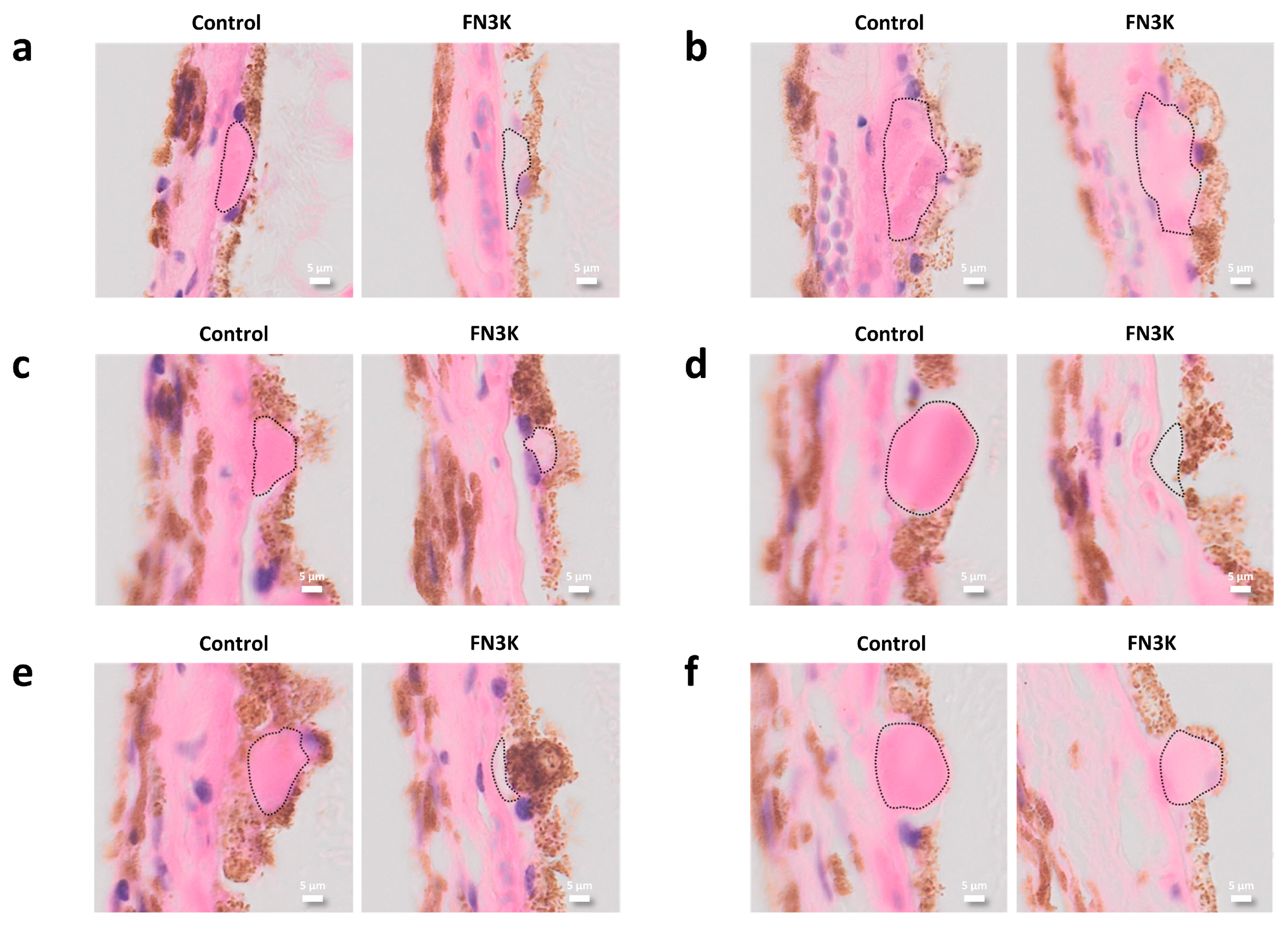
3.2. Murine Eyes Treated Intravitreally with FN3K Show Less Drusenoid Material and Lesions on Stained Tissue Sections
3.3. FN3K Treatment Reduces the Color Intensity and Area of Drusenoid Lesions on Stained Human Tissue Sections
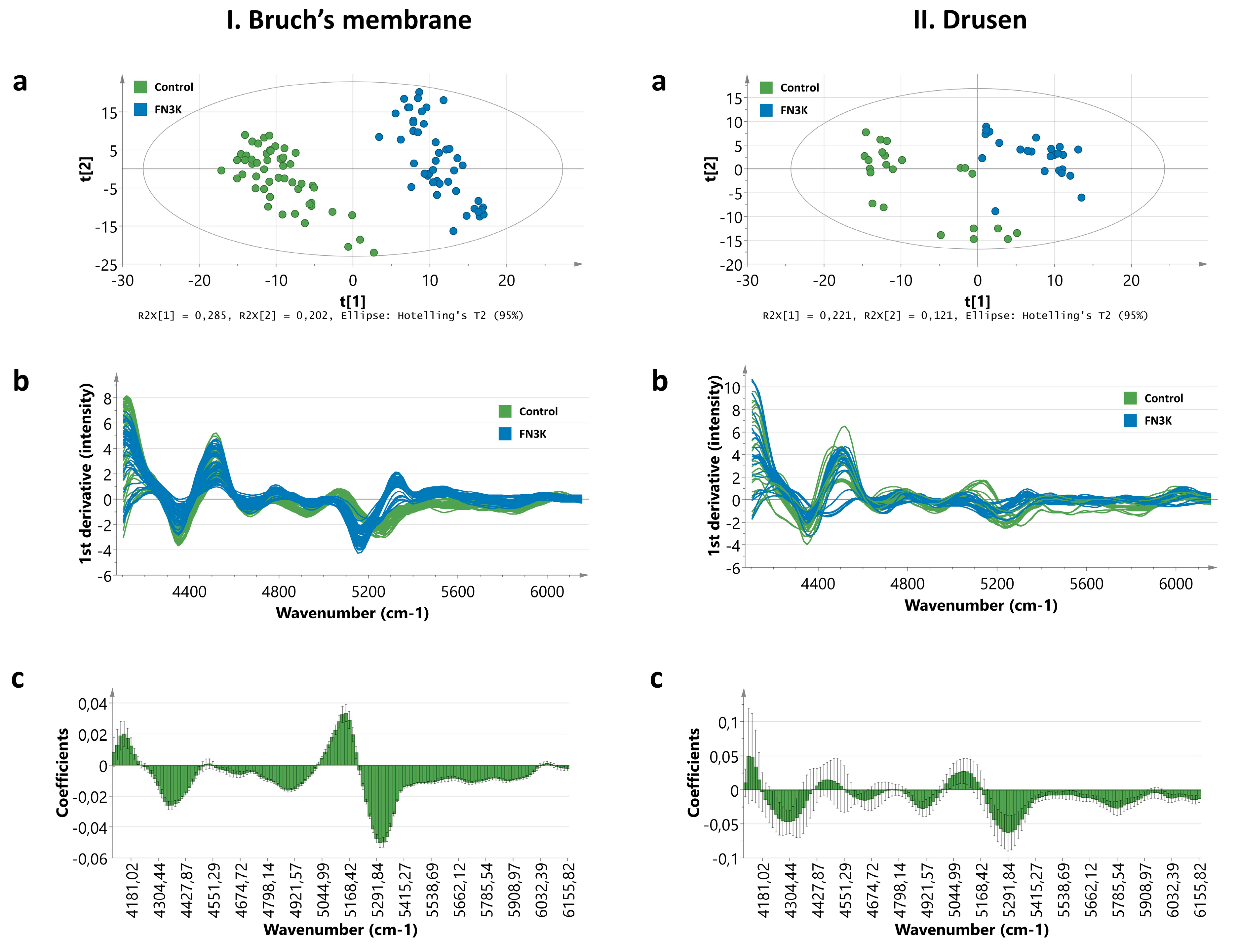
3.4. Near-Infrared Microspectroscopy on Stained Tissue Sections Unravels Biochemical Changes After FN3K Treatment
3.4.1. Bruch’s Membrane
3.4.2. Drusen
3.5. Ex Vivo Intravitreal FN3K Treatment on Human Eyes Strongly Reduces Size of Subretinal Drusenoid Deposits on Optical Coherence Tomography
4. Discussion
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mitchell, P.; Liew, G.; Gopinath, B.; Wong, T.Y. Age-related macular degeneration. Lancet 2018, 392, 1147–1159. [Google Scholar] [CrossRef]
- Yonekawa, Y.; Miller, J.; Kim, I. Age-Related Macular Degeneration: Advances in Management and Diagnosis. J. Clin. Med. 2015, 4, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Bowes Rickman, C.; Farsiu, S.; Toth, C.A.; Klingeborn, M. Dry age-related macular degeneration: Mechanisms, therapeutic targets, and imaging. Investig. Ophthalmol. Vis. Sci. 2013, 54, ORSF68–ORSF80. [Google Scholar] [CrossRef] [PubMed]
- Borrelli, E.; Diadori, A.; Zalaffi, A.; Bocci, V. Effects of major ozonated autohemotherapy in the treatment of dry age related macular degeneration: A randomized controlled clinical study. Int. J. Ophthalmol. 2012, 5, 708–713. [Google Scholar] [CrossRef]
- Schutt, F.; Bergmann, M.; Holz, F.G.; Kopitz, J. Proteins modified by malondialdehyde, 4-hydroxynonenal, or advanced glycation end products in lipofuscin of human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3663–3668. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, T.; Murata, T.; Hangai, M.; Nagai, R.; Horiuchi, S.; Lopez, P.F.; Hinton, D.R.; Ryan, S.J. Advanced glycation end products in age-related macular degeneration. Arch. Ophthalmol. 1998, 116, 1629–1632. [Google Scholar] [CrossRef]
- Glenn, J.V.; Beattie, J.R.; Barrett, L.; Frizzell, N.; Thorpe, S.R.; Boulton, M.E.; McGarvey, J.J.; Stitt, A.W. Confocal Raman microscopy can quantify advanced glycation end product (AGE) modifications in Bruch’s membrane leading to accurate, nondestructive prediction of ocular aging. FASEB J. 2007, 21, 3542–3552. [Google Scholar] [CrossRef]
- Glenn, J.V.; Mahaffy, H.; Wu, K.; Smith, G.; Nagai, R.; Simpson, D.A.C.; Boulton, M.E.; Stitt, A.W. Advanced glycation end product (AGE) accumulation on Bruch’s membrane: Links to age-related RPE dysfunction. Investig. Ophthalmol. Vis. Sci. 2009, 50, 441–451. [Google Scholar] [CrossRef]
- Yoon, K.D.; Yamamoto, K.; Ueda, K.; Zhou, J.; Sparrow, J.R. A novel source of methylglyoxal and glyoxal in Retina: Implications for age-related macular degeneration. PLoS ONE 2012, 7, e41309. [Google Scholar] [CrossRef]
- Delpierre, G.; Collard, F.; Fortpied, J.; Van Schaftingen, E. Fructosamine 3-kinase is involved in an intracellular deglycation pathway in human erythrocytes. Biochem. J. 2002, 365, 801–808. [Google Scholar] [CrossRef]
- Avemaria, F.; Carrera, P.; Lapolla, A.; Sartore, G.; Chilelli, N.C.; Paleari, R.; Ambrosi, A.; Ferrari, M.; Mosca, A. Possible role of fructosamine 3-kinase genotyping for the management of diabetic patients. Clin. Chem. Lab. Med. 2015, 53, 1315–1320. [Google Scholar] [CrossRef] [PubMed]
- Conner, J.R.; Beisswenger, P.J.; Szwergold, B.S. The expression of the genes for fructosamine-3-kinase and fructosamine-3-kinase-related protein appears to be constitutive and unaffected by environmental signals. Biochem. Biophys. Res. Commun. 2004, 323, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Schoonooghe, S.; Kaigorodov, V.; Zawisza, M.; Dumolyn, C.; Haustraete, J.; Grooten, J.; Mertens, N. Efficient production of human bivalent and trivalent anti-MUC1 Fab-scFv antibodies in Pichia pastoris. BMC Biotechnol. 2009, 9, 70. [Google Scholar] [CrossRef] [PubMed]
- Hayer-Hartl, M. Assay of malate dehydrogenase. A substrate for the E. coli chaperonins GroEL and GroES. Methods Mol. Biol. 2000, 140, 127–132. [Google Scholar] [CrossRef]
- Delpierre, G.; Rider, M.H.; Collard, F.; Stroobant, V.; Vanstapel, F.; Santos, H.; Van Schaftingen, E. Identification, cloning, and heterologous expression of a mammalian fructosamine-3-kinase. Diabetes 2000, 49, 1627–1634. [Google Scholar] [CrossRef]
- Szwergold, B.S.; Howell, S.; Beisswenger, P.J. Human fructosamine-3-kinase: Purification, sequencing, substrate specificity, and evidence of activity in vivo. Diabetes 2001, 50, 2139–2147. [Google Scholar] [CrossRef]
- Ashraf, J.M.; Ahmad, S.; Choi, I.; Ahmad, N.; Farhan, M.; Tatyana, G.; Shahab, U. Recent advances in detection of AGEs: Immunochemical, bioanalytical and biochemical approaches. IUBMB Life 2015, 67, 897–913. [Google Scholar] [CrossRef]
- Steenbeke, M.; De Bruyne, S.; Van Aken, E.; Glorieux, G.; Van Biesen, W.; Himpe, J.; De Meester, G.; Speeckaert, M.; Delanghe, J. UV Fluorescence-Based Determination of Urinary Advanced Glycation End Products in Patients with Chronic Kidney Disease. Diagnostics 2020, 10, 34. [Google Scholar] [CrossRef]
- Davis, M.D.; Gangnon, R.E.; Lee, L.-Y.; Hubbard, L.D.; Klein, B.E.K.; Klein, R.; Ferris, F.L.; Bressler, S.B.; Milton, R.C. The Age-Related Eye Disease Study severity scale for age-related macular degeneration: AREDS Report No. 17. Arch. Ophthalmol. Chic. Ill 1960 2005, 123, 1484–1498. [Google Scholar] [CrossRef]
- Espinosa-Heidmann, D.G.; Suner, I.J.; Catanuto, P.; Hernandez, E.P.; Marin-Castano, M.E.; Cousins, S.W. Cigarette smoke-related oxidants and the development of sub-RPE deposits in an experimental animal model of dry AMD. Investig. Ophthalmol. Vis. Sci. 2006, 47, 729–737. [Google Scholar] [CrossRef]
- Fernandez, D.C.; Bhargava, R.; Hewitt, S.M.; Levin, I.W. Infrared spectroscopic imaging for histopathologic recognition. Nat. Biotechnol. 2005, 23, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Varma, V.K.; Kajdacsy-Balla, A.; Akkina, S.K.; Setty, S.; Walsh, M.J. A label-free approach by infrared spectroscopic imaging for interrogating the biochemistry of diabetic nephropathy progression. Kidney Int. 2016, 89, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- De Bruyne, S.; Speeckaert, M.M.; Delanghe, J.R. Applications of mid-infrared spectroscopy in the clinical laboratory setting. Crit. Rev. Clin. Lab. Sci. 2018, 55, 1–20. [Google Scholar] [CrossRef] [PubMed]
- De Bruyne, S.; Speeckaert, R.; Boelens, J.; Hayette, M.-P.P.M.-P.; Speeckaert, M.; Delanghe, J. Infrared spectroscopy as a novel tool to diagnose onychomycosis. Br. J. Dermatol. 2019, 180, 637–646. [Google Scholar] [CrossRef]
- Bergen, A.A.; Arya, S.; Koster, C.; Pilgrim, M.G.; Wiatrek-Moumoulidis, D.; van der Spek, P.J.; Hauck, S.M.; Boon, C.J.F.; Emri, E.; Stewart, A.J.; et al. On the origin of proteins in human drusen: The meet, greet and stick hypothesis. Prog. Retin. Eye Res. 2019, 70, 55–84. [Google Scholar] [CrossRef]
- Thompson, R.B.; Reffatto, V.; Bundy, J.G.; Kortvely, E.; Flinn, J.M.; Lanzirotti, A.; Jones, E.A.; McPhail, D.S.; Fearn, S.; Boldt, K.; et al. Identification of hydroxyapatite spherules provides new insight into subretinal pigment epithelial deposit formation in the aging eye. Proc. Natl. Acad. Sci. USA 2015, 112, 1565–1570. [Google Scholar] [CrossRef]
- Garg, U.; Althahabi, R.; Amirahmadi, V.; Brod, M.; Blanchard, C.; Young, T. Hyaluronidase as a Liquefying Agent for Chemical Analysis of Vitreous Fluid. J. Forensic Sci. 2004, 49, 388–391. [Google Scholar] [CrossRef]
- Buhren, B.A.; Schrumpf, H.; Hoff, N.-P.; Bölke, E.; Hilton, S.; Gerber, P.A. Hyaluronidase: From clinical applications to molecular and cellular mechanisms. Eur. J. Med. Res. 2016, 21, 5. [Google Scholar] [CrossRef]
- Rüschen, H.; Aravinth, K.; Bunce, C.; Bokre, D. Use of hyaluronidase as an adjunct to local anaesthetic eye blocks to reduce intraoperative pain in adults. Cochrane Database Syst. Rev. 2018, 3, CD010368. [Google Scholar] [CrossRef]
- Repetto, R.; Siggers, J.H.; Stocchino, A. Mathematical model of flow in the vitreous humor induced by saccadic eye rotations: Effect of geometry. Biomech. Model. Mechanobiol. 2010, 9, 65–76. [Google Scholar] [CrossRef]
- Owen, L.A.; Shakoor, A.; Morgan, D.J.; Hejazi, A.A.; McEntire, M.W.; Brown, J.J.; Farrer, L.A.; Kim, I.; Vitale, A.; DeAngelis, M.M. The Utah Protocol for Postmortem Eye Phenotyping and Molecular Biochemical Analysis. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1204–1212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Bruyne, S.; Van Dorpe, J.; Himpe, J.; Van Biesen, W.; Delanghe, S.; Speeckaert, M.M.; Delanghe, J.R. Detection and Characterization of a Biochemical Signature Associated with Diabetic Nephropathy Using Near-infrared Spectroscopy on Tissue Sections. J. Clin. Med. 2019, 8, 1022. [Google Scholar] [CrossRef] [PubMed]
- Westenskow, P.D. Nicotinamide: A novel treatment for age-related macular degeneration? Stem Cell Investig. 2017, 4, 1–7. [Google Scholar] [CrossRef]
- Dunmore, S.J.; Al-Derawi, A.S.; Nayak, A.U.; Narshi, A.; Nevill, A.M.; Hellwig, A.; Majebi, A.; Kirkham, P.; Brown, J.E.; Singh, B.M. Evidence that differences in fructosamine-3-kinase activity may be associated with the glycation gap in human diabetes. Diabetes 2018, 67, 131–136. [Google Scholar] [CrossRef]
- Semba, R.D.; Cotch, M.F.; Gudnason, V.; Eiriksdottir, G.; Harris, T.B.; Sun, K.; Klein, R.; Jonasson, F.; Ferrucci, L.; Schaumberg, D.A. Serum carboxymethyllysine, an advanced glycation end product, and age-related macular degeneration: The age, gene/environment susceptibility- Reykjavik study. JAMA Ophthalmol. 2014, 132, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Howes, K.A.; Liu, Y.; Dunaief, J.L.; Milam, A.; Frederick, J.M.; Marks, A.; Baehr, W. Receptor for advanced glycation end products and age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2004, 45, 3713–3720. [Google Scholar] [CrossRef]
- Handa, J.T.; Verzijl, N.; Matsunaga, H.; Aotaki-Keen, A.; Lutty, G.A.; Te Koppele, J.M.; Miyata, T.; Hjelmeland, L.M. Increase in the advanced glycation end product pentosidine in Bruch’s membrane with age. Investig. Ophthalmol. Vis. Sci. 1999, 40, 775–779. [Google Scholar] [PubMed]
- Yamada, Y.; Ishibashi, K.; Ishibashi, K.; Bhutto, I.A.; Tian, J.; Lutty, G.A.; Handa, J.T. The expression of advanced glycation endproduct receptors in rpe cells associated with basal deposits in human maculas. Exp. Eye Res. 2006, 82, 840–848. [Google Scholar] [CrossRef]
- Glenn, J.V.; Stitt, A.W. The role of advanced glycation end products in retinal ageing and disease. Biochim. Biophys. Acta. 2009, 1790, 1109–1116. [Google Scholar] [CrossRef]
- Boyer, N.P.; Higbee, D.; Currin, M.B.; Blakeley, L.R.; Chen, C.; Ablonczy, Z.; Crouch, R.K.; Koutalos, Y. Lipofuscin and N-retinylidene-N-retinylethanolamine (A2E) accumulate in retinal pigment epithelium in absence of light exposure: Their origin is 11-cis-retinal. J. Biol. Chem. 2012, 287, 22276–22286. [Google Scholar] [CrossRef]
- Schlanitz, F.G.; Baumann, B.; Kundi, M.; Sacu, S.; Baratsits, M.; Scheschy, U.; Shahlaee, A.; Mittermüller, T.J.; Montuoro, A.; Roberts, P.; et al. Drusen volume development over time and its relevance to the course of age-related macular degeneration. Br. J. Ophthalmol. 2017, 101, 198–203. [Google Scholar] [CrossRef] [PubMed]
- de Amorim Garcia Filho, C.A.; Yehoshua, Z.; Gregori, G.; Nunes, R.P.; Penha, F.M.; Moshfeghi, A.A.; Zhang, K.; Feuer, W.; Rosenfeld, P.J. Change in drusen volume as a novel clinical trial endpoint for the study of complement inhibition in age-related macular degeneration. Ophthalmic Surg. Lasers Imaging Retina 2014, 45, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Crabb, J.W.; Miyagi, M.; Gu, X.; Shadrach, K.; West, K.A.; Sakaguchi, H.; Kamei, M.; Hasan, A.; Yan, L.; Rayborn, M.E.; et al. Drusen proteome analysis: An approach to the etiology of age-related macular degeneration. Proc. Natl. Acad. Sci. USA 2002, 99, 14682–14687. [Google Scholar] [CrossRef] [PubMed]
- Forest, D.L.; Johnson, L.V.; Clegg, D.O. Cellular models and therapies for age-related macular degeneration. Dis. Models Mech. 2015, 8, 421–427. [Google Scholar] [CrossRef]
- Bird, A.C. Therapeutic targets in age-related macular disease. J. Clin. Investig. 2010, 120, 3033–3041. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Bruyne, S.; Van den Broecke, C.; Vrielinck, H.; Khelifi, S.; De Wever, O.; Bracke, K.; Huizing, M.; Boston, N.; Himpe, J.; Speeckaert, M.; et al. Fructosamine-3-Kinase as a Potential Treatment Option for Age-Related Macular Degeneration. J. Clin. Med. 2020, 9, 2869. https://doi.org/10.3390/jcm9092869
De Bruyne S, Van den Broecke C, Vrielinck H, Khelifi S, De Wever O, Bracke K, Huizing M, Boston N, Himpe J, Speeckaert M, et al. Fructosamine-3-Kinase as a Potential Treatment Option for Age-Related Macular Degeneration. Journal of Clinical Medicine. 2020; 9(9):2869. https://doi.org/10.3390/jcm9092869
Chicago/Turabian StyleDe Bruyne, Sander, Caroline Van den Broecke, Henk Vrielinck, Samira Khelifi, Olivier De Wever, Ken Bracke, Manon Huizing, Nezahat Boston, Jonas Himpe, Marijn Speeckaert, and et al. 2020. "Fructosamine-3-Kinase as a Potential Treatment Option for Age-Related Macular Degeneration" Journal of Clinical Medicine 9, no. 9: 2869. https://doi.org/10.3390/jcm9092869
APA StyleDe Bruyne, S., Van den Broecke, C., Vrielinck, H., Khelifi, S., De Wever, O., Bracke, K., Huizing, M., Boston, N., Himpe, J., Speeckaert, M., Vral, A., Van Dorpe, J., Van Aken, E., & Delanghe, J. R. (2020). Fructosamine-3-Kinase as a Potential Treatment Option for Age-Related Macular Degeneration. Journal of Clinical Medicine, 9(9), 2869. https://doi.org/10.3390/jcm9092869







